Abstract

Interest in insects as waste biomass bioconverters and their use as valuable resources for fat, proteins, and chitin has increased considerably in the last few years. In this study, proteins were extracted from defatted black soldier fly (BSF) (Hermetia illucens) exuviae by green hydrolysis using superheated water at 150 °C for 20 h, and the remaining chitin was deacetylated into chitosan and used as a finishing agent for polyester fabrics. A total amount of 7% fat, 40% proteins, and 20% chitin was obtained from BSF exuviae. Different hydrolysis times ranging from 1 to 20 h were tried until the complete purification of chitin. The purity of chitin and the obtained chitosan after deacetylation was assessed by Fourier transform infrared spectroscopy and thermal analysis. A preliminary study was successfully carried out to use the obtained chitosan as a finishing agent for polyester pretreated fabrics using citric acid as a grafting agent. The presence of chitosan on the fabric was verified by scanning electron microscopy and by dyeing of the pretreated polyester fabric using a reactive dye with sulfonated groups that are able to be absorbed by electrostatic attraction because of the created cationic nature of the fiber surface treated by chitosan.
1. Introduction
Insects occupy 95% of the animal kingdom and have great potential as a natural resource for chitin and chitosan production.1 Many insects naturally feed on organic wastes, convert biomass nutrients into their biomass, and reduce the amount of waste materials.2 Fly species are well known for biodegradation of organic waste and, in particular, the house fly (Musca domestica L.) and the black soldier fly (BSF, Hermetia illucens L.). The BSF is the most extensively studied insect for this purpose. BSF is a species native to South America but currently cosmopolitan. The adult state is a medium-sized fly (15–20 mm) and tendentially black in color, characterized by a short life (a few days). The adults lay their eggs, preferring the colonization of the decomposing organic material such as fruits, manure, and byproducts of the agroindustry and aquaculture. One of the main advantages of using BSF as a waste bioconverter is that adult fly does not eat, thus avoiding any disease transmission risk.3 The commercial end products from BSF farming are derived from the larvae and prepupae containing high fat and protein. They can be converted into poultry and fish-feed products, replacing classical protein sources for animal feed such as fish meal or soybean meal.4 In order to maintain a constant colony size during the breeding of BSF, about 10% of the pupae are allowed to get converted into flies, which, when molting out, leave behind the shells or exoskeletons.
Hence, chitin, which may be available for commercial extraction, is obtained from the pupae shells or exuviae. The pupa exuviae of BSF is a rich source of chitin.5 Chitin is the second most abundant polysaccharide found in the world after cellulose. It is a linear polymer in which an acetamide, at position C-2, substitutes the hydroxyl group present in cellulose. The chemical structure of chitin is (C8H13O5N)n.6−8 Chitin is found in crustacean’s exoskeleton (shrimps, crabs, lobsters, crawfishes, and squids), in cell walls of fungi, yeasts, and green algae, and in insects (cockroaches, beetles, grasshoppers, and flies).9−14 Chitin has more applications after it is transformed into chitosan by partial deacetylation under alkaline conditions.15 Chitin and chitosan are biodegradable, biocompatible, natural, and nontoxic compounds. They have become of great interest in various fields, including biomedical, chemical, cosmetics, agriculture, pharmaceutical, food, nutrition, paper industry, and wastewater treatment and in producing films and biodiesel.12,16−18 Chitosan is an off-white, innocuous, flavorless, semitransparent, and amorphous solid, soluble in acid, but insoluble in alkali and water or in an ordinary organic solvent. The majority of the chitosan produced lacked purity, which is primarily essential in the pharmaceutical and biomedical industries, but not mandatory in other fields such as the textile industry, where applications of low purity chitosan include fiber production, textile dyeing for cotton, silk, wool, polypropylene, and polyester fibers, coatings, and antimicrobial/antibacterial/antistatic/antiodor/antiwrinkle finishing.19−21 Chitosan has broad-spectrum antibacterial activity, including on Staphylococcus aureus, Escherichia coli, and Bacillus subtilis. For this reason, chitosan has been used as a finishing agent or to prepare antibacterial fibers.19 The prime focus for chitosan as an antimicrobial treatment has been on cotton. To improve durability, chitosan has been cross-linked to cotton using chemicals such as dimethyloldihydroxyethyleneurea22 citric acid, 1,2,3,4-butanetetracarboxylic acid, or glutaric dialdehyde. These chemicals cross-link chitosan to cotton through hydroxyl groups. Antibacterial fibers were produced by Japan Fuji Textile Co., Ltd., who developed a stable ultrafine chitosan powder with the particle size of ca. 5 μm, which was subsequently added to a viscose solution for blending, and fibers named chitopoly with high antibacterial properties were produced.23 Treatment of polyester fabrics with chitosan imparts, in addition to antibactericity, a significant antistatic effects shown in the study by Matsukawa et al.,24 who treated polyester fabrics with chitosan, hydrolyzing the surfaces with caustic soda solution to incorporate in the polyester functional groups (−COOH). In wool finishing, chitosan has been used as a shrink resist agent25 with antimicrobial and deodorant activities26 and as an agent for improving the dyeability of wool.27
Conventionally, to extract chitin from crustacean shells, chemical processing consisting of demineralization and deproteinization has been applied. The raw material is treated with dilute hydrochloric acid to remove mineral salts, mainly calcium carbonate, and with strong bases to remove proteins.28
An alternative approach to the harsh chemical deproteinization is the use of enzymatic deproteinization or microbial fermentation. Doan et al. extracted chitin from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis, obtaining 95% protein removal after 4 days of treatment.29 Hamdi et al. extracted chitin from blue crab (Portunus segnis) shells using several microbial, visceral, and commercial proteases and tested them for their deproteinization efficiency. A high level of protein removal recorded of about 82% and 85% was obtained using Bacillus safensis proteases and the crude enzyme extract of P. segnis, respectively.30 The general method to obtain chitin from insects is the classical one using HCl for demineralization and NaOH for protein removal.31,32 Other approaches have been tried using natural deep eutectic solvents and proteases. Zhou et al. investigated the selectivity of demineralization and deproteinization of natural deep eutectic solvents to obtain chitin from H. illucens. It was found that intermolecular and intramolecular hydrogen bonds facilitate the removal of proteins in solvent use.33 The possibility of using an enzymatic assisted extraction of proteins from H. illucens prepupae was explored by Caligiani and co-workers,34 obtaining maximum nitrogen solubilization with Bacillus licheniformis protease of about 60%.
For the first time in this study, a different approach was successfully tried for the deproteinization of chitin using superheated water. Superheated water is defined as the liquid water under pressure in the range of atmospheric boiling point 100 °C and critical temperature 374 °C. In this temperature range, as the temperature of water increases, the density of water molecules decreases. The hydrogen bonds are weakened, and further dissociation of water molecules into hydroxonium ions (H3O+) and hydroxide ions (OH–) by a combination of oxygen–hydrogen stretching within a molecule and liberation vibrations between molecules occurred.35 Superheated water is a green solvent and can be used as a replacement for existing solvents. The superheated water hydrolysis has been applied for a long time in various fields such as the food industry for cooking above 100 °C, coffee extraction, paper industry for pulp processing, for waste treatment (called wet-air oxidation), extraction of flavors and fragrance, extraction of biomass, removing metal and organic compounds from polymers, and so on. Superheated water is highly effective in terms of hydrolysis or dissolution of proteins to get oligopeptides.36 It was used for the hydrolysis of wool to obtain organic nitrogen fertilizers and foaming agents for the foam dyeing of wool and cotton fabrics.37−39 Further advantages of using superheated water for protein extraction and chitin purification are protein extraction in water medium only at neutral pH and are sterilized by a temperature above 130–135 °C, so that they can be used without any further treatment.
