Abstract
Background
Echinococcus multilocularis is one of the most severe and lethal parasitic diseases of humans, most often reported in Europe and Asia. Only 1 previous case has been documented in the contiguous United States from Minnesota in 1977. European haplotypes have been identified in carnivores and domestic dogs as well as recently in patients in western and central Canada.
Methods
We used immunohistochemical testing with the monoclonal antibody Em2G11 and a species-specific enzyme-linked immunosorbent assay affinity-purified antigen Em2, as well as COX1 gene sequencing.
Results
Using pathology, immunohistochemical staining, specific immunodiagnostic testing, and COX1 gene sequencing, we were able to definitively identify E. multilocularis as the causative agent of our patient’s liver and lung lesions, which clustered most closely with the European haplotype.
Conclusions
We have identified the first case of a European haplotype E. multilocularis in the United States and the first case of this parasitic infection east of the Mississippi River. Given the identification of this haplotype in Canada, this appears to be an emerging infectious disease in North America.
Keywords: Echinococcus multilocularis, parasitic infection, liver masses, Echinococcus multilocularis COX gene, alveolar echinococcus spectrum of disease
We have identified the first case of a European haplotype Echinococcus multilocularis in the United States. Given the recent identification of this haplotype in Canada, this may be an emerging infectious disease in North America.
(See the Editorial Commentary by Kern on pages 1124–26.)
Cestodes are a subgroup of the flatworms (Platyhelminthes) that cause human parasitic infection in the form of intestinal tapeworms (Diphyllobothrium, Hymenolepis, Taenia) or invasive larval cysts (Taenia and Echinococcus). Within the genus Echinococcus, Echinococcus granulosus (cystic echinococcosis) and Echinococcus multilocularis (alveolar echinococcosis [AE]) are the 2 most commonly encountered species in humans, who act as intermediate hosts and become infected following ingestion of microscopic parasite eggs shed in the feces of definitive hosts (dogs, wolves, foxes, and other canines) infected with adult tapeworms. Parasite eggs hatch in the stomach and release oncospheres (first larval stage) that penetrate the intestinal mucosa, enter the circulation, and subsequently vesiculate or encyst predominantly in the host liver to form mature fertile metacestodes [1]. Worldwide, E. granulosus is highly endemic in regions of South America, the Mediterranean littoral, Eastern Europe, the Near and Middle East, East Africa, Central Asia, China, and Russia; the annual incidence ranges from < 1 to 200 per 100 000 inhabitants. In contrast, the distribution of E. multilocularis is more restricted to the Northern Hemisphere and is endemic to portions of Western Europe, Central and Eastern Europe, and a large geographic belt spanning from Russia to northern Japan. The annual AE incidence ranges from 0.03 to 1.2 per 100 000 inhabitants but may be much higher in endemic foci [2]. Approximately 91% of the estimated 18 000 new AE cases per year globally originate in China, and the incidence of E. multilocularis has doubled in the previously recognized endemic areas of France, Germany, and Switzerland, where the majority of European cases occur and constitute 42%, 24%, and 21 % of cases, respectively [3, 4].
In the United States (US), northwest Alaska has been considered the primary endemic focus of E. multilocularis infection for autochthonous cases [3, 5]. Although the North American strain of E. multilocularis has been long known to reside in wildlife in the northern US, outside Alaska there has been only 1 case, reported from a resident in Minnesota in 1977 [6]. We describe a patient from Vermont with liver and lung lesions found to be of a European E. multilocularis haplotype.
