Abstract
Purpose
In children with a mitral annulus too small to accommodate traditional prostheses, surgical implantation of stent-based valves is a promising option. However, no reliable pre-operative methods exist to guide patient selection, device sizing and positioning. We describe a novel methodology to visualize and quantify device fit in three-dimensional echocardiogram (3DE)-derived heart models.
Description
Heart models were created from existing pre-operative 3DE using custom software. Valve models were virtually implanted into the models and both device fit and left ventricular outflow tract (LVOT) area were quantified.
Evaluation
The 3DE of three patients who underwent Melody valve placement in the mitral position were retrospectively modeled — one with left ventricular outflow tract obstruction(LVOTO), one with perivalvar leak, and one without complications. In all cases 2D measurements underestimated 3D annular dimensions, and the patient with clinical LVOT obstruction had the lowest predicted LVOT area/Aortic area ratio (0.5).
Conclusions
3DE based pre-operative modeling of surgical implantation of stent-based valves in the mitral position may improve quantification of mitral valve dimensions and inform risk stratification for potential LVOTO.
Keywords: Classifications, Valve replacement, 3D echocardiography, Congenital heart disease
Introduction
The surgical management of clinically significant atrioventricular valve disease in small children remains challenging with high morbidity and rates of reintervention.(1, 2) Management is further complicated by the limited commercial device options for mitral annulus sizes less than 15mm. The Melody valve (Medtronic, Minneapolis, MN) is a stent-mounted valved bovine jugular vein graft that can be surgically implanted in the mitral position, and offers a promising option for this high risk population.(1, 3) These serially expandable valves confer the additional benefit of allowing subsequent serial transcatheter balloon dilation to accommodate somatic growth.
Appropriate patient selection, device sizing, and device positioning are crucial to avoid post-operative complications such as left ventricular outflow tract obstruction (LVOTO) and perivalvar leak.(3) Determining patient candidacy and optimal device positioning is currently done using linear measurements in 2D echocardiograms and surgical inspection. Current methods do not allow 3-dimensional (3D) quantification in the beating heart or intuitive visualization of important relationships of the device to surrounding structures.
To elucidate optimal candidate selection and device placement, we have developed a novel methodology for modeling virtual device placement into 3DE-derived patient-specific heart models and volume-rendered images to visualize and quantify device fit. This modeling was retrospectively applied to three children who had undergone implantation of Melody valve in the mitral position.
Technology and Technique
Patient Selection and Image Acquisition
Databases of patients with Melody valve implantation in the mitral position were queried to identify patients at the two participating institutions who were eligible for inclusion, defined as 1) pre-operative transthoracic 3DE within 3 months of surgery, and 2) existing 3DE including the mitral valve, LVOT, and aortic valve. Two patients with complications were selected; the first had clinically significant LVOTO (mean gradient ≥40 mm Hg), the second had perivalvar leak within 30 days after surgery. A third, with no perioperative complications, was randomly selected as a reference (Table 1). In all cases, images were acquired using X5 and X7 probes on Philips EPIQ 7 ultrasound systems (Philips Medical Systems, Andover, MA). The institutional review boards at the participating institutions approved this study.
Table 1.
Patient Demographics
| Variable | Patient 1 | Patient 2 | Control |
|---|---|---|---|
| Sex | Female | Female | Male |
| Age at Surgery (days) | 275 | 174 | 235 |
| Weight (kg) | 5.2 | 3.0 | 6.6 |
| Height (cm) | 67 | 47 | 64 |
| BSA (m2) | 0.31 | 0.20 | 0.35 |
| Anatomy | Parachute mitral valve | Arcade mitral valve | Parachute mitral valve |
| Previous Mitral Intervention | Mitral repair | Mitral repair | Mitral repair |
| Indication for Mitral Valve Replacement | Severe MS, moderate MR | Moderate MR, severe MS | Severe MS |
| Melody System Size (mm) | 18 | 22 | 18 |
| Actual Measured Valve Diameter (mm) | 12 | 9 | 12 |
| Estimated Deployed Length (mm)* | 26 | 21 | 26 |
| Complication | Moderate perivalvar leak | LVOTO peak gradient 60mmHg | None |
Estimated lengths based on manufacturer deployment specifications
Annular Modeling and Quantification
3DE images were imported into 3D Slicer as previously described.(4) Annular modeling and quantification were performed using custom code and 3D Slicer(Video 1).(4) The mitral and aortic valve annuli were modeled in end diastole (ED) and end systole (ES). The annulus and relevant points were marked as shown in Figure 1. Annular metrics were calculated for each mitral annulus in each phase (Figure 1D) as previously described.(4) For comparison, 2D echocardiographic mitral valve measurements were measured by an observer blinded to the 3D modeling results.
