Abstract
Gliomas constitute 80% of malignant brain tumors. The survival rate of patients diagnosed with malignant gliomas is only 34.4%, as seen in both adults as well as children. The biggest challenge in treatment of gliomas is the impenetrable blood–brain barrier. With the availability of only a very few choices of chemotherapeutics in the treatment of gliomas, it is imperative that a novel strategy to effectively deliver drugs into the brain is researched and applied. The most popular strategy that is gaining importance is the receptor-mediated uptake of targeted nanoparticles comprising of ligands specific to the receptors. This review discusses briefly one such receptor called the transferrin receptor that is highly expressed in the brain and can be applied effectively for targeted nanoparticle delivery systems in gliomas.
Introduction
Gliomas are tumors that derive from glial cells and are the most common form of brain cancer. They usually occur in the cerebral hemispheres, but they can also occur anywhere in the central nervous system (CNS). Gliomas make up about 30% of all types of brain and CNS tumors and 80% of all malignant brain tumors. The average survival rate for those with malignant brain tumors is only 34.4%.1 They can occur in both children and adults, and efforts are needed to develop a highly effective means of treatment to reduce the number of deaths from gliomas. Even with aggressive chemotherapy and surgical procedures, gliomas are incurable because the total resection of the tumor is impossible without damaging the surrounding healthy cells. Due to the fact that gliomas are located in the brain, and both complete resection and chemotherapy are ineffective, novel methods of treatment are needed to make gliomas curable.
One of the methods that is extensively researched and found to be promising is targeted brain delivery via receptor-mediated uptake into the brain across the blood–brain barrier (BBB). Transferrin (Tf) receptors (TfR) have gained a lot of popularity in this regard and have been widely explored for drug delivery into the brain. Tf is a glycoprotein that plays a central role in iron metabolism, and it is responsible for ferric ion (Fe3+) delivery (Figure 1). Fe3+ is the form of iron that binds to Tf (which in turn binds to the Tf receptor and is generated upon the oxidation of the Fe2+). Tf also removes toxic iron from the blood and the brain. Tf belongs to the transferrin family, which includes melano-, ovo-, and serum-Tf, but for this review, Tf will be explored in general.
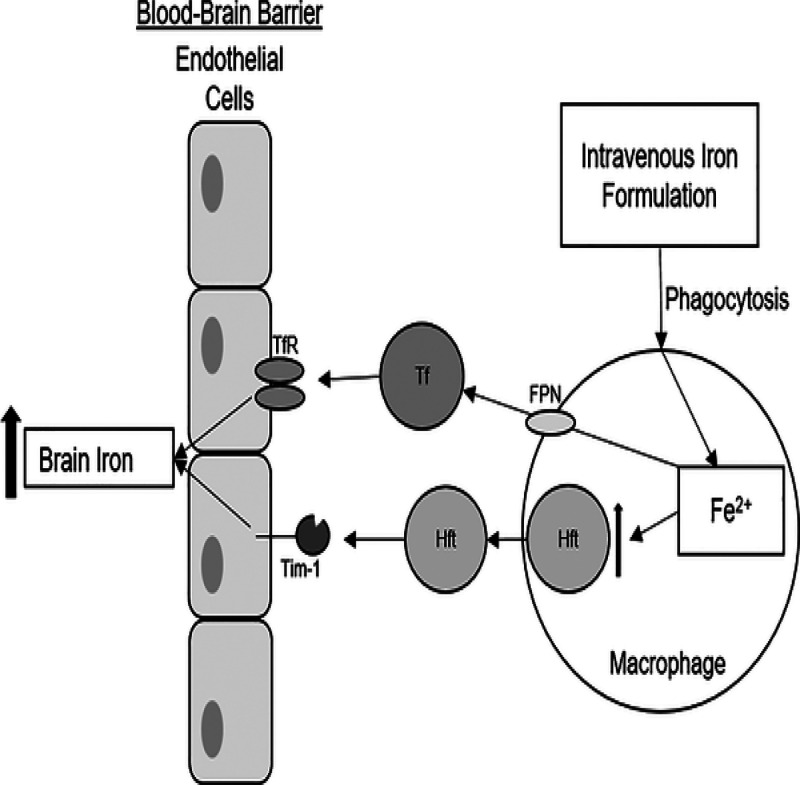
Figure 1.

Fe2+ first becomes oxidized by losing an electron and turns into Fe+3 (ferric iron). Ferric iron will then bind to transferrin, which subsequently binds to the transferrin receptor. Binding to the TfR allows for iron to enter the brain through the BBB.2 Adapted with permission from ref (2). Copyright 2018 Brian Lindshield, Kansas State University. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Transferrin Receptors
Tf is highly researched in relation to gliomas and their treatment because of its ability to cross the BBB. The BBB is a highly selective semipermeable border of capillary endothelial cells which allows the movement of only a few select molecules across it, Tf being one. Transferrin receptors are expressed extensively at several sites in the body such as RBCs (red blood cells), endothelial cells in the brain, and also on different types of cancerous cells.3 In glioma, Tf receptors are known to be overexpressed on the brain capillary endothelial cells and tumor cells because of which this receptor system provides a good avenue for targeted therapy.