CASE REPORT
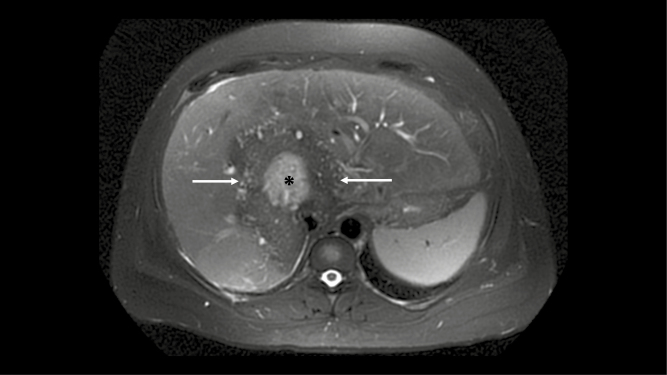
The patient was a 36-year-old woman with a past medical history notable for a thyroidectomy secondary to Graves disease. A complete metabolic panel and thyroid-stimulating hormone (TSH) level obtained in the course of routine monitoring for her thyroid disease were notable for a TSH level < 0.02 μIU/mL (0.46–4.68 μIU/mL), total bilirubin of 1.8 mg/dL (0.2–1.3 mg/dL), increased aspartate aminotransferase level of 45 U/L (14–36 U/L), and an alkaline phosphatase level of 357 U/L (38–126 U/L). A right upper quadrant ultrasonogram obtained to determine the cause of her increased liver enzymes demonstrated a 10-cm heterogeneous area in the right hepatic lobe, and subsequent abdominal magnetic resonance imaging (MRI) confirmed a 10.6 × 10.1 × 12-cm irregular, lobular hepatic mass in the right and left hepatic lobes with partial occlusion of the portal vein and dilated left upper quadrant veins suggestive of portal hypertension (Figure 1). A mass in the posterior left lung base measuring 3.5 × 3.0 × 3.4 cm was also identified on chest MRI (Figure 2).
Figure 1.
Axial T2-weighted magnetic resonance imaging of the liver showing a large hypointense hepatic mass with a central region of high signal (asterisk), suggesting fluid.
Figure 2.
Coronal T1-weighted postcontrast magnetic resonance imaging through the posterior lungs shows a lobulated mass (arrow) in the left lower lobe.
Fine needle aspiration of the lung mass demonstrated necrotizing granulomas and associated chronic inflammation. Gomori methenamine silver (GMS) and Ziehl-Neelsen stains were negative for fungi and acid-fast organisms, respectively. Core biopsy of the liver mass demonstrated necrotic debris with a surrounding fibrotic wall and adjacent, relatively unremarkable liver parenchyma. The fibrotic wall contained granulomas, lymphocytes, plasma cells, and only few eosinophils. Within the necrosis, numerous refractile structures of varying shapes and sizes were identified. These structures were nonpolarizable but stained with both GMS and periodic acid-Schiff (PAS) stains. The overall morphologic appearance was suggestive of invasive parasite cysts, although definitive diagnosis was not possible at that time. All bacterial, fungal, and acid-fast bacilli smears and cultures were negative from both liver and lung.
She was referred to the infectious diseases clinic where she described mild, short-lived, intermittent right upper quadrant tenderness and nausea. Her epidemiologic history was notable in that she was born in Maryland, lived in Vermont most of her life, has a healthy dog at home, and went fox hunting as a teenager in Maryland. She had traveled extensively including in the eastern, northwestern, and southwestern US; Costa Rica; Rome/Naples, and Montreal. She had not traveled to Western or Central Europe, Asia, South America, or Africa.
An extensive workup was undertaken to determine the etiology of her lung and liver lesions, and revealed negative serologic studies for Entamoeba histolytica, Ascaris lumbricoides, Trichinella species, Schistosoma species, Toxocara canis, Strongyloides stercoralis, Bartonella species, Coxiella burnetii (Q fever), Francisella tularensis, Brucella species, and human immunodeficiency virus, as well as negative Blastomyces species and Histoplasma species urinary antigens and negative stool ova and parasites on 3 separate occasions. Toxoplasma gondii immunoglobulin G (IgG) was positive at 302 IU/mL (≥ 12 IU/mL is positive), consistent with previous infection. Echinococcus species IgG was also positive.