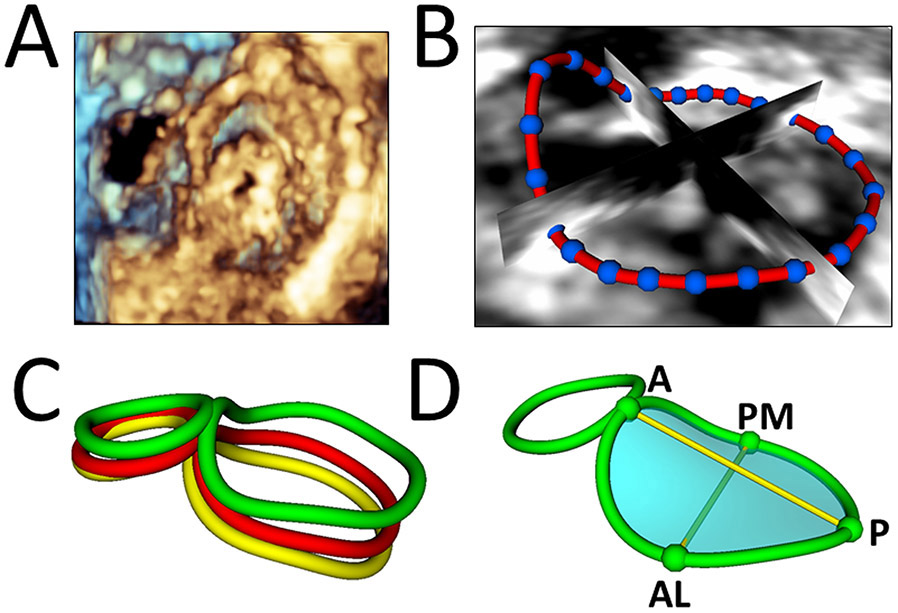
Figure 1. Creation of Mitral Valve Annular Contour and Quantification.
A. 3D volume-rendered view of mitral valve. B. Defining mitral annulus. C. Mitral valve annular contour curves defined at end systole (yellow), mid systole (red), and end diastole (green). D. Mitral valve annular quantification at end diastole. A = Anterior; P = Posterior; AL = Anterolateral; PM = Posteromedial.
3D Segmentation and Volume Rendering of 3D Echocardiograms
Static 3D segmentations of the left ventricle and LVOT were created in ED and ES using the Segments module in 3D Slicer (Figure S1). Volume rendering of 3D images was performed using customized settings and code in 3D Slicer, similar to our previous description.(5)
Device Placement
Virtual cylindrical valve models with the same diameter as the implanted Melody valves were placed in the segmented heart models in 3D Slicer (Figure 2). Due to intra-operative manual modification (e.g., trimming end of stent, pericardial skirt creation), precise quantification of the actual device length was not available. Therefore, unmodified expanded dimensions were utilized for simulation(Table 1). The devices were placed and oriented in two different conformations: 1) orthogonal to the mitral annular plane and 2) angled toward the tip of the posterior-medial papillary muscle. In addition to device placement with traditional controls, we placed virtual devices in virtual reality (VR) using the SlicerVR extension (Video 2).(5)
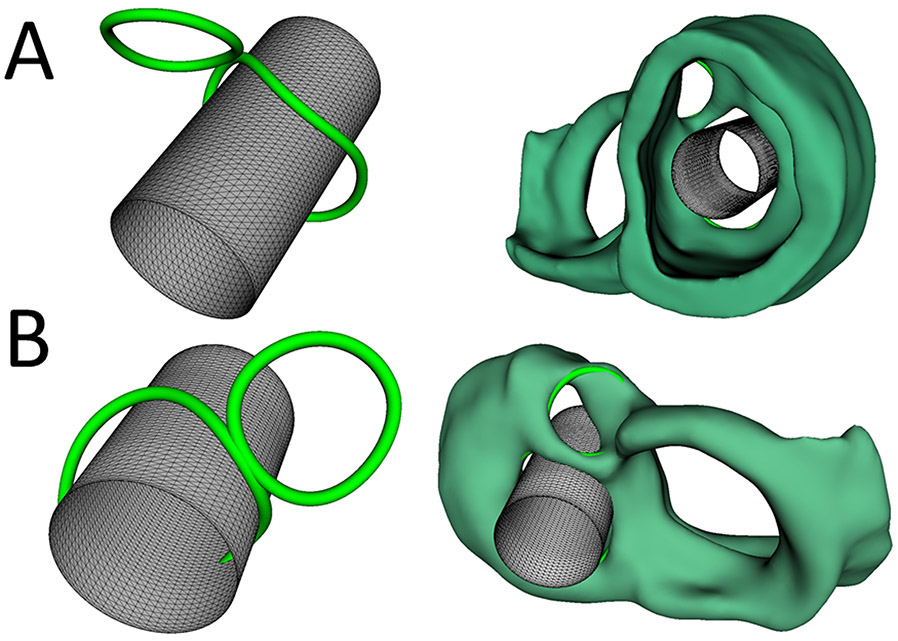
Figure 2. Virtual Implantation of Melody Valve in the Mitral Valve Position.