There are two transferrin receptors (TfR) known as TfR1 and TfR2.4 TfR1 is a human protein that is encoded by the TFRC gene. It is required for iron import bound to Tf into cells by endocytosis as mentioned in Figure 1. It is found highly expressed in the brain capillary endothelium and neurons and is overexpressed in gliomas. On the other hand, TfR2 is encoded by the TFR2 gene and is also involved in the uptake of Tf-bound iron into cells (expressed to a much lesser extent in the brain endothelial cells than TfR1).5 Maintaining homeostasis is important because a low level of the Tf disrupts the normal liver functioning and high level leads to loss of Tf from the body. Further, a high Tf level, such as seen in neurodegenerative disorders like Parkinson’s and Alzheimer’s diseases, indicates iron deficiency anemia.6 This shows that dyshomeostasis can occur when Tf levels are disturbed. Disorders of iron metabolism, especially overexpression of iron and increased iron acquisition and retention, can induce tumorigenesis and increase the growth and metastasis of cancer.
Structure and Composition of Transferrin Receptor
Tf is 80 kDa glycoprotein that consists of 679 amino acids and two carbohydrate chains. The structure of Tf comprises two homologous lobes, dubbed the N-lobe and the C-lobe, which are connected by a peptide (Figure 2).7 In both of the lobes, ferric iron is bound very tightly. Both the N-lobe and the C-lobe can be divided even further into the subdomains N1, N2 and C1, C2, respectively, with a cleft between the two, as shown in Figure 2. In the cleft, ferric iron is bound to the four amino acids found in Tf: two tyrosines, one aspartic acid, and one histidine. The amino acids that bind to the site are similar for both the N- and C-lobes. In Tf, the peptide between the two lobes is unstructured, and disulfide bonds are formed in between N1, N2 and C1, C2.
Figure 2.
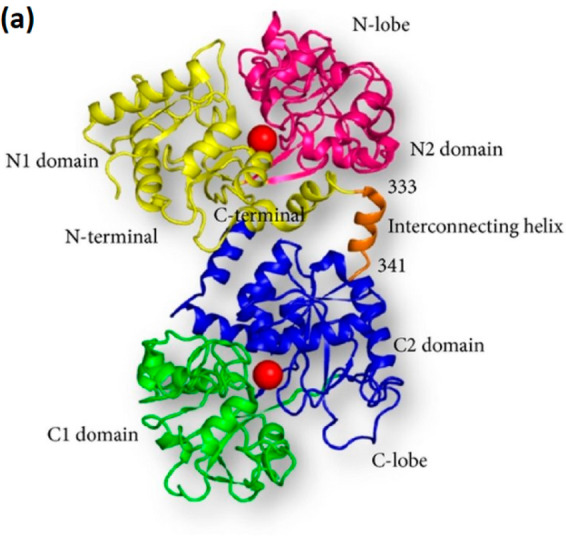
Structure of TfR: Transferrin is made up of α-helices and β-sheets. There are N- and C-lobes, with them both being divided further into N1, N2 and C1, C2 respectively.7 Reprinted with permission from ref (7). Copyright 2013 Sujata Sharma et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The N- and C-lobes are composed of α-helices and β-sheets with an iron binding site between the two lobes. The structure of Tf allows it to have conformational changes when it takes up or releases iron. This is possible because of the domains that rotate around the axis.7 Also because of its ability to reversibly bind Fe3+, Tf can exist without iron as apotransferrin (apo-Tf) or with iron as holo-Tf. The iron free protein, apo-Tf, is a single chain glycoprotein that has a high affinity for ferric iron. Holo-Tf is also a single chain protein that also has a high affinity for Fe3+.8
TfR1 is composed of two disulfide-linked monomers joined by two disulfide bonds. Each one monomer binds to one holo-Tf. This creates an iron Tf-TfR complex, which enters the cell by endocytosis. Both TfR1 and TfR2 are both type II membrane proteins that function with two disulfide bonds and share 66% homology. Both receptors bind Tf, and many of the amino acids involved in the binding of TfR1 to Tf are conserved in TfR2.9 TfR1 binds holo-Tf, and it is responsible for the endocytosis of holo-Tf. TfR2 is also capable of iron uptake, but its ability to bind to Tf is not as strong as that with TfR1 (25 times less than that of TfR1).10 Their different binding abilities to Tf would suggest different functions (but this will not be discussed in detail because it is beyond the scope of this review).
Function of Transferrin Receptor
The main function of Tf is to transport iron in the body and, for the purpose of this review, to transport iron into the brain. Tf is the major source of iron delivery to the brain, and it delivers iron to cells by binding to the TfR on the cell surface. Both TfR1 and TfR2 bind Tf for transport into cells. As previously mentioned, TfR1 has a higher affinity for Tf. Additionally, TfR1 is highly expressed in almost all cells, whereas TfR2 is only expressed in a few cells, such as liver and erythroid cells.10 Although TfR1 is the major iron binding pathway to acquire iron for most cells, several studies have indicated that the uptake of iron changes depending on the cell type.11 If the TfRs are dysfunctional, the iron transport is affected throughout the body because TfR can no longer bind Tf and transport iron into cells. This will cause iron deficiency in the body and lead to anemia. A low expression of the Tf receptor results in a poor production of Tf or an excessive loss of it through urine. This will cause the iron in the blood to decrease. However, if there is increased iron acquisition and retention, this can induce tumorigenesis and increase the growth of cancer.6
Mechanism of Action
Tf receptors are found on neurons, glial cells, and endothelial cells.12 Both TfR1 and TfR2 (to some extent) are found in the normal brain cells such as neurons and capillary endothelial cells and are overexpressed in brain cancers such as gliomas. However, this review discusses more about TfR1 due to the relevance to gliomas. TfR1 are usually found on endothelial cells from glioblastomas and on the BBB.4 TfRs that are found in the brain have the ability to cross the BBB, which is a selectively permeable border. Tf crosses the BBB through the transcytosis processes via brain capillary endothelial cells.4 Transcytosis is a type of transcellular transport wherein the TfR is captured by the vesicles on one side of the cell, taken across the cell, and then released on the other side. Transcytosis is an active process and has been found to be pH- and temperature-dependent. To enter the brain, iron is transported by Tf, which is attached to the TfR (Figure 3).