The aim of this study is to investigate the influence of superheated water hydrolysis treatment on BSF exuvia to develop a sustainable process for chitin extraction from it. An application in the textile field of chitosan obtained from BSF exuvia chitin deacetylation as a finishing agent for polyester fabrics has also been successfully carried out.
2. Results and Discussion
2.1. BSF Exuviae Composition
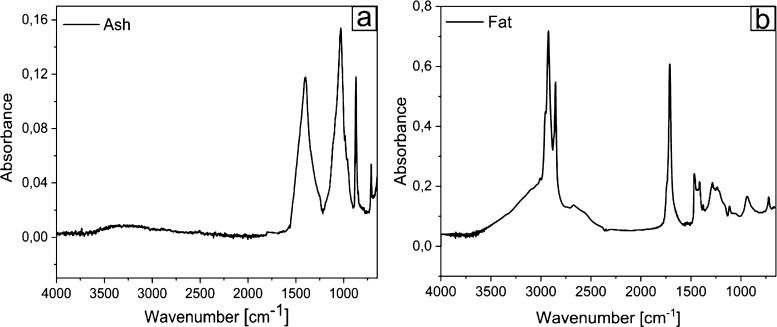
The humidity content of BSF exuviae was found to be 8%, and the ash, lipid, and chitin amount were found to be 10, 7, and 20%, respectively, on the dried BSF exuviae weight. In accordance with Smets et al.,40 the ash content of the different development stages of BSF ranges around 8–10%. Figure 1a shows the spectrum of ash with the characteristic peaks of calcite (CaCO3): the band at 1400 cm–1 is ascribed to the asymmetric CO3 stretching band, 1030 cm–1 to symmetric CO3 stretching, 870 cm–1 to CaO out-plane bending, and 710 cm–1 to CaO in plane bending.41 The determined lipid amount is in agreement with data obtained from the study by Liu and co-workers,42 who observed a rapid increase of crude fat content from day 1 to day 14 of larvae of BSF from 4.8 to 28.4% in dry mass and fat content of pupa from 7.2 to 8.2%. In our case, a low-fat amount (7% on dry weight) is found in exuvia, where BSF does not need to accumulate any source of energy. Figure 1b exhibits IR spectra of BSF fat. The assignment of functional groups responsible for IR absorption is as follows: 2954 cm–1 (−CH3 asymmetrical stretch), 2922 and 2853 cm–1 (symmetrical and asymmetrical stretching of −CH2), 1743 cm–1 (−C=O stretch), and the intense peaks located at 1713 cm–1 corresponding to carbonyl and is a characteristic of esters. Below 1500 cm–1, several other signals ascribed to −CH2 bending, −CH3 bending, and C–O stretching were obtained.43,44
Figure 1.
FTIR spectra: BSF ash (a) and fat (b).
The amount of chitin obtained in BSF exuvia was 20% on the dry exuviae weight, comparatively higher with respect to the amount found in the study by Smets et al. in the larva, prepupa, and pupa of H. illucens,(40) respectively, of 3.85, 4.72, and 6.31%. Similarly, Wang et al. determined a chitin content of 3.6, 3.1, 14.1, and 2.9% in the BSF larvae, prepupae, puparium, and adults, respectively. In accordance with previous authors,32 a high amount of chitin was found in BSF exuviae because when the prepupa was pupated, the epidermis became harder and chitinized.
2.2. Isolation of Chitin Using Superheated Water Hydrolysis to Remove Proteins
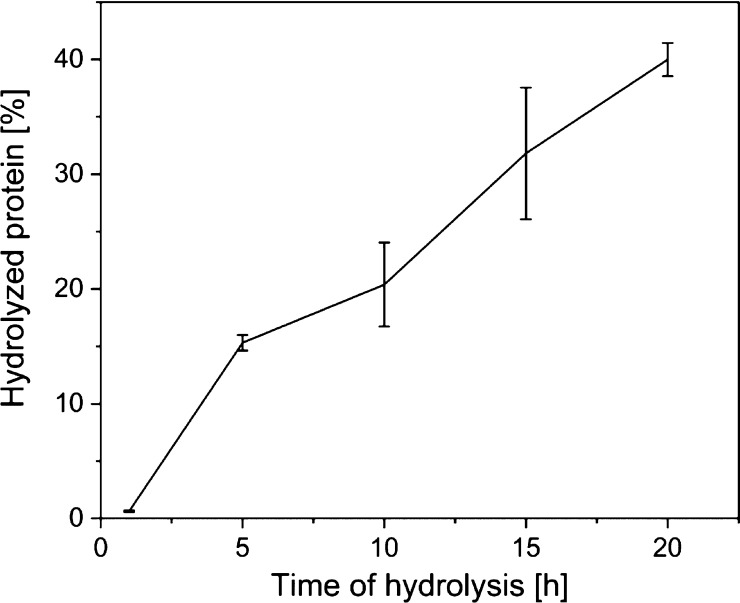
For the deproteinization step, instead of using the classical method with NaOH, the defatted and demineralized powder of BSF exuviae was hydrolyzed using superheated water at 150 °C for different times: 1, 5, 10, 15, and 20 h, and hydrolyzed proteins were collected and weighted. Increasing the time of hydrolysis, the percentage of extraction of proteins increases (see Figure 2) and the obtained chitin becomes purer.
Figure 2.
Amount of the extracted protein (average of four samples) after 1, 5, 10, 15, and 20 h of hydrolysis.
2.3. Morphological Analysis
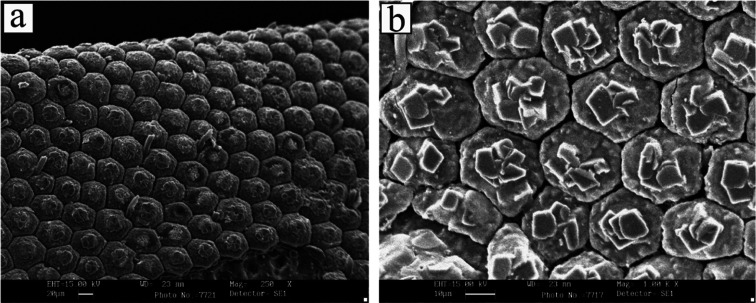
Scanning electron microscopy (SEM) images (see Figure 3) show the morphological aspect of BSF exuviae after demineralization and fat and protein removal. The chitin shows a nonporous structure consisting of well-organized repeating units of the hexagonal form.
Figure 3.
SEM image of chitin extracted from BSF. Magnification 250× (a) and 1000× (b).