A. Virtual implantation of the Melody valve in the mitral valve position viewed from the ventricle without 3D heart segmentation (left) and with 3D heart segmentation (right). B. Virtual implantation of the Melody valve in the mitral valve position viewed from the atrium without 3D heart segmentation (left) and with 3D heart segmentation (right).
Quantification of Left Ventricular Outflow Tract Area
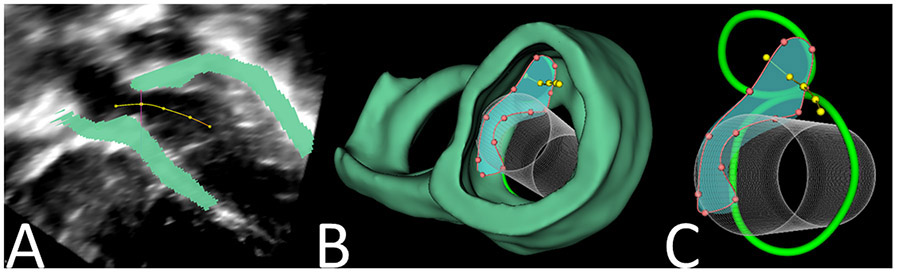
A centerline curve was defined by serially placing points in different positions through the LVOT, beginning at the center of the aortic valve and continuing through the left ventricle (Figure 3A). A cross-sectional plane orthogonal to this line was defined at each point. The smallest cross-sectional LVOT area was defined (Figure 3B and 3C) and quantified for each patient in each device conformation at ED and ES(Figure 4, Video 3).
Figure 3. Demonstration of Cross-Section Analyzer Tool for LVOT Area Quantification.
A. Points placed along center-line of LVOT. B/C. Cross-sectional area of LVOT visualized with 3D heart segmentation and virtual implantation of Melody Valve.
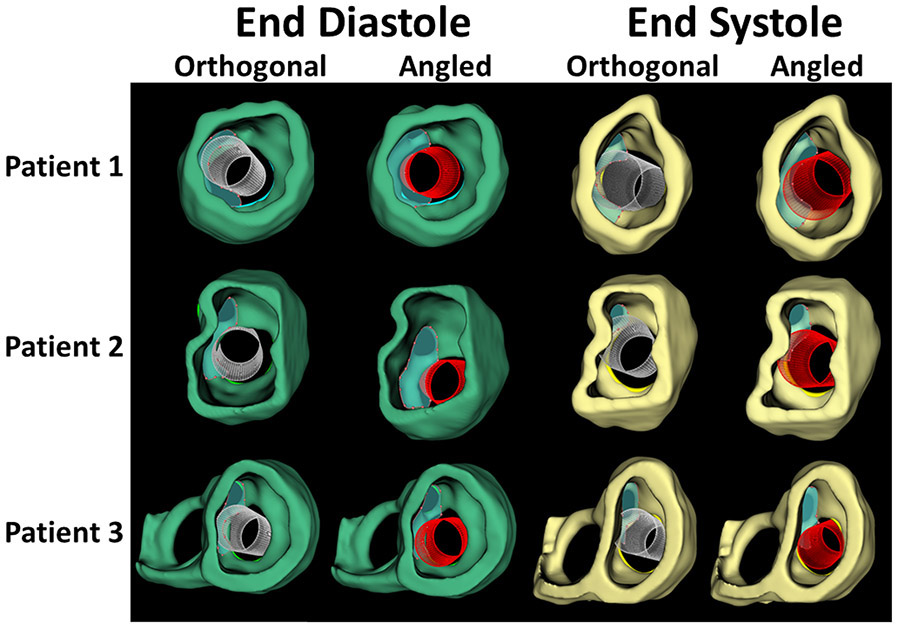
Figure 4.
Comparison of Orthogonal and Angled Conformations of Virtual Melody Valve Implants in End Diastole (green) and End Systole (yellow).