Figure 3.
Tf crosses the BBB through transcytosis, a form of endocytosis. Fe+2 (free iron) is exported from the macrophage through ferroportin (FPN) and binds to Tf, which then binds to the TfR on the BBB. The TfR receptor then delivers iron to the brain, and the amount of iron in the brain increases.13 Reprinted with permission from ref (13). Copyright 2018 Chiou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Transferrin Receptors in Gliomas
Transferrin receptors are highly overexpressed on the surface of brain capillary endothelial cells and tumor cells in brain cancer including gliomas. TfR1 in gliomas increases iron accumulation and promotes tumor progression by two mechanisms: an increase in the proliferation rate and glutamate production.14 TfR2 is also highly expressed in glioblastomas (GBM). The overexpression of TfR2 increases the cell proliferation rate in GBM. Overall, the overexpression of the TfR on gliomas worsens the prognosis for gliomas and GBM. The fact that the TfRs are overexpressed in brain cancers can be used to the advantage of designing targeted therapies for brain cancers including gliomas.
Nanoparticles Targeting Transferrin Receptors
Chemotherapy, radiotherapy, and resection of the glioma do prolong life; however, once diagnosed, there is still a poor prognosis and a limited survival. As mentioned before, Tf is found on brain capillary endothelial cells and gliomas, and it has the ability to cross the BBB. Therefore, targeting the transferrin receptor system provides an avenue for the entry of drug molecules and nanoparticles (NPs) into the brain.14 Transferrin-conjugated nanoparticles can enter the brain through the BBB, which many other forms of treatment cannot do. Thus, NPs are very useful because of their specific advantageous features, which include high drug loading, prolonged blood circulation time, enhanced stability, and better targeting ability. The NPs are also made ultrasmall so that they are able to move in the body easily. Different drugs are encapsulated in NPs, which will then allow them to target the glioma and allow the drug to be released at the site of the cancer. Some common drugs encapsulated in NPs to treat gliomas are doxorubicin (DOX) and paclitaxel (PTX). The NP delivery system should be able to bind to specific tissues and deliver its drug load, while making sure to avoid drug-induced damage to healthy tissues. Each NP can be made out of different material: metallic, polymeric, liposomes, and carbon-based NPs are all common types that are used for targeted drug delivery. These targeted delivery systems are discussed in the following sections.
Metallic Nanoparticles
Metal NPs are made up of pure metals (silver, gold, zinc, iron, and platinum) or their metal compounds (oxides, phosphates, fluorides, and chlorides). They are used for their stability, size, and ability to be easily conjugated with antibodies, ligands, and drugs. However, there are risks to using metallic NPs as they interfere with homeostasis by affecting bodily functions and forming salts in the body. Silver NPs (AgNPs) are one type of metallic NPs used. They are utilized for their high electrical conductivity, low sintering temperatures, and stability. Along with being used for targeted therapy, they are also used in diagnostics. Liu et al. conducted a study in which the goal was to evaluate and compare the radio sensitizing efficacies of gold NPs (AuNPs) and AgNPs on gliomas. It was found that the AgNPs showed radio sensitizing ability more powerful than that of the AuNPs at the same concentrations and mass. The AgNPs also lead to a higher rate of apoptosis. This shows that AgNPs can be used in therapy.15 In a different study, Liang et al. investigated the effect of AgNPs and the effect when AgNPs was used with Temozolomide (TMZ) on human glioma U251 cells. The AgNPs showed dose-dependent cytotoxicity on U251 cells and showed the ability of TMZ to increase the drug sensitivity on U251 cells. This study concluded that AgNPs could have a potential application in treating gliomas.16 Another metallic NP used for targeted therapy are iron-oxide NPs. They have attracted interest due to their biocompatibility, nontoxicity, and superparamagnetic properties. Xu et al. conducted a study in which DOX-loaded multifunctional superparamagnetic iron oxide nanoparticles (DOX-SPIONs) were designed in order to watch the cellular uptake of DOX-SPIONs by C6 rat glioma cells. The cellular uptake of DOX-SPIONs by C6 cells under a magnetic field was greatly enhanced over the uptake of free DOX. This resulted in stronger in vitro cytotoxicity. Once the safety of the DOX-SPIONs was also verified, it was concluded that multifunctional SPIONs could be used as potential carriers for theranostic treatment.17
While many other metallic NPs, such as platinum and zinc, are used in targeted therapy, usually gold NPs are utilized in relation to gliomas and the TfR. Gold NPs are highly efficient because of their low cytotoxicity, tunable size and shape, and stable attachment of ligands and molecules. They are also very biocompatible and have been used as drug carriers in various diseases.18 Gold NPs also have a high surface area to volume ratio which allows a high drug loading capacity and drug stability. These NPs can also control the release of the drug with internal or external stimuli.19 In addition, these NPs target cancer biomarkers that are overexpressed, which in glioblastomas are the Tf receptors. Dixit et al. used targeted gold NPs (AuNPs) to the brain, which were a very useful drug delivery system due to the selectivity of the NPs to target the tumor. The NPs were targeted toward Tf receptors and were loaded with the photodynamic pro-drug, Pc 4. Tf-conjugated AuNPs delivered the drug which continued to accumulate over a 4 h period. This along with the information that AuNPs were successful in only attacking the glioma cells without also affecting the healthy tissue suggests that TfR-targeted AuNPs may have important therapeutic implications for targeted therapy in gliomas.