MATERIALS AND METHODS
Hematoxylin and eosin, PAS, and GMS stains were performed according to standard protocols (Figures 3 and 4). Pathologic images and slides were sent for consultation to 2 of the authors (B. P. and T. F. E. B.) for expert opinion, and the histopathology was consistent with the larval stage of E. multilocularis. Immunohistochemical testing was performed using the monoclonal antibody Em2G11, an in vitro–produced monoclonal IgG1 antibody from a mouse hybridoma cell line (Figure 5) [7]. The antibody is available on request from Peter Deplazes at the Institute of Parasitology, University of Zurich, Switzerland. For immunohistochemistry, standard protocols were used [8]. In brief, for antigen retrieval, the sections were heated in citrate buffer at pH 6 in a microwave oven for 20 minutes. The primary antibody was used in a concentration of 0.2057 mg/mL in phosphate-buffered saline; slides were incubated with 50 µL per section in a humid chamber at room temperature for 30 minutes. As a detection system, we used the EnVision Kit (Dako, Carpinteria, California) according to the manufacturer’s protocols.
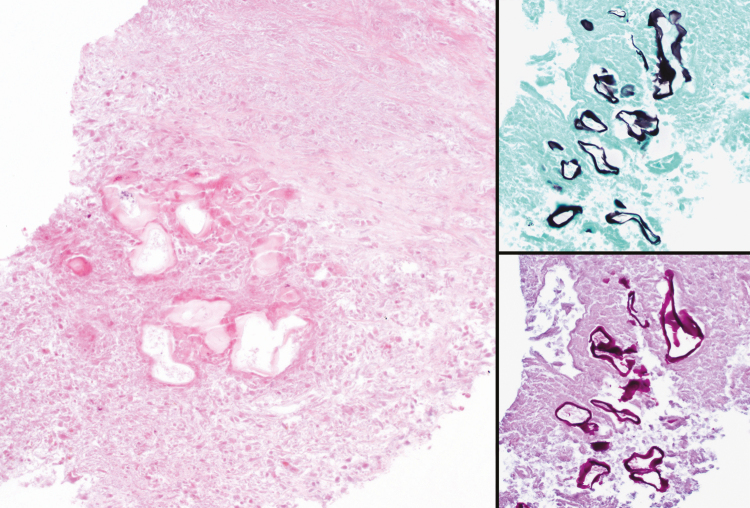
Figure 3.
Liver biopsy demonstrating large areas of necrosis associated with infiltrating parasitic cysts (hematoxylin and eosin stain, ×200) (left image). The cysts are lined with a thin laminated layer, which is strongly highlighted with Gomori methenamine silver (upper right image) and periodic acid-Schiff (lower right image) stains. No protoscoleces are seen, as is common for human cases of alveolar echinococcosis.
Figure 4.
Portion of resected lung nodule showing similar-appearing parasitic cysts within a necrotizing granuloma (hematoxylin and eosin stain, ×400). This biopsy was obtained following albendazole therapy.
Figure 5.
Immunohistochemical staining of the section shown in Figure 2 using the monoclonal antibody Em2G11, which recognizes a glycoprotein in the laminated layer of the larval stage of Echinococcus multilocularis. Strongly positive red staining is seen in association with the parasitic cysts and focally in the surrounding necrotic regions, marking shed particles of the laminated layer known as small particles of E. multilocularis.
IMMUNODIAGNOSIS
Echinococcus multilocularis
Given that the Echinococcus species serology used initially does not differentiate between antibodies reacting with E. granulosus and E. multilocularis, a species-specific enzyme-linked immunosorbent assay (ELISA) affinity-purified E. multilocularis antigen Em2 and recEm18-antigen were used, which together exhibit a high diagnostic sensitivity of 97% and an overall specificity of 98%–100% for E. multilocularis [9]. The patient yielded clearly positive ELISA serologies in both tests (48 antibody units [AU] in the Em2 ELISA and 28 AU in the recEm18 ELISA; positive ≥ 1 AU).
Immunoblotting was based on an in vitro–produced E. multilocularis soluble vesicle fluid antigen, with a diagnostic sensitivity and specificity of 98% [10]. The patient yielded a strongly positive result and all bands characteristic for AE were reactive.