In general, the morphology of chitin depends on insect species, growth stage, and gender.45 In BSF exuviae, a similar kind of the microfibrillar structure with wide application in the textile industry46 was found by other authors.13
2.4. FTIR Analysis
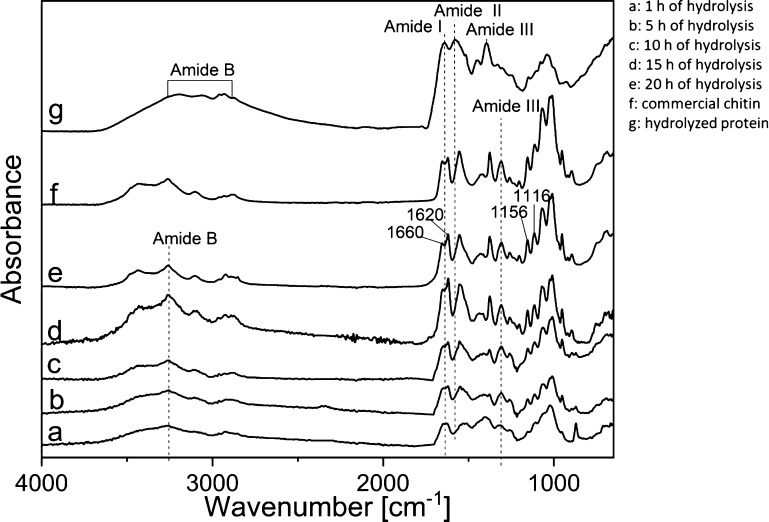
Fourier transform infrared (FTIR) spectra of commercial chitin and defatted and demineralized BSF exuviae hydrolyzed for different times using superheated water are shown in Figure 4. There are no new chemical groups or free residues formed from the hydrolysis treatment. The spectrum of BSF exuviae treated for 20 h is very close to the spectrum of commercial chitin and corresponds to α-chitin. It shows characteristic vibration bands at 1660, 1620, and 1555 cm–1, which correspond to C=O amide stretch. The amide I band of the chitin splits into two parts at 1660 and 1620 cm–1, indicating the α crystalline form of chitin because the bond splitting can be attributed to the occurrence of intermolecular hydrogen bond CO–NH, and the absorbance at around 1620 cm–1 is due to the intramolecular hydrogen bond CO–H OCH2. Additionally, there are C–N vibrations observed around 1307 cm–1 (amide III). The other significant peaks observed in BSF exuviae treated for 20 h with superheated water are N–H stretching of amide groups (3258 cm–1); O–H stretching vibration (2971 cm–1); C–O–C asymmetric stretching (1156 cm–1), and C–O–C symmetric stretching (1116 cm–1).13,18,47,48
Figure 4.
FTIR spectra: defatted and demineralized BSF exuviae after a hydrolysis time of 1 (a), 5 (b), 10 (c), 15 (d), and 20 h (e). Spectra of chitin obtained from Sigma-Aldrich (f) and spectra of the hydrolyzed protein (g).
We can see that only after 20 h of hydrolysis, the spectrum of BSF exuviae is comparable to the spectrum of commercial chitin. The changes in different peak absorptions may be attributed to the breakage of the bond because of the hydrolysis treatment. After 20 h of hydrolysis, we can see in Figure 4 spectrum e the absence of a peak/shoulder at 1540 cm–1, which is attributed to the lack of protein content in the extracted chitin from BSF exuviae.47 However, because of the overlapping of chitin with protein peaks in FTIR spectroscopy, it is possible that small amount of proteins still remains in the purified chitin. The split of amide bands at 1659 and 1622 cm–1 is clearly seen after 20 h of hydrolysis treatment in BSF exuviae, and it is indicative of the presence of chitin from BSF exuviae in α form, with antiparallel chains responsible for the rigidity of the polymer.1 The spectrum of the BSF exuviae protein hydrolyzate collected after removal of chitin and drying shows classical characteristics of bands in three different regions, represented as amide I 1600–1700 cm–1, amide III 1200–1400 cm–1, and amide B 2900–3200 cm–1.49 It is to be highlighted that by removing chitin from the insect exocuticle using superheated water, it is possible to recover the obtained protein hydrolyzate and use it in different fields without any additional purification.50
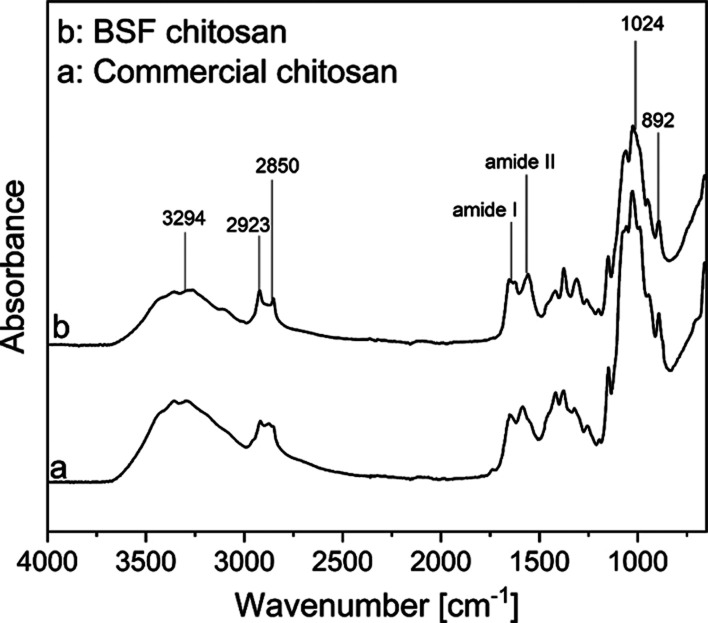
After the deacetylation of chitin from BSF exuviae using NaOH, the obtained chitosan is very close to the commercial one obtained from Sigma-Aldrich (Figure 5), as shown by the complete overlapping of peaks in FTIR spectra.
Figure 5.
FTIR spectra: commercial chitosan (a) and (b) chitosan from deacetylation of BSF chitin.
Both spectra show the characteristic chitosan absorption peaks, and in particular, the absorption at 892 cm–1 is associated with the ring stretching and at 1024 cm–1 with the CO stretching. The absorbance band of C=O stretching in secondary amide is evident at the peak of 1559 cm–1 and the amide I band absorbance at 1653 cm–1, as shown in Figure 5. The peaks at 2850, 2923, and 3294 cm–1 correspond to symmetric CH3 stretching, asymmetric CH2 stretching, and N–H stretching, respectively.51,52
2.5. Thermal Analysis
Thermal analysis, including differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA), was carried out to confirm the effective purification of chitin from defatted and demineralized BSF exuviae after 20 h of hydrolysis using superheated water at 150 °C and the deacetylation of it to obtain chitosan.
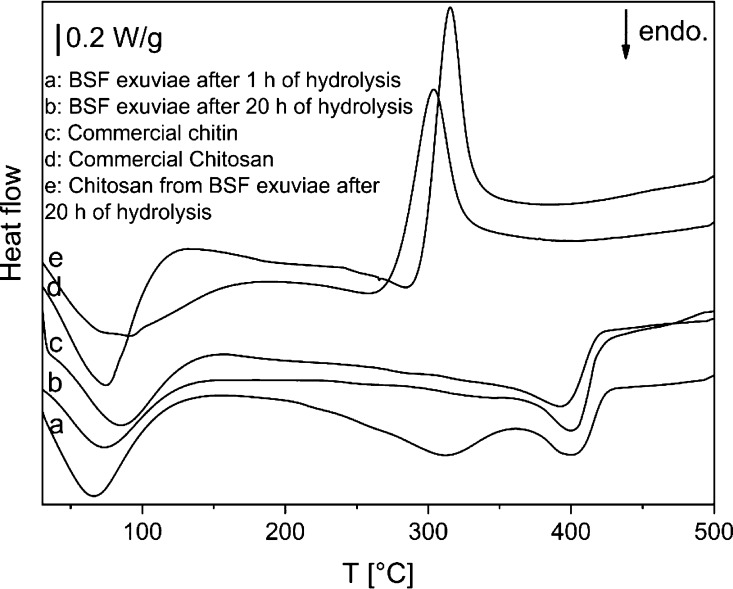
The DSC thermograms shown in Figure 6 show defatted and demineralized BSF exuviae after 1 h of hydrolysis and 20 h of hydrolysis in comparison with commercial chitin and the obtained chitosan from the second sample compared with commercial chitosan.