Polymeric Nanoparticles
Polymeric NPs have immense potential as drug carriers as they are stable, easily prepared, and easily conjugated with other molecules or drugs. There are different loading approaches for polymeric NPs, and they include loading in polymeric micelles, nanospheres, and nanocapsules. Polymeric NPs include poly(butylcyanoacrylate), (polylactic-co-glycolic acid) (PLGA), and polylactic acid (PLA) NPs. PLGA NPs are one of the most useful biodegradable molecules because the hydrolysis of the NP leads to lactic and glycolic acid. PLGA NPs are also approved by the Food and Drug Administration (FDA), which allows them to be used in pharmaceutical applications for humans. Surface modulation is possible with PLGA NPs, and therefore, this allows for the coating of NPs with polymers to enhance the penetrating performance of PLGA NPs. This also increases the circulating time of the NPs in the blood for better penetration of the BBB.
A study done by Jose et al. concluded that the anticancer activity of the Tf-conjugated PLGA NPs loaded with docetaxel is very promising because of their ability to arrest cancer activity at the G2/M phase of mitosis.20 In a different study done by Cui et al., Tf-conjugated PLGA NPs were loaded with doxorubicin and paclitaxel for glioma treatment.37 In this study, the NPs were effectively prepared and delivered, and the delivery was made even more effective with the presence of a magnetic barrier, and more importantly, with the use of Tf as the targeting ligand. The NPs showed great tumor growth inhibition, and the use of Tf as the targeting ligand was extremely useful. Overall, PLGA NPs are great to use in relation to Tf receptors. In another study done by Cui et al., Tf-targeted magnetic (MNP) PLGA NPs were loaded with PTX and were evaluated in U-87 glioma cells in vitro. In the study, it was discovered that, compared to unmodified NPs or free PTX, Tf-conjugated PTX-MNP-PLGA NPs had the greatest antiproliferation and the greatest cellular uptake efficiency in U-87 cells. The study concluded that Tf-conjugated PTX-MNP-PLGA NPs can be used to treat gliomas.21
Another type of polymeric NP used besides PLGA NPs includes PLA NPs. In a study done by Sun et al., Tf-conjugated polyethylene glycol (PEG)-PLA nanoparticles were prepared. C6 rat glioma cell lines were observed, and it was proven through fluorescence microscopy that PEG-PLA NPs could enter into the tumor in vivo. In the study, it was discovered that PEG-PLA NPs have the ability to target the glioma tumor both in vitro and in vivo. This strongly suggests that the bio-PEG-PLA NPs can be used in targeted therapy in relation to gliomas.22 Guo et al. examined the effects of Tf-modified PEG-PLA NPs conjugated with resveratrol (Tf-PEG-PLA-RSV) in vitro and in vivo in relation to glioma therapy on C6 and U87 glioma cells. The cytotoxicity of PEG-PLA-RSV in C6 and U87 cells was much higher than that of free RSV, and the Tf-PEG-PLA-RSV enhanced the cytotoxicity of the polymer conjugates. The use of Tf-PEG-PLA-RSV NPs increased the lifespan of the C6 glioma-bearing rats. This suggests that these NPs have a potential of targeted therapy to gliomas.23 Overall, polymeric NPs have great potential as drug carriers for targeted therapy.
Liposome Nanoparticles
Liposomes (LPs) can have one or multiple lipid bilayers and are often composed of phospholipids. They have been utilized recently due to their excellent biocompatibility, biodegradability, and low toxicity. LPs represent a versatile system for drug delivery in cancer therapy, and as of now, several drug-loaded LPs have been approved by the FDA for cancer therapy (Figure 4). In addition, receptor-targeted LPs have been developed to reduce side effects and to enhance antitumor efficiency.
Figure 4.
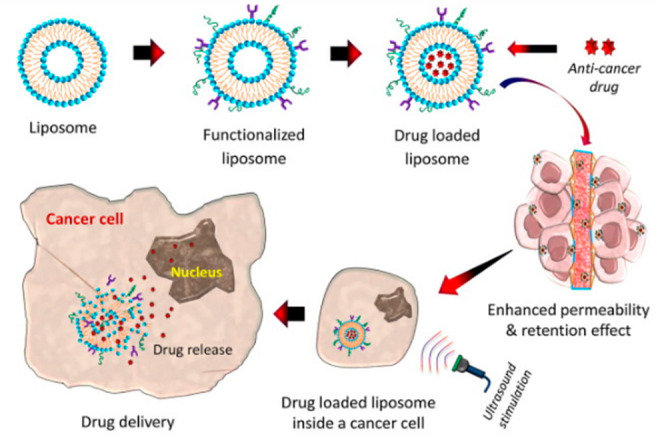
Illustration of the liposome-based drug delivery system.24 Reprinted from ref (24). Copyright 2019 Hossen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited].