Clinical Experience
Study design
Pre-operative 3DE studies the two Melody MV replacement(MVR) patients with post-operative complications (one with LVOTO, the other with perivalvar leak) were analyzed and subsequently compared to that of a patient with no complications. Patient demographics are shown in Table 1.
Results
The mitral and aortic valve annuli, as well as the surrounding cardiac structures were modeled and quantified in ED and ES for each of the three subjects (Table 2, Table 3). The annular measurements recorded from the 2D images were, on average, lower than those obtained from the 3D-based quantification (Table 2). The average ratio of the 2D-to-3D linear measurement was 0.77 in the 4-chamber view, and 0.87 in the parasternal long axis view. The circumference of the Melody valves calculated from the measured diameters of the stents were all notably less than the measured mitral annular circumference (mean difference ED circumference = 1.8cm, Figure 3, Figure 4).
Table 2.
Mitral Valve and Melody Device Measurements
| Patient 1 | Patient 2 | Control | ||||
|---|---|---|---|---|---|---|
| Complication | Perivalvar Leak | LVOTO | None | |||
| Time in Cardiac Cycle | ED | ES | ED | ES | ED | ES |
| Measured Melody Dimensions | ||||||
| Diameter (cm) | 1.2 | 1.2 | 0.9 | 0.9 | 1.2 | 1.2 |
| Circumference (cm) | 3.8 | 3.8 | 2.8 | 2.8 | 3.8 | 3.8 |
| Area (cm2) | 1.1 | 1.1 | 0.6 | 0.6 | 1.1 | 1.1 |
| 2D Mitral Annular Measurements | ||||||
| 2D Diameter from 4-Chamber (cm) | 1.1 | 1.3 | 1.1 | 1.1 | 1.5 | 1.2 |
| 2D Diameter in Long Axis (cm) | 1.9 | 1.8 | 1.1 | 1.1 | 1.2 | 1.3 |
| 3D Mitral Annular Measurements | ||||||
| A-P Diameter (cm) | 1.8 | 1.8 | 1.2 | 1.3 | 1.8 | 1.7 |
| AL-PM Diameter (cm) | 1.9 | 1.7 | 1.3 | 1.2 | 1.4 | 1.4 |
| Circumference (cm) | 6.1 | 5.6 | 4.2 | 4.0 | 5.4 | 5.1 |
| Area (cm2) | 2.8 | 2.4 | 1.3 | 1.2 | 2.2 | 2.0 |
ED: End diastole; ES: End systole; Ao: Aorta; A-P; Anterior-Posterior; AL-PM; Anterolateral-Posteromedial.
Table 3.
Aortic Annulus and LVOT Quantification
| Patient 1 | Patient 2 | Control | ||||
|---|---|---|---|---|---|---|
| Complication | Perivalvar Leak | LVOTO | None | |||
| Time in Cardiac Cycle | ED | ES | ED | ES | ED | ES |
| 3D Aortic Annular Area (cm2) | 0.7 | 0.6 | 0.4 | 0.4 | 0.9 | 0.9 |
| 3D LVOT Area (Orthogonal) (cm2) | 1.6 | 1.0 | 0.6 | 0.2 | 1.2 | 0.7 |
| LVOT (Orthogonal) / Ao Ratio | 2.4 | 1.6 | 1.3 | 0.5 | 1.4 | 0.8 |
| 3D LVOT Area (Angled) (cm2) | 1.8 | 1.2 | 0.8 | 0.3 | 1.6 | 0.8 |
| LVOT (Angled) / Ao Ratio | 2.8 | 2.0 | 1.8 | 0.9 | 1.8 | 1.0 |
LVOT: Left ventricular outflow tract; LVOTO: Left ventricular outflow tract obstruction; ED: End diastole; ES: End systole; Ao: Aorta.
After simulated Melody valve implantation, the LVOT area was quantified in ED and ES for each patient and compared to the aortic valve area (Table 3, Video 3). The LVOT area was first quantified with the simulated valve orthogonal to the least squares plane of the annulus. The LVOT area was smaller in ES than in ED for all patients. For Patient 2 in ES, quantification of the LVOT area after device placement in the orthogonal device conformation was smaller than the area of the aortic valve, with an LVOT-to-aorta area ratio of 0.5. Notably, this patient had clinically significant LVOTO (Table 1, Video 3).
During actual implantation of the stent-based valves in these patients, the stent frame was purposefully angled toward the posterior-medial papillary muscle and secured with sutures. Virtual posterior angulation of the device resulted in an increase in calculated LVOT area and LVOT-to-aorta area ratio in all cases (Table 3, Video 2, Video 3).