Species Determination: Partial COX1 Amplification and Sequencing
DNA was extracted from formalin-fixed, paraffin-embedded liver tissue. Using nematode- and cestode-directed quantitative polymerase chain reaction (PCR) primers and probes [11], the extracted DNA was determined to be cestode in origin. Partial COX1 gene amplification was performed using primers designed to amplify a 127-bp fragment across cestode genomes [11]. PCR products were confirmed to be approximately 130 bp, then inserted into pCR 2.1 TOPO (Invitrogen, Carlsbad, California) and cloned. Six separate isolates were sequenced in the forward and reverse direction (Macrogen USA, Rockville, Maryland). The 128-bp consensus sequence result was searched using the National Center for Biotechnology Information Basic Local Alignment Search Tool (BLAST) and matched E. multilocularis (accession number KY446489) with 97% identity. The sequence was as follows:
TGGGTTATTGTTTGCTATGTTTTCAATAGTGTGTTTAGGGAGTAGTGTTTGGGGTCATCATATGTTTACTGTTGGGTTGGATGTGAAGACGGCGGTTTTTTTTAGTTCTGTTACTATGATAATAGGGG.
E. multilocularis Complete COX1 Gene Sequencing
Approximately 0.5 g of resected tissue from the lung of this patient was frozen and shipped on dry ice overnight to the National Institutes of Health and stored at −80°C until extraction. DNA was extracted with Wizard Genomic DNA Purification Kit (Promega, catalog number A1120) per the manufacturer’s instructions. Initial attempts at shotgun next-generation sequencing revealed that > 96% of the DNA matched to the human genome. Thus, targeted amplification and sequencing of the E. multilocularis COX1 gene was performed. PCR was performed using 22 sets of described overlapping primers [12] using Illustra PuReTaq Ready-To-Go beads (GE Life Sciences, catalog number 27955901) per the manufacturer’s instructions with thermocycling conditions as follows: initial denaturation at 95°C for 5 minutes followed by 40 cycles of 95°C for 45 seconds, 50°C for 45 seconds, and 72°C for 45 seconds, subsequently followed by an 8-minute elongation step at 72°C and held at 4°C. Resulting fragments were subjected to Sanger sequencing (Psomagen, Rockville, Maryland) in both the forward and reverse directions. High-quality reads were assembled in Sequencher version 5.4.6 software (Ann Arbor, Michigan) against published E. multilocularis COX1 complete coding sequence. Sequencing data of 2 nucleotides (bp 250–251) was of such low quality that no bases were called. The entire COX1 sequence was deposited at GenBank (accession number MN387224).
A phylogenetic tree was constructed with this sequence (MN387224) and all other publicly available E. multilocularis COX1 sequences with > 1400 bp sequenced (72 other sequences), and bootstrapping was performed in MegAlign version 15.0.0 software (DNASTAR, Madison, Wisconsin).
RESULTS
In comparing this clinical sample to all reported sequences of E. multilocularis for the COX1 sequence, this patient’s isolate clusters most closely with wildlife isolates found in Poland, Austria, and, to a lesser extent, those found in Canada recently (Figure 6A). In assessing nucleotide substitutions in described haplotypes [13], of the 43 potential base pairs, our sequence was identical at 42 positions to the European E2, E4, and E5 haplotypes (Figure 6B). Position 822 was equally represented by nucleotides A and G (represented by “R”) and of high quality (Phred scores of 62 each); A is seen in European haplotypes and G is seen in Asian and North American strains. Notably, this is a wobble position.
Figure 6.
Echinococcus multilocularis phylogenetic relationships and haplotype classification based on COX1 sequencing. A, Phylogenetic tree of all reported E. multilocularis COX1 sequences with > 1400 bp. Bootstrap values (trials = 1000; seed = 111) are depicted. Sequence from the Vermont patient is shown in red. B, Echinococcus multilocularis haplotype by nucleotide polymorphism location found in this patient’s isolate, compared with the most similar European (E), Asian (A), and North American (N) haplotypes. Most sequenced isolates are from wildlife, and the diversity of countries represented in the phylogenetic tree does not reflect clinical disease burden in the respective countries. The IUPAC representation of sequences is shown, with R denoting ambiguity between A and G. Dots represent identical nucleotides at the given position with this isolate. Aside from ambiguity at 822, the Vermont patient’s E. multilocularis haplotype is identical to E2, E4, and E5 by COX1 sequencing. Abbreviation: IUPAC, International Union of Pure and Applied Chemistry.