In a study done by Lam et al., Tf LPs were found to accumulate in the endothelial walls of the brain microvessels, diffuse across the BBB, and aggregate in the surrounding brain milieu. This shows that NPs functionalized with Tf can offer a promising treatment for glioma patients. The NPs were loaded with TMZ, and there was an increased efficiency of drug delivery to the tumor site. The Tf-conjugated NPs reduced tumor size, signals, and growth. In addition, the mice that were treated with drug-loaded Tf-liposome nanoparticles had brain tumors that showed increased markers for DNA damage and apoptosis. Functionalizing the liposomes with Tf also proved to be safe for the body. All of the above attributes pave the path for Tf-liposomes to be a prospective treatment option in the future for treating glioma patients.25 In a study done by Jhanvi et al., Tf-PEG-PLA NPs were used. The drug encapsulated was Resveratrol (RES). Liposomal encapsulation was used to protect it in circulation and improve stability. As it was concluded that RES-liposomes developed in this project were robust, versatile, and scalable, additional studies must be conducted to learn more about the RES-liposomes. However, as RES is effective against many cancers and Tf is overexpressed on glioma cancer cells, Tf-targeted RES-loaded liposomes may prove to be an effective nanotherapy after additional studies are completed.26 In a different study done by Qin et al., arginine-glycine-aspartic acid (RGD) and Tf were used as the targeted ligands, and C6 rat glioma cell lines were studied. LPs were used to conjugate RGD and TFs to establish the brain glioma delivery system (RGD-TF-LP), which was loaded with PTX. The PTX-RGD/TF-LP achieved the lowest cell viability and the greatest cellular uptake when compared to that of the other delivery systems studied. This shows that the LPs had a beneficial effect, and although further studies regarding this delivery system are required, the system is a promising one.27 Zheng et al. examined a brain drug delivery system based on Tf and cell-penetrating peptide dual-functioned liposome, Tf/TAT-LP. Tf/TAT-LP was made and loaded with DOX. It was found that the Tf/TAT-LP-DOX presented great antiproliferative activity against U87 glioma cells, and therefore, it is a promising brain drug delivery system.28 In another study done by Lv et al., a cisplatin (Cis)-LP was modified with transferrin (Tf) to study the characteristics of potential sequential targeting to gliomas. They discovered that the Tf modified Cis-LPs had a high transport efficiency across the BBB and a high cytotoxicity in C6 cells. It was concluded that Tf-modified Cis-LPs exhibited a higher cytotoxicity to glioma cells in vitro and a more potent sequential target in contrast to cisplatin solution and cisplatin liposome. This highlights the ability of the Tf-modified Cis-LPs to cross the BBB and target glioma cells and proves that they could be a future targeted delivery system.29
Carbon-Based Nanoparticles
Among the carbon-based nanoparticles, diamond- and graphite-based nanoparticles are widely researched because they present easily accessible surfaces for modifications. However, their toxicological profile in biological systems is yet to be fully understood.30 Studies performed by Strojny et al.30 and Zakrzewska et al.31 showed that these nanoparticles are safe and have excellent cytotoxic effects in glioblastoma, respectively. This phenomenon was observed both in diamond- and graphite-based nanoparticles.31 However, it is important to note that these studies do not completely address the reality of carbon-based nanoparticles as they interact with transferrin. According to Pardo et al., carbon nanotubes with transferrin on its surface have been found to contribute to oxidative damage, which leads to cell death or possible tumor formation/progression. Moving forward, it is important for those interested in using carbon-based NPs in conjunction with transferrin receptors and transferrin ligands alike to determine whether or not their prospective effectiveness outweighs potential harm.
Ceramic Nanoparticles
Ceramic nanoparticles are now being explored recently due to their characteristic properties such as high resistance to heat and chemicals. Ceramic nanoparticles are stable and porous and can be made from a range of starting materials such oxides, carbonates, carbides, and phosphates of metals. The porous nature is favorable to high payload capacities. However, they posses a disadvantage of slow degradation and clearance from the body. There is still a long way for these nanoparticles to become fully useful as theranostic purposes.32
Antibody-Conjugated Nanoparticles
Targeted antibodies that bind to TfR are the most recent and sought-after therapeutic agents that are being explored now for the treatment of gliomas. The major advantage of antibody-conjugated nanoparticles is higher accumulation due to better targeting and higher binding affinity to the receptor. Several of these antibodies have been reported to be effective preclinically. Some of the examples of such antibodies are OX26 and its variants like 8D3 and RI7217.33 Several types of nanoparticles such as metallic nanoparticles,34 liposomes,35 polymeric nanoparticles36 have been applied to carry the antibodies for more successful delivery of drug payload for gliomas. Much progress is being made to make the antibody-conjugated nanoparticles efficient. Some of them have reached clinical trials without much success (NCT00083447),12 but clearly there is much to achieve with many studies reporting unsuccessful deliveries.