Figure 6.
DSC curves of defatted and demineralized BSF exuviae after 1 h of hydrolysis (a) and 20 h of hydrolysis (b) in comparison with commercial chitin (c); thermograms of the obtained chitosan from sample b (e) and commercial chitosan (d).
All curves show an endothermic peak between 66 °C and 86 °C associated with the evaporation of water. The commercial chitin and the defatted and demineralized BSF exuviae after 20 h of hydrolysis show an endothermic peak at 395–400 °C related to the maximum decomposition of chitin, and there are no significant differences between the two curves. The data obtained are similar to those reported by Kittur and et al.53 BSF exuviae after 1 h of hydrolysis, in addition to the pyrolysis peak, also have another endothermic peak at 310 °C probably associated with the presence of proteins not removed by the short hydrolysis time. Both curves (d and e) show an exothermic peak at about 320 °C attributable to the chitosan degradation. This includes saccharide ring dehydration, depolymerization, and decomposition of deacetylated and acetylated chitosan units.54,55
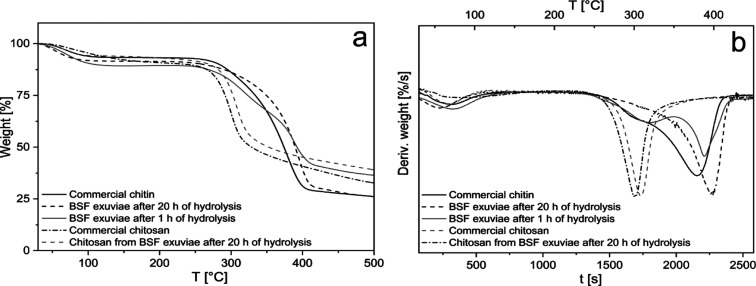
The TG curves and their derivatives of defatted and demineralized BSF exuviae after 1 h of hydrolysis and 20 h of hydrolysis in comparison with commercial chitin and obtained chitosan compared with commercial chitosan are shown in Figure 7.
Figure 7.
(a) TG curves of defatted and demineralized BSF exuviae after 1 h of hydrolysis and 20 h of hydrolysis in comparison with commercial chitin and thermograms of obtained chitosan compared with commercial chitosan and (b) derivative curves of thermograms shown in (a).
In Figure 7a, all the curves indicate a weight loss of 5–10% because of water evaporation in the interval between 30 and 100 °C. As regards the curves related to chitin, the second weight loss between 240 and 430 °C (65, 60, and 47% of weight loss for commercial chitin and BSF-treated exuviae after 20 and 1 h of hydrolysis, respectively) can be assigned to degradation of the chitin chains14,56 and protein chains for the last curve.
The weight loss is shown in the first derivatives (Figure 7b) of commercial chitin and BSF exuviae purified chitin after 20 h of hydrolysis are quite similar, instead of treated BSF exuviae after 1 h hydrolysis shows a shoulder at 310 °C corresponding to protein degradation and the final weight loss is lower than in the other curves.9
TGA of commercial and obtained chitosans from treated BSF exuviae after 20 h of hydrolysis are both smooth curves, showing in addition to weight loss from water evaporation, a further weight loss step corresponding to one peak on the derivative curves (Figure 7b), indicating that the thermal degradation of chitosan is a one-step reaction corresponding to a weight loss of 48% for both chitosans. This behavior is similar to that obtained by other authors.57 In the derivative curves, it is possible to observe a peak shift of 10 °C probably associated with a different degree of deacetylation of two chitosans.
2.6. Polyester Fabric Finishing Using Chitosan Obtained from BSF Exuviae
A preliminary test was carried out to use the obtained chitosan as a finishing agent for polyester fabrics. Indeed, because of its antimicrobial property, coupled with its nontoxicity, biodegradability, and biocompatibility, chitosan provides increasing interest in adding functionalities to the textile surfaces; it has been used in wool, cotton, cellulose, and polyester finishing.58 However, the weak binding of chitosan with the textile fibers constitutes the main problems in its application. Citric acid and other low toxic oxidizing agents have been shown to promote an effective cross-linking between chitosan and textile substrates such as cotton cellulose59 and woollen fabrics60 in an esterification reaction.
Alkali treatment of polyester fabrics is a well-known and conventional process in the textile industry to improve the reactivity and hydrophilic character of the polyester. In this process, sodium hydroxide can hydrolyze the ester groups in the polyester chains, and the modified polyester fragments with terminating carboxyl or hydroxyl groups remain in the polyester fiber structure.61
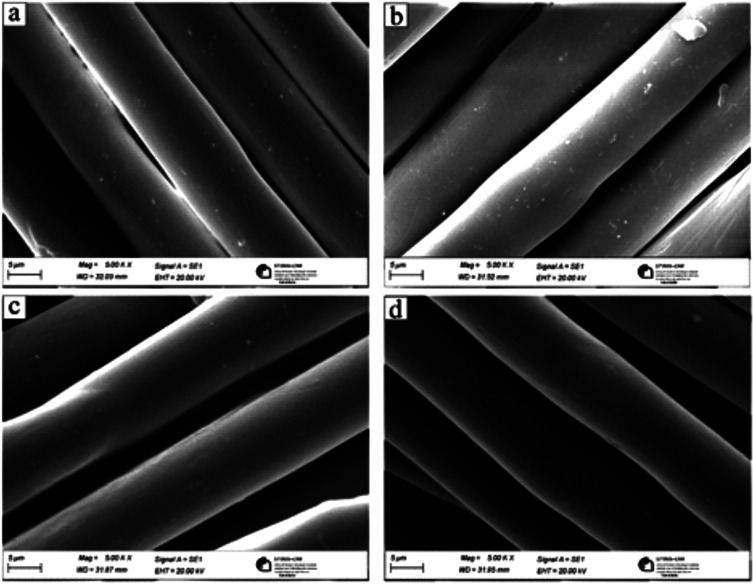
In this experiment, chitosan obtained by purification of BSF exuviae was grafted onto pretreated polyester fabrics using citric acid as a grafting agent. The presence of the chitosan film on the surface of polyester fabrics is shown in Figure 9. Indeed, poly(carboxylic acid)s, such as citric acid can be used as cross-linking agents and have at least two carboxyl groups that can react with active groups located in chitosan, such as amino (−NH2) or hydroxyl (−OH) groups.62 Chitosan and citric acid resulted in electrostatic bonding and ionic cross-linking between the protonated amine and carboxylate ion of a nonheat-treated chitosan citrate.63
Figure 9.
SEM image of (a) untreated polyester. (b) Polyester treated with the debaca process. (c) Polyester fabrics treated with debaca and chitosan. (d) Polyester fabrics treated with debaca, chitosan, and stained with reactive dye.
The presence of chitosan on the polyester fabric was verified by dyeing the polyester fabric using Remazol Red dye, a reactive dye which can be absorbed by electrostatic attraction because of the created cationic nature of the fiber surface treated by chitosan with sulfonated groups able to bind the OH groups of chitosan.
In Figure 8, the pretreated sample of polyester fabrics finished with chitosan from BSF exuvia followed by dyeing/staining with the reactive dye is shown and compared with a polyester sample pretreated with only debaca (without chitosan) and dyed with reactive dye. The color difference clearly shows the successful deposition of chitosan on the fabric.
Figure 8.