Devices could be placed and visually assessed on screen and in VR. Subjectively, VR improved appreciation of depth and subjectively simplified device placement and evaluation of device size and orientation (Video 2).
Comment
We believe this is the first description of a 3DE-based modeling method for determining the size and fit for surgically-implanted stent-based valves in pediatric patients. Based on our pilot study, we assert three main findings: 1) 3D measurement of the mitral annulus may yield more accurate measurements for device sizing compared to 2D; 2) pre-operative 3DE-based modeling holds promise for improved selection of optimal device size and position for surgically implanted stent-based valves, particularly in determination of potential LVOTO; and 3) angling the ventricular end of stent-based devices away from the LVOT appears to significantly increase LVOT area with stent-based valve implant.
3DE-based modeling holds promise for informing patient candidacy and improving device selection. The presence of a non-circular annulus, and the potential for misrepresentation of the annular size are relevant to the potential for both perivalvar leak and coronary compression. The circumference and area of the Melody valve calculated from the measured diameter of the valve were less than the measured mitral annular circumference in all three patients, even those that did not develop perivalvar leak. Our findings are consistent with recent literature suggesting systematic underestimation of valve circumference based on 2D compared to 3D measurements in both children and adults.(6, 7) In actual surgical implantation, there is typically a pericardial “skirt” placed around the valve which may add a degree of forgiveness in sizing that may not be present in purely transcatheter applications.(3) Variations in the technique of implantation (e.g. proportion of valve in atrium vs. ventricle) is another determinant of LVOTO which could be explored using modeling.(3) Finally, although our modeling is focused on a specific application in children, this cardiac modeling and visualization process may aid more generally in the planning of transcatheter MVR in adult populations(Video 2).
In conclusion, 3D image-based preoperative modeling of surgical implantation of stent-based valves in the mitral position may hold promise for quantification of accurate mitral valve dimensions and for risk stratification of the potential for LVOTO. Modeling and visualization may also be beneficial for the planning of transcatheter MVR in adult patients.
Supplementary Material
Supplemental Figure S1. Creation of 3D Heart Model. A-C. 2D Slice-plane views of segmentation in 3D Slicer. D. 3D segmented model in 3D Slicer viewed from the atrium.
Acknowledgements & Disclosures
All authors declare no relevant conflicts of interest or disclosures. This work was supported the Department of Anesthesia and Critical Care at the Children's Hospital of Philadelphia(CHOP), A CHOP Cardiac Center Innovation Grant, and by CANARIE’s Research Software Program.
References
- 1.Abdullah I, Ramirez FB, McElhinney DB, Lock JE, del Nido PJ, Emani S. Modification of a stented bovine jugular vein conduit (melody valve) for surgical mitral valve replacement. Ann Thorac Surg 2012;94(4):e97–98. [DOI] [PubMed] [Google Scholar]
- 2.Alsoufi B, Manlhiot C, McCrindle BW et al. Results after mitral valve replacement with mechanical prostheses in young children. J Thorac Cardiovasc Surg 2010;139(5):1189–1196, 1196 e1181-1182. [DOI] [PubMed] [Google Scholar]
- 3.Pluchinotta FR, Piekarski BL, Milani V et al. Surgical atrioventricular valve replacement with melody valve in infants and children. Circ Cardiovasc Interv 2018;11(11):e007145. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen AV, Lasso A, Nam HH et al. Dynamic three-dimensional geometry of the tricuspid valve annulus in hypoplastic left heart syndrome with a fontan circulation. J Am Soc Echocardiogr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasso A, Nam HH, Dinh PV et al. Interaction with volume-rendered three-dimensional echocardiographic images in virtual reality. J Am Soc Echocardiogr 2018;31(10):1158–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolley MA, Ghelani SJ, Adar A, Harrild DM. Three-dimensional mitral valve morphology and age-related trends in children and young adults with structurally normal hearts using transthoracic echocardiography. J Am Soc Echocardiogr 2017;30(6):561–571. [DOI] [PubMed] [Google Scholar]
- 7.Foster GP, Dunn AK, Abraham S, Ahmadi N, Sarraf G. Accurate measurement of mitral annular dimensions by echocardiography: Importance of correctly aligned imaging planes and anatomic landmarks. J Am Soc Echocardiogr 2009;22(5):458–463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Creation of 3D Heart Model. A-C. 2D Slice-plane views of segmentation in 3D Slicer. D. 3D segmented model in 3D Slicer viewed from the atrium.