Conclusion
Gliomas are deadly, and the majority of people who are diagnosed with it will not survive. However, there are possible treatments that include Tf and its receptor. The overexpression of Tf receptors in brain tumors facilitates more Tf to cross the BBB through endocytosis as the tumor needs more iron to compensate for their rapid growth. Also, transcytosis, endocytosis, and paracellular transport contribute to iron homeostasis of the brain. In this function combined with the overexpression of Tf receptors on human glioma cell lines, Tf-conjugated NPs (of any material) targeting the TfR on brain endothelial cells are a possible avenue of therapy to treat gliomas. The NPs will be able to enter the brain through the BBB and help to decrease the area of the glioma as well as decrease cell proliferation. In the future, more studies should be done to discover whether Tf-conjugated NPs can completely attack and destroy gliomas. Once studies are done in vivo, the next step would be clinical trials. Tf-conjugated NPs are able to effectively reach and destroy the glioma by targeting the TfR, and if they prove to be successful in the clinical trials in the future, many lives could be saved. As of now, a diagnosis of glioma means almost certain death, and Tf-conjugated NPs targeting TfR on gliomas being part of clinical trials will allow for advancements in the treatment of gliomas.
Acknowledgments
T.K., E.M., and S.P. would like to acknowledge the Biomedical Career Advancement Program (BCAP) in Iyer’s Lab, and the Department of Pharmaceutical Sciences, Wayne State University (WSU). K.T. would like to acknowledge AGRADE scholarship and Graduate Professional Scholarship from the Wayne State University Graduate School, EACPHS, Wayne State University (WSU). S.S. acknowledges the support of Burroughs Wellcome Fund Collaborative Research Travel Grant (BWFCRTG). A.K.I. acknowledges the U.S. Department of Defense CDMRP KCRP Idea Development Award #W81XWH1810471, U.S. National Institutes of Health, National Cancer Institute (NIH/NCI) Grant No. R21CA179652, and Wayne State University FRAP Grant Nos. 142174 and 176575.
Biographies
Tejaswi Koneru is a senior at Walled Lake Central High School. She has conducted research on the genes involved in opioid addiction through miRcore at the University of Michigan and has also researched the transferrin receptor and its use in targeted therapy at Wayne State University. She organizes many community service events as the President of her school’s National Honors Society, tutors students in different subjects, and volunteers at Beaumont Hospital in Farmington Hills, MI.
Eva McCord is a senior at Grosse Pointe South High School and has been a student researcher at Wayne State University since the summer of 2020. Before being honored with a stipend by WSU’s Biomedical Career Advancement Program, she received a scholarship from The Joyce Ivy Foundation to study neuroscience at Harvard College’s Secondary School Program in 2019. She helped to found her school’s Science Olympiad team, on which she serves as president, and is a 2020 National Top 25 Epidemiology Finalist.
Shreya Pawar is a senior at Troy High School and a student researcher at Wayne State University. She has researched the details of biology of cancer and potential treatments under a lab at the University of Michigan as well as the prospects of nanoparticle therapy in Alzheimer’s disease and glioma at Wayne State University. She volunteers as a Science Olympiad coach at her former middle school. She serves as President of her school’s Entrepreneurship Club and has won awards in Health Occupation Students of America (HOSA) and Model United Nations. She is also involved in her school’s National Honors Society chapter.
Katyayani Tatiparti is a Ph.D. candidate in Use-Inspired Biomaterials & Integrated Nano Delivery (U-BiND) Systems Laboratory at EACPHS, Wayne State University, Detroit, Michigan, USA. She has a Master of Pharmacy (2012) and a PG Diploma in Patents Law (2016). She completed her MS in U-BiND Systems Laboratory. She has published more than 10 peer-reviewed articles. Her current research focuses on designing ultrasmall nanoparticles targeting Alzheimer’s disease.
Samaresh Sau is a Research Assistant Professor in the Department of Pharmaceutical Sciences, WSU. He was awarded a Ph.D. from the CSIR-Indian Institute of Chemical Technology, India. He worked at Purdue University/Endocyte Inc., where he developed clinically translatable drug formulations for cancer and autoimmune diseases. His research interests are nanoparticles, small molecules, and antibody–drug conjugates for cancer and Alzheimer’s disease. He has 50 articles and 5 book chapters published and two U.S. patents filed.
Arun Iyer is the Director of U-BiND Systems Laboratory and Associate Professor of Pharmaceutical Sciences at WSU. He completed his PhD under Prof. Hiroshi Maeda (2016 Nobel Prize Nominee). He received the U.S. DoD Early Career Investigator Award and the prestigious CRS T. Nagai Award for outstanding contribution in drug delivery science and research. He has expertise in polymeric biomaterials and nanomedicine for targeting cancer, infection and neurodegenerative diseases. His research has been funded by agencies such as NCI/NIH, NIAID/NIH. and DoD.
The authors declare no competing financial interest.
References
- Goodenberger M. L.; Jenkins R. B. Genetics of Adult Glioma. Cancer Genet. 2012, 205 (12), 613–621. 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Lindshield B.Iron Transport & Storage. Kansas State University Human Nutrition Flexbook; New Prairie Press, 2018. [Google Scholar]
- Mo X.; Liu E.; Huang Y.. The Intra-Brain Distribution of Brain Targeting Delivery Systems. In Brain Targeted Drug Delivery System; Gao H., Gao X., Eds.; Academic Press: Cambridge, 2019; pp 409–438. [Google Scholar]
- Leitner D. F.; Connor J. R. Functional Roles of Transferrin in the Brain. Biochim. Biophys. Acta, Gen. Subj. 2012, 1820 (3), 393–402. 10.1016/j.bbagen.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Human Protein Atlas . TFR2; https://www.proteinatlas.org/ENSG00000106327-TFR2/tissue (accessed March 4, 2021).