Pretreated polyester fabrics after dyeing using Ramazol Red as a reference (left) and pretreated polyester fabrics after finishing with chitosan and dyeing (right).
2.6.1. Morphological Analysis of Polyester Fabrics
In Figure 9, the morphological structures of the polyester untreated and treated samples were studied at 5000× magnification. In the micrograph 9a of the untreated polyester, the fibers were observed to have a smooth surface without any surface damage, while the polyester fibers after treatment with the debaca process shown in micrograph 9b (benzylalkoniumchoride and sodium hydroxide) resulted in the surface pitted with ditches and less deep cavities. The pits on the surface of polyester fibers treated with the debaca process tend to be less deep and flatter cavities. These results are in correlation with previous studies’ related surface modification of polyesters with sodium hydroxide.64 This effect leads to microlevel roughness of the polyester fiber surface, which allows for better adhesion of chitosan finish/coating on the surface, as shown in micrograph 9c. In Figure 9c, the surface of the polyester fibers appeared to be smooth and all the surface roughness cavities/pits on polyester fibers were evenly covered/filled with chitosan.
The similar surface morphology was observed to be on micrograph 9d of chitosan finish polyester fabrics after dyeing with reactive dye, where the smooth surface without any cavities/surface roughness/pits confirms the presence of chitosan, which indirectly resulted in high color intensity of polyester fabrics dyed/stained with reactive dye, as shown in Figure 8.
3. Conclusions
In the present study, chitin was isolated from defatted and demineralized BSF exuviae using superheated water hydrolysis at 150 °C for 20 h, where superheated water acts as a green solvent for protein removal. Different hydrolysis times were investigated, keeping a constant temperature of 150 °C until a maximum removal of proteins was reached. The purity of obtained chitin, which is α type, was assessed by FTIR spectroscopy and thermal analysis, as well as the purity of chitosan produced by chitin deacetylation. From the biorefinery of BSF exuviae, a total amount of 7% fat from solvent extraction, 40% proteins in aqueous solution after superheated water hydrolysis, and 20% of purified chitin as final remains were obtained. It is to be highlighted that proteins were obtained in water-based medium instead of in strong alkaline solution can be used as an example animal food without any further purification. A preliminary study was successfully carried out to use the obtained chitosan as a finishing agent for polyester fabrics, pretreated with debaca treatment to increase the reactivity and using citric acid as a cross-linking agent.
4. Experimental Section
4.1. Materials
BSF exuviae were provided by Lipitalia Spa, Rosta, Turin, Italy. All chemicals were of analytical grade and purchased from Sigma-Aldrich, unless otherwise specified. Distilled water used in all the experiments was prepared using the Millipore apparatus. A commercially 100% polyester fabric ISO 105-F04 was obtained from Ausiliari Tessili Srl, Cornaredo, Milan, Italy.
4.2. Methods
4.2.1. BSF Exuviae Collection and Preparation
The larvae of BSF were bred in the greenhouse of Lipitalia and fed with waste from the large-scale retail trade. BSF exuviae were manually selected by eliminating impurities such as dead flies or soil, washed three times with distilled water, and dried in an oven at 55 °C for 24 h. After cleaning, the BSF exuviae were ground coarsely with a home mixer (Bosch MSM66150 Immersion Mixer, 600 W) prior to further analysis and refining (see Figure 10).
Figure 10.
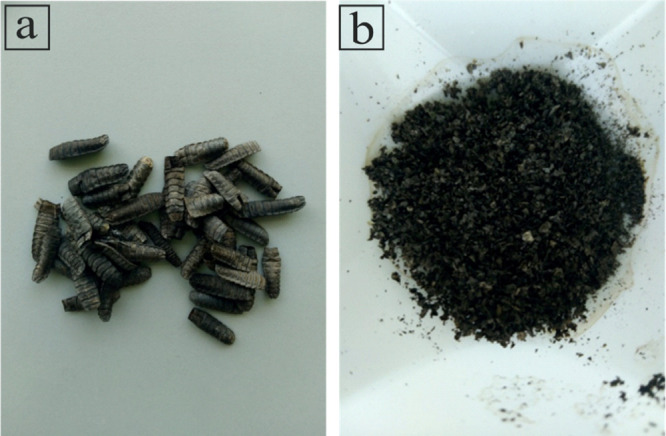
(a) BSF exuviae and (b) BSF exuviae powder.
4.2.2. BSF Exuviae Composition
The moisture content was determined by drying the BSF exuviae in a ventilated oven at 105 °C up to reaching a constant weight, and the results were calculated using the formula
| 1 |
where Ai = initial weight of the BSF exuviae sample and Af = final weight of the BSF exuviae sample.
The crude fat content of BSF exuviae was determined using a Soxhlet apparatus. The dried BSF exuviae powder was loaded in cellulose extraction thimbles and refluxed for 4 h using diethyl ether as a solvent. The amount of fat recovered from BSF exuviae powder at the end of solvent extraction was calculated and reported to the initial dry weight of the BSF exuviae powder sample using the formula
| 2 |
where Wi = initial dry weight of the BSF exuviae powder sample and Wf = weight of the extracted fat.
For the determination of ash content,33 3 g of BSF exuviae were kept in a furnace at 550 °C for 5 h + 5 h until complete mineralization, and the weight of the ash was calculated by
| 3 |
where Af = final weight of the ash and Ai = initial dry weight of the BSF exuviae powder sample.
4.2.3. Isolation of Chitin Using Superheated Water Hydrolysis to Remove Proteins
The BSF exuviae defatted powder (after Soxhlet extraction using diethyl ether) was treated with 2 N HCl for 24 h at room temperature under stirring at 120 rpm with a material to liquor ratio (MLR) of 1:10 to eliminate the mineral deposits and catechol.65 The sample was filtered, and the residue was washed to neutral with distillate water on a stainless steel sieve (120 mesh).
The defatted and demineralized BSF exuviae were then fed in a specifically designed and built laboratory-scale reactor along with water.39 The experiment was carried out at 150 °C, equivalent to a pressure of 5 bar, with a MLR of 1:10 for different times 1, 5, 10, 15, and 20 h. The sample was filtered, and the residue was recovered on a stainless steel sieve (120 mesh) and rinsed. The filtrate was collected, and 5 mL of the filtrate was dried in the oven until the constant weight to determine the amount of extracted proteins. Four replicates for each condition were performed. Hydrolysis treatment resulted in homogeneous impregnation of water inside the BSF exuviae, followed by solubilization of proteins in water.
The chitin content in the sample was determined by the ratio between the final weight (after complete protein removal) and the initial weight (before defatting) of the dried sample.
The protein removal in the samples was determined as the ratio between the weight of proteins recovered and dried after protein removal and the initial weight (before defatting) of the dried sample. However, some protein loss occurs during the rinsing stage.
4.2.4. Chitosan Production
The deacetylation step on the obtained chitin was performed by using 50% NaOH at 100 °C for 20 h under a nitrogen atmosphere in a thermostatic bath for 20 h under mechanical agitation (100 oscillations per minute), MLR 1:30. The suspension was cooled and washed to neutral with distillate water on a stainless steel sieve (120 mesh) until neutral pH.66,8
4.2.5. Polyester Fabric Finishing Using Chitosan Obtained from BSF Exuviae
The obtained chitosan was used as a finishing agent on polyester fabrics. Polyester fabrics were pretreated to increase water absorbency in an aqueous solution containing 3 g/L benzalkonium chloride as a catalyst and an amount of NaOH to give 5% weight loss on polyester fabrics. The reaction was carried out in a hermetically sealed container for 1 h at 105 °C, with the MLR being 1:2567 (debaca treatment).
The amount of NaOH used was determined by the formula
| 4 |
Chitosan solution was prepared by dissolving 1% w/w of chitosan in 3% citric acid at 50 °C for 4 h under magnetic stirring. The polyester fabric was then impregnated in the chitosan solution for 30 min, squeezed through a padding mangle with 100% wet pickup, and dried in a ventilated oven at 105 °C. The presence of chitosan on the polyester fabric was confirmed by SEM and by dyeing of polyester fabrics with reactive dye Remazol Red—1% o.w.f—in a thermostatic water bath under stirring (shaking speed 100 oscillations per minute) at 60 °C for 30 min, MLR 1:50. After dyeing, fabric samples were washed with cold water and then with hot water, followed by soaping using 2 g/L nonionic soap at boiling.
4.3. Characterization of BSF Exuviae, Chitin, Chitosan, and Treated Fabrics
4.3.1. Scanning Electron Microscopy
Morphological investigation of chitin from exuviae and of chitosan-finished polyester fabrics was carried out by a LEO 135 VP scanning electron microscope (Leica Electron Optics) with an acceleration voltage of 15 kV, 50 pA of current probe, and 30 mm working distance. The samples were mounted on aluminium specimen stubs with double-sided adhesive tape. Samples were sputter-coated with a 20–30 nm thick gold layer in rarefied argon, using a sputter coater with a current of 20 mA for 4 min.
4.3.2. FTIR Spectroscopy
FTIR spectra were obtained by a Thermo Nicolet iZ10 spectrometer equipped with a Smart Endurance TM (ZnSe crystal) in attenuated total reflectance mode with 100 scans in the range of 4000–650 cm–1 with a resolution of 4 cm–1 and gain of 8.0. The FTIR spectra of chitin and chitosan samples obtained from BSF exuviae were compared with the FTIR spectra of commercial chitin and chitosan.
4.3.3. Thermal Analysis
Thermal analysis was carried out on samples of chitin from BSF exuviae with different degrees of purification and on obtained chitosan using commercial samples as a reference.
4.3.4. Differential Scanning Calorimetry
DSC analysis was performed with a Mettler Toledo DSC 821, flushing the calorimeter cell with 100 mL/min of nitrogen, from 25 to 500 °C at a heating rate of 10 °C/min, and the instrument was calibrated with indium as a standard. The data were collected on a computer using the Mettler Toledo Star System.
4.3.5. Thermal Gravimetric Analysis
TGA was conducted with a Mettler Toledo TGA/DSC 1 Stare System. Accurately weighed material samples were placed in an alumina cup and hermetically sealed. The measurements were carried out from 25 to 500 °C under a protective nitrogen gas atmosphere and under an airflow of 10 ml/min at a scanning rate of 10 °C/min. The data were collected on a computer with the Mettler Toledo Star System.
Acknowledgments
The authors thank the company Lipitalia 2000 S.p.A. for his financial support in the framework of the project HI-BIORAFINERY (POR FESR 2014/2020 Piedmont Region—Italy).
The authors declare no competing financial interest.
References
- Liu S.; Sun J.; Yu L.; Zhang C.; Bi J.; Zhu F.; Qu M.; Jiang C.; Yang Q. Extraction and Characterization of Chitin from the Beetle Holotrichia Parallela Motschulsky. Molecules 2012, 17, 4604–4611. 10.3390/molecules17044604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čičková H.; Newton G. L.; Lacy R. C.; Kozánek M. The Use of Fly Larvae for Organic Waste Treatment. Waste Manag. 2015, 35, 68–80. 10.1016/j.wasman.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Sheppard D. C.; Tomberlin J. K.; Joyce J. A.; Kiser B. C.; Sumner S. M. Rearing Methods for the Black Soldier Fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. 10.1603/0022-2585-39.4.695. [DOI] [PubMed] [Google Scholar]
- Makkar H. P. S.; Tran G.; Heuzé V.; Ankers P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. 10.1016/j.anifeedsci.2014.07.008. [DOI] [Google Scholar]
- Wu C.-S.; Wang S.-S. Bio-Based Electrospun Nanofiber of Polyhydroxyalkanoate Modified with Black Soldier Fly’s Pupa Shell with Antibacterial and Cytocompatibility Properties. ACS Appl. Mater. Interfaces 2018, 10, 42127–42135. 10.1021/acsami.8b16606. [DOI] [PubMed] [Google Scholar]
- Guinesi L. S.; Cavalheiro É. T. G. The Use of DSC Curves to Determine the Acetylation Degree of Chitin/Chitosan Samples. Thermochim. Acta 2006, 444, 128–133. 10.1016/j.tca.2006.03.003. [DOI] [Google Scholar]
- Wanjun T.; Cunxin W.; Donghua C. Kinetic Studies on the Pyrolysis of Chitin and Chitosan. Polym. Degrad. Stab. 2005, 87, 389–394. 10.1016/j.polymdegradstab.2004.08.006. [DOI] [Google Scholar]
- Yaghobi N. Enhancement of Chitins Degree of Deacetyla- Tion by Multistage Alkali Treatments. Iran. Polym. J. 2004, 13, 131. [Google Scholar]
- Abdel-Rahman R. M.; Hrdina R.; Abdel-Mohsen A. M.; Fouda M. M. G.; Soliman A. Y.; Mohamed F. K.; Mohsin K.; Pinto T. D. Chitin and Chitosan from Brazilian Atlantic Coast: Isolation, Characterization and Antibacterial Activity. Int. J. Biol. Macromol. 2015, 80, 107–120. 10.1016/j.ijbiomac.2015.06.027. [DOI] [PubMed] [Google Scholar]
- Abdou E. S.; Nagy K. S. A.; Elsabee M. Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. 10.1016/j.biortech.2007.01.051. [DOI] [PubMed] [Google Scholar]
- El Knidri H.; Belaabed R.; Addaou A.; Laajeb A.; Lahsini A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. 10.1016/j.ijbiomac.2018.08.139. [DOI] [PubMed] [Google Scholar]
- Saravanan D.; Gomathi T.; PN S. Comparative Study of Thermal Stability Using Natural Polymer Blend by Cross Linking. Arch. Appl. Sci. Res. 2011, 3, 342–350. [Google Scholar]
- Purkayastha D.; Sarkar S. Physicochemical Structure Analysis of Chitin Extracted from Pupa Exuviae and Dead Imago of Wild Black Soldier Fly (Hermetia Illucens). J. Polym. Environ. 2020, 28, 445–457. 10.1007/s10924-019-01620-x. [DOI] [Google Scholar]
- Paulino A. T.; Simionato J. I.; Garcia J. C.; Nozaki J. Characterization of Chitosan and Chitin Produced from Silkworm Crysalides. Carbohydr. Polym. 2006, 64, 98–103. 10.1016/j.carbpol.2005.10.032. [DOI] [Google Scholar]