- Shen Y.; Li X.; Dong D.; Zhang B.; Xue Y.; Shang P. Transferrin Receptor 1 in Cancer: A New Sight for Cancer Therapy. Am. J. Cancer Res. 2018, 8 (6), 916–931. [PMC free article] [PubMed] [Google Scholar]
- Sharma S.; Sinha M.; Kaushik S.; Kaur P.; Singh T. P. C-Lobe of Lactoferrin: The Whole Story of the Half-Molecule. Biochem. Res. Int. 2013, 271641. 10.1155/2013/271641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. W.The Erythrocyte: Physiology, Metabolism, and Biochemical Disorders. 2017.
- Giannetti A. M.; Snow P. M.; Zak O.; Björkman P. J. Mechanism for Multiple Ligand Recognition by the Human Transferrin Receptor. PLoS Biol. 2003, 1 (3), 341–350. 10.1371/journal.pbio.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven M. D.; Jue S.; Enns C. A. Transferrin Receptors TfR1 and TfR2 Bind Transferrin through Di Ff Ering Mechanisms. Biochemistry 2018, 57, 1552–1559. 10.1021/acs.biochem.8b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkouvatsos K.; Papanikolaou G.; Pantopoulos K. Regulation of Iron Transport and the Role of Transferrin. Biochim. Biophys. Acta, Gen. Subj. 2012, 1820 (3), 188–202. 10.1016/j.bbagen.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Johnsen K. B.; Burkhart A.; Thomsen L. B.; Andresen T. L.; Moos T. Targeting the Transferrin Receptor for Brain Drug Delivery. Prog. Neurobiol. 2019, 181 (May), 101665. 10.1016/j.pneurobio.2019.101665. [DOI] [PubMed] [Google Scholar]
- Chiou B.; Neal E. H.; Bowman A. B.; Lippmann E. S.; Simpson I. A.; Connor J. R. Pharmaceutical Iron Formulations Do Not Cross a Model of the Human Blood-Brain Barrier. PLoS One 2018, 13 (6), e0198775. 10.1371/journal.pone.0198775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari A.; Larocca L. M.; Deaglio S.; Finisguerra V.; Boe A.; Raggi C.; Ricci-Vitani L.; Pierconti F.; Malavasi F.; De Maria R.; Testa U.; Pallini R. Transferrin Receptor 2 Is Frequently and Highly Expressed in Glioblastomas. Transl. Oncol. 2010, 3 (2), 123–134. 10.1539/tlo.09274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Jin H.; Guo Z.; Ma J.; Zhao J.; Li D.; Wu H.; Gu N. Silver Nanoparticles Outperform Gold Nanoparticles in Radiosensitizing U251 Cells in Vitro and in an Intracranial Mouse Model of Glioma. Int. J. Nanomed. 2016, 11, 5003–5014. 10.2147/IJN.S115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P.; Shi H.; Zhu W.; Gui Q.; Xu Y.; Meng J.; Guo X.; Gong Z.; Chen H. Silver Nanoparticles Enhance the Sensitivity of Temozolomide on Human Glioma Cells. Oncotarget 2017, 8, 7533–7539. 10.18632/oncotarget.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. L.; Mao K. L.; Huang Y. P.; Yang J. J.; Xu J.; Chen P. P.; Fan Z. L.; Zou S.; Gao Z. Z.; Yin J. Y.; Xiao J.; Lu C. T.; Zhang B. L.; Zhao Y. Z. Glioma-Targeted Superparamagnetic Iron Oxide Nanoparticles as Drug-Carrying Vehicles for Theranostic Effects. Nanoscale 2016, 8, 14222–14236. 10.1039/C6NR02448C. [DOI] [PubMed] [Google Scholar]
- Dixit S.; Novak T.; Miller K.; Zhu Y.; Kenney M. E.; Broome A. M. Transferrin Receptor-Targeted Theranostic Gold Nanoparticles for Photosensitizer Delivery in Brain Tumors. Nanoscale 2015, 7, 1782–1790. 10.1039/C4NR04853A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Lu G.. Applications of Gold Nanoparticles in Cancer Imaging and Treatment. In Noble and Precious Metals - Properties, Nanoscale Effects and Applications; Seehra M. S., Bristow A. D., Eds.; IntechOpen, 2017. [Google Scholar]
- Jose S.; Cinu T. A.; Sebastian R.; Shoja M. H.; Aleykutty N. A.; Durazzo A.; Lucarini M.; Santini A.; Souto E. B. Transferrin-Conjugated Docetaxel–PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle. Oncol. 2017, 92 (4), 221–228. 10.3390/polym11111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.; Xu Q.; Chow P. K. H.; Wang D.; Wang C. H. Transferrin-Conjugated Magnetic Silica PLGA Nanoparticles Loaded with Doxorubicin and Paclitaxel for Brain Glioma Treatment. Biomaterials 2013, 34, 8511–8520. 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- Cui Y. N.; Xu Q. X.; Davoodi P.; Wang D. P.; Wang C. H. Enhanced Intracellular Delivery and Controlled Drug Release of Magnetic PLGA Nanoparticles Modified with Transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. 10.1038/aps.