- Younes I.; Rinaudo M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S. Y.; Chong S. S.; Chin K. S. Biodiesel Derive Bio-Oil of Hermetia Illucens Pre-Pupae Catalysed by Sulphonated Biochar. E3S Web Conf. 2018, 34, 02004. 10.1051/e3sconf/20183402004. [DOI] [Google Scholar]
- Roy J.; Salaün F.; Giraud S.; Ferri A.; Guan J.. Biological Activities and Application of Marine Polysaccharides: Chitosan-Based Sustainable Textile Technology: Process, Mechanism, Innovation, and Safety; IntechOpen, 2016; Chapter 12. [Google Scholar]
- Sagheer F. A. A.; Al-Sughayer M. A.; Muslim S.; Elsabee M. Z. Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. 10.1016/j.carbpol.2009.01.032. [DOI] [Google Scholar]
- Huang L.; Xiao L.; Yang G.. Chitosan Application in Textile Processing. Current Trends in Fashion Technology & Textile Engineering; Juniper Publishers Inc., 2018; Vol. 4. [Google Scholar]
- Hahn T.; Bossog L.; Hager T.; Wunderlich W.; Breier R.; Stegmaier T.; Zibek S.. Chitosan Application in Textile Processing and Fabric Coating. Chitin and Chitosan; John Wiley & Sons, Ltd., 2019; pp 395–428. [Google Scholar]
- Enescu D. Use of Chitosan in Surface Modification of Textile Materials. Roum. Biotechnol. Lett. 2008, 13, 4037. [Google Scholar]
- Teli M. D.; Sheikh J.; Bhavsar P. Multifunctional Finishing of Cotton Using Chitosan Extracted from Bio-Waste. Int. J. Biol. Macromol. 2013, 54, 125–130. 10.1016/j.ijbiomac.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Lee E. J.; Huh B. K.; Kim S. N.; Lee J. Y.; Park C. G.; Mikos A. G.; Choy Y. B. Application of Materials as Medical Devices with Localized Drug Delivery Capabilities for Enhanced Wound Repair. Prog. Mater. Sci. 2017, 89, 392–410. 10.1016/j.pmatsci.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa S.; Kasai M.; Mizuta Y. Modification of Polyester Fabrics Using Chitosan. Sen’i Gakkaishi 1995, 51, 17–22. 10.2115/fiber.51.17. [DOI] [Google Scholar]
- Julià M.; Pascual E.; Erra P. Influence of the Molecular Mass of Chitosan on Shrink-resistance and Dyeing Properties of Chitosantreated Wool. Color. Technol. 2006, 116, 62–67. 10.1111/j.1478-4408.2000.tb00023.x. [DOI] [Google Scholar]
- Park W. H.; Lee K. Y.; Choi J. H.; Ha W. S.; Chang B. H. Characterization of Chitosan-Treated Wool Fabric (I) – Antimicrobial and Deodorant Activities. J. Korean Fiber Soc. 1996, 33, 855–860. 10.3348/jkrs.1996.35.6.855. [DOI] [Google Scholar]
- Julià M. R.; Cot M.; Erra P.; Jocic D.; Canal J. The Use of Chitosan on Hydrogen Peroxide Pretreated Wool. Text. Chem. Color. 1998, 30, 78–83. [Google Scholar]
- Roberts G. A. F.Structure of Chitin and Chitosan BT—Chitin Chemistry; Macmillan Education UK: London, 1992; pp 1–53. [Google Scholar]
- Doan C. T.; Tran T. N.; Nguyen V. B.; Vo T. P. K.; Nguyen A. D.; Wang S.-L. Chitin Extraction from Shrimp Waste by Liquid Fermentation Using an Alkaline Protease-Producing Strain, Brevibacillus Parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. 10.1016/j.ijbiomac.2019.03.117. [DOI] [PubMed] [Google Scholar]
- Hamdi M.; Hajji S.; Affes S.; Taktak W.; Maâlej H.; Nasri M.; Nasri R. Development of a Controlled Bioconversion Process for the Recovery of Chitosan from Blue Crab (Portunus Segnis) Exoskeleton. Food Hydrocolloids 2018, 77, 534–548. 10.1016/j.foodhyd.2017.10.031. [DOI] [Google Scholar]
- Purkayastha D.; Sarkar S. Physicochemical Structure Analysis of Chitin Extracted from Pupa Exuviae and Dead Imago of Wild Black Soldier Fly (Hermetia Illucens). J. Polym. Environ. 2020, 28, 445–457. 10.1007/s10924-019-01620-x. [DOI] [Google Scholar]
- Wang H.; Rehman K. u.; Feng W.; Yang D.; Rehman R. u.; Cai M.; Zhang J.; Yu Z.; Zheng L. Physicochemical Structure of Chitin in the Developing Stages of Black Soldier Fly. Int. J. Biol. Macromol. 2020, 149, 901–907. 10.1016/j.ijbiomac.2020.01.293. [DOI] [PubMed] [Google Scholar]
- Zhou P.; Li J.; Yan T.; Wang X.; Huang J.; Kuang Z.; Ye M.; Pan M. Selectivity of Deproteinization and Demineralization Using Natural Deep Eutectic Solvents for Production of Insect Chitin (Hermetia Illucens). Carbohydr. Polym. 2019, 225, 115255. 10.1016/j.carbpol.2019.115255. [DOI] [PubMed] [Google Scholar]
- Caligiani A.; Marseglia A.; Leni G.; Baldassarre S.; Maistrello L.; Dossena A.; Sforza S. Composition of Black Soldier Fly Prepupae and Systematic Approaches for Extraction and Fractionation of Proteins, Lipids and Chitin. Food Res. Int. 2018, 105, 812–820. 10.1016/j.foodres.2017.12.012. [DOI] [PubMed] [Google Scholar]
- McNeil S. J.; Sunderland M. R.; Zaitseva L. I. Closed-Loop Wool Carpet Recycling. Resour. Conserv. Recycl. 2007, 51, 220–224. 10.1016/j.resconrec.2006.09.006. [DOI] [Google Scholar]
- Yin J.; Rastogi S.; Terry A. E.; Popescu C. Self-Organization of Oligopeptides Obtained on Dissolution of Feather Keratins in Superheated Water. Biomacromolecules 2007, 8, 800–806. 10.1021/bm060811g. [DOI] [PubMed] [Google Scholar]
- Zoccola M.; Montarsolo A.; Mossotti R.; Patrucco A.; Tonin C. Green Hydrolysis as an Emerging Technology to Turn Wool Waste into Organic Nitrogen Fertilizer. Waste Biomass Valorization 2015, 6, 891–897. 10.1007/s12649-015-9393-0. [DOI] [Google Scholar]
- Bhavsar P.; Patrucco A.; Montarsolo A.; Mossotti R.; Rovero G.; Giansetti M.; Tonin C. Superheated Water Hydrolysis of Waste Wool in a Semi-Industrial Reactor to Obtain Nitrogen Fertilizers. ACS Sustainable Chem. Eng. 2016, 4, 6722. 10.1021/acssuschemeng.6b01664. [DOI] [Google Scholar]
- Bhavsar P. S.; Zoccola M.; Patrucco A.; Montarsolo A.; Mossotti R.; Giansetti M.; Rovero G.; Maier S. S.; Muresan A.; Tonin C. Superheated Water Hydrolyzed Keratin: A New Application as a Foaming Agent in Foam Dyeing of Cotton and Wool Fabrics. ACS Sustainable Chem. Eng. 2017, 5, 9150–9159. 10.1021/acssuschemeng.7b02064. [DOI] [Google Scholar]
- Smets R.; Verbinnen B.; Van De Voorde I.; Aerts G.; Claes J.; Van Der Borght M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia Illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valor. 2020, 11, 6455. 10.1007/s12649-019-00924-2. [DOI] [Google Scholar]
- Tinti A.; Tugnoli V.; Bonora S.; Francioso O. Recent Applications of Vibrational Mid-Infrared (IR) Spectroscopy for Studying Soil Components: A Review. J. Cent. Eur. Agric. 2015, 16, 1–22. 10.5513/jcea01/16.1.1535. [DOI] [Google Scholar]
- Liu X.; Chen X.; Wang H.; Yang Q.; ur Rehman K.; Li W.; Cai M.; Li Q.; Mazza L.; Zhang J.; Yu Z.; Zheng L. Dynamic Changes of Nutrient Composition throughout the Entire Life Cycle of Black Soldier Fly. PLoS One 2017, 12, e0182601 10.1371/journal.pone.0182601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P.; Wang H.; Chen C.; Ge F.; Liu D.; Li S.; Han B.; Xiong X.; Zhao S. The Use of Fourier Transform Infrared Spectroscopy for Quantification of Adulteration in Virgin Walnut Oil. J. Spectrosc. 2013, 2013, 305604. 10.1155/2013/305604. [DOI] [Google Scholar]
- Mahmood T.; Malik M.; Bano A.; Umer J.; Shaheen A. Nanocatalytic Conversion of Waste Palm Oil Grade III and Poplar Plant’s Wood Sawdust into Fuel. Innovat. Energy Res. 2017, 106, 1–7. 10.4172/2576-1463.1000170. [DOI] [Google Scholar]
- Kaya M.; Erdogan S.; Mol A.; Baran T. Comparison of Chitin Structures Isolated from Seven Orthoptera Species. Int. J. Biol. Macromol. 2015, 72, 797–805. 10.1016/j.ijbiomac.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Ravi Kumar M. N. V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. 10.1016/s1381-5148(00)00038-9. [DOI] [Google Scholar]
- Waśko A.; Bulak P.; Polak-Berecka M.; Nowak K.; Polakowski C.; Bieganowski A. The First Report of the Physicochemical Structure of Chitin Isolated from Hermetia Illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. 10.1016/j.ijbiomac.2016.07.038. [DOI] [PubMed] [Google Scholar]
- Dahmane E. M.; Taourirte M.; Eladlani N.; Rhazi M. Extraction and Characterization of Chitin and Chitosan from Parapenaeus Longirostris from Moroccan Local Sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. 10.1080/1023666x.2014.902577. [DOI] [Google Scholar]
- Chatsuwan N.; Puechkamut Y.; Pinsirodom P. Characterization, Functionality and Antioxidant Activity of Water-Soluble Proteins Extracted from Bombyx Mori Linn. Curr. Appl. Sci. Technol. 2018, 18, 83–96. 10.14456/cast.2018.4. [DOI] [Google Scholar]
- Bhavsar P.; Fontana G. D.; Tonin C.; Patrucco A.; Zoccola M. Superheated Water Hydrolyses of Waste Silkworm Pupae Protein Hydrolysate: A Novel Application for Natural Dyeing of Silk Fabric. Dyes Pigm. 2020, 183, 108678. 10.1016/j.dyepig.2020.108678. [DOI] [Google Scholar]
- Pendekal M.; Tegginamat P. Development and Characterization of Chitosan-Polycarbophil Interpolyelectrolyte Complex-Based 5-Fluorouracil Formulations for Buccal, Vaginal and Rectal Application. DARU J. Pharm. Sci. 2012, 20, 67. 10.1186/2008-2231-20-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvvada Y.; Vankayalapati S.; Sukhavasi S. Extraction of Chitin and Chitosan from Exoskeleton of Shrimp for Application in the Pharmaceutical Industry. Int. Curr. Pharm. J. 2012, 1, 258. 10.3329/icpj.v1i9.11616. [DOI] [Google Scholar]
- Kittur F. S.; Harish Prashanth K. V.; Udaya Sankar K.; Tharanathan R. N. Characterization of Chitin, Chitosan and Their Carboxymethyl Derivatives by Differential Scanning Calorimetry. Carbohydr. Polym. 2002, 49, 185–193. 10.1016/s0144-8617(01)00320-4. [DOI] [Google Scholar]
- Mathew S.; Brahmakumar M.; Abraham T. E. Microstructural Imaging and Characterization of the Mechanical, Chemical, Thermal, and Swelling Properties of Starch–Chitosan Blend Films. Biopolymers 2006, 82, 176–187. 10.1002/bip.20480. [DOI] [PubMed] [Google Scholar]
- Grząbka-Zasadzińska A.; Amietszajew T.; Borysiak S. Thermal and Mechanical Properties of Chitosan Nanocomposites with Cellulose Modified in Ionic Liquids. J. Therm. Anal. Calorim. 2017, 130, 143–154. 10.1007/s10973-017-6295-3. [DOI] [Google Scholar]
- Abdelghani H.; Satha H.; Boufi S. Chitin from Agaricus Bisporus: Extraction and Characterization. Int. J. Biol. Macromol. 2017, 117, 1334. 10.1016/j.ijbiomac.2017.11.172. [DOI] [PubMed] [Google Scholar]
- Hong P.-Z.; Li S.-D.; Ou C.-Y.; Li C.-P.; Yang L.; Zhang C.-H. Thermogravimetric Analysis of Chitosan. J. Appl. Polym. Sci. 2007, 105, 547–551. 10.1002/app.25920. [DOI] [Google Scholar]
- Lim S.-H.; Hudson S. M. Review of Chitosan and Its Derivatives as Antimicrobial Agents and Their Uses as Textile Chemicals. J. Macromol. Sci., Part A: Pure Appl.Chem. 2003, 43, 223–269. 10.1081/mc-120020161. [DOI] [Google Scholar]
- Chung Y.-S.; Lee K.-K.; Kim J.-W. Durable Press and Antimicrobial Finishing of Cotton Fabrics with a Citric Acid and Chitosan Treatment. Text. Res. J. 1998, 68, 772–775. 10.1177/004051759806801011. [DOI] [Google Scholar]
- Hsieh S.-H.; Huang Z. K.; Huang Z. Z.; Tseng Z. S. Antimicrobial and Physical Properties of Woolen Fabrics Cured with Citric Acid and Chitosan. J. Appl. Polym. Sci. 2004, 94, 1999–2007. 10.1002/app.21104. [DOI] [Google Scholar]
- Hilal N. M.; Gomaa S. H.; ELsisi A. A. Improving Dyeing Parameters of Polyester/Cotton Blended Fabrics by Caustic Soda, Chitosan, and Their Hybrid. Egypt. J. Chem. 2020, 63, 2379–2393. 10.21608/ejchem.2020.25571.2498. [DOI] [Google Scholar]
- Dutkiewiez J.Antimicrobial structures. U.S. Patent 6,197,322 B1, 2001.
- Khouri J.; Penlidis A.; Moresoli C. Viscoelastic Properties of Crosslinked Chitosan Films. Processes 2019, 7, 157. 10.3390/pr7030157. [DOI] [Google Scholar]
- Solbrig C. M.; Obendorf S. K. Alkaline Hydrolysis of Titanium Dioxide Delustered Poly(Ethylene Terephthalate) Yarns 1. Text. Res. J. 1991, 61, 177–181. 10.1177/004051759106100308. [DOI] [Google Scholar]
- Majekodunmi S. O. Current Development of Extraction, Characterization and Evaluation of Properties of Chitosan and Its Use in Medicine and Pharmaceutical Industry. Am. J. Polym. Sci. 2016, 6, 86–91. 10.5923/j.ajps.20160603.04. [DOI] [Google Scholar]
- Yuan Y.; Chesnutt B. M.; Haggard W. O.; Bumgardner J. D. Deacetylation of Chitosan: Material Characterization and in Vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. 10.3390/ma4081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperotto Rimar SpA Finishing Effects on Polyester Fabrics by Alkali Treatment Using the Debaca Process, 2021. [Google Scholar]