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T.; Wu H.; Li Y.; Huang Y.; Yao L.; Chen X.; Han X.; Zhou Y.; Du Z. Targeting Transferrin Receptor Delivery of Temozolomide for a Potential Glioma Stem Cell-Mediated Therapy. Oncotarget 2017, 8, 74451–74465. 10.18632/oncotarget.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.; Li A.; Jia Z.; Yuan Y.; Dai H.; Li H. Transferrin Modified PEG-PLA-Resveratrol Conjugates: In Vitro and in Vivo Studies for Glioma. Eur. J. Pharmacol. 2013, 718 (1–3), 41–47. 10.1016/j.ejphar.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Hossen S.; Hossain M. K.; Basher M. K.; Mia M. N. H.; Rahman M. T.; Uddin M. J. Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 2019, 15, 1–18. 10.1016/j.jare.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam F. C.; Morton S. W.; Wyckoff J.; Vu Han T. L.; Hwang M. K.; Maffa A.; Balkanska-Sinclair E.; Yaffe M. B.; Floyd S. R.; Hammond P. T. Enhanced Efficacy of Combined Temozolomide and Bromodomain Inhibitor Therapy for Gliomas Using Targeted Nanoparticles. Nat. Commun. 2018, 9, 1991. 10.1038/s41467-018-04315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri A.; Deshpande P.; Pattni B.; Torchilin V. Transferrin-Targeted, Resveratrol-Loaded Liposomes for the Treatment of Glioblastoma. J. Controlled Release 2018, 277, 89–101. 10.1016/j.jconrel.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L.; Wang C. Z.; Fan H. J.; Zhang C. J.; Zhang H. W.; Lv M. H.; Cui S. De. A Dual-Targeting Liposome Conjugated with Transferrin and Arginine-Glycine-Aspartic Acid Peptide for Glioma-Targeting Therapy. Oncol. Lett. 2014, 8 (5), 2000–2006. 10.3892/ol.2014.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C.; Ma C.; Bai E.; Yang K.; Xu R. Transferrin and Cell-Penetrating Peptide Dual-Functioned Liposome for Targeted Drug Delivery to Glioma. Int. J. Clin. Exp. Med. 2015, 8 (2), 1658–1668. [PMC free article] [PubMed] [Google Scholar]
- Lv Q.; Li L. M.; Han M.; Tang X. J.; Yao J. N.; Ying X. Y.; Li F. Z.; Gao J. Q. Characteristics of Sequential Targeting of Brain Glioma for Transferrin-Modified Cisplatin Liposome. Int. J. Pharm. 2013, 444 (1–2), 1–9. 10.1016/j.ijpharm.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Strojny B.; Kurantowicz N.; Sawosz E.; Grodzik M.; Jaworski S.; Kutwin M.; Wierzbicki M.; Hotowy A.; Lipińska L.; Chwalibog A. Long Term Influence of Carbon Nanoparticles on Health and Liver Status in Rats. PLoS One 2015, 10 (12), e0144821. 10.1371/journal.pone.0144821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska K. E.; Samluk A.; Wierzbicki M.; Jaworski S.; Kutwin M.; Sawosz E.; Chwalibog A.; Pijanowska D. G.; Pluta K. D. Analysis of the Cytotoxicity of Carbon-Based Nanoparticles, Diamond and Graphite, in Human Glioblastoma and Hepatoma Cell Lines. PLoS One 2015, 10 (3), e0122579. 10.1371/journal.pone.0122579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.; Harshita B. S. P.; Mishra P.; Talegaonkar S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21 (42), 6165–6188. 10.2174/1381612821666151027153246. [DOI] [PubMed] [Google Scholar]
- Paris-Robidas S.; Emond V.; Tremblay C.; Soulet D.; Calon F. In Vivo Labeling of Brain Capillary Endothelial Cells after Intravenous Injection of Monoclonal Antibodies Targeting the Transferrin Receptor. Mol. Pharmacol. 2011, 80 (1), 32–39. 10.1124/mol.111.071027. [DOI] [PubMed] [Google Scholar]
- Johnsen K. B.; Bak M.; Kempen P. J.; Melander F.; Burkhart A.; Thomsen S.; Nielsen M. S.; Moos T.; Andresen T. L. Antibody Affinity and Valency Impact Brain Uptake of Transferrin Receptor-Targeted Gold Nanoparticles. Theranostics 2018, 8 (12), 3416–3436. 10.7150/thno.25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.; Jung H.; Oh J.; Song D. Use of PEGylated Immunoliposomes to Deliver Dopamine Across the Blood – Brain Barrier in a Rat Model of Parkinson ’ s Disease. CNS Neurosci. Ther. 2016, 22, 817–823. 10.1111/cns.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M. J.; Kennedy P. J; Martins S.; Sarmento B. Delivery of SiRNA Silencing P-Gp in Peptide- Functionalized Nanoparticles Causes Efflux Modulation at the Blood – Brain Barrier. Nanomedicine 2017, 12 (12), 1385–1399. 10.2217/nnm-2017-0023. [DOI] [PubMed] [Google Scholar]