DISCUSSION
Our patient was diagnosed with E. multilocularis based on the histopathologic, serologic, and molecular test results, and her lung lesion would categorize her as having stage IV disease [2]. It is important to note that patients with E. multilocularis may test positive for antibodies using commercially available serologic tests for E. granulosus; therefore, additional serologic or molecular testing for E. multilocularis is helpful in definitive identification.
Approximately 8 weeks following her initial abdominal imaging, our patient was started on albendazole 400 mg twice daily and had initial improvement in her liver enzymes. Three months following the initiation of her therapy, albendazole levels were 6.9 μmol/L (0.65–3.0 μmol/L) (Inselspital, Universitatsspital, Bern, Switzerland). Given that she was tolerating her therapy without any clinical or adverse laboratory reactions, she was maintained on her original regimen. She underwent resection of her pulmonary lesion, which was subsequently complicated by a chylothorax requiring multiple thoracenteses to achieve resolution. Repeat abdominal imaging 3 and 10 months following initiation of antiparasitic therapy showed no improvement in her liver mass; therefore, she was evaluated for surgical removal of her liver lesions. However, given the central location with involvement of the liver hilum, hepatic veins, and portal venous obstruction, it was the considered opinion that her lesion was not amenable to simple or ex vivo liver resection, and liver transplantation was thought to be the only viable surgical option. She currently remains stable on albendazole while awaiting liver transplantation.
Alveolar echinococcosis is a zoonosis and is acquired through contact with definitive hosts or an environment contaminated with respective parasite eggs. Although risk factors for transmission in endemic countries are not completely defined, epidemiologic studies have identified several risks including agricultural occupation; activity in gardening, forestry, or hunting; dog/cat ownership; residence in a rural landscape; and/or hunting/trapping [14]. The infection rate in humans is relatively low given the high prevalence in wild carnivores [15]. A serological survey of the South Dakota Trappers Association demonstrated that all 115 trappers tested were negative using an ELISA purified Em2 antigen despite 74% of trapped foxes with evidence of E. multilocularis [16]. Low infection rates may be due to a relatively high degree of innate resistance to infection, low exposure rates, and immunogenetic factors [15].
Although the timing and mechanism of transmission are unclear, our patient’s fox hunting without direct contact may have provided the potential for exposure to echinococcal eggs. The patient had not traveled to areas where alveolar echinococcosis is endemic; therefore, we believe this is only the second reported autochthonous case acquired in the US in the last 40 years and the first case reported from the East Coast.
Northwestern Canada, northwestern Alaska, and the north-central US are generally considered to be the endemic foci of the E. multilocularis parasite in North America among definitive and intermediate hosts (voles, lemmings, deer mice, and woodrats). Historically, 2 distinct regions were recognized and identified as the Northern Tundra Zone, consisting of the northwestern coastal regions of Alaska and of the western Canadian Arctic corresponding to the range of the Arctic fox [3]. The North Central Zone includes the Canadian provinces of Alberta, Saskatchewan, and Manitoba and the north-central US (Montana, North Dakota, South Dakota, Minnesota, Iowa, Wisconsin, Illinois, Nebraska, Michigan, Indiana, Ohio, and Missouri) [3, 17–20]. Alveolar echinococcosis has not been identified in the wildlife of the southern or eastern US, Mexico, or Central America. However, the epidemiology of E. multilocularis in the US, including prevalence and transmission dynamics, is relatively unknown [17].
Retrospective genetic analysis using the COX1 gene of the larval E. multilocularis originating from the patient identified in Minnesota in 1977 showed that this specimen was almost identical to a specimen obtained from a South Dakota fox rather than isolates contiguous to Alaska [12] and identified as the N2 Central Region haplotype [21].
European haplotypes have been isolated in wild carnivores and domestic dogs in western and central Canada [21], and a recent report identified European haplotypes of E. multilocularis in patients from Canada [22]. Expansion of the known endemic areas of canine disease may also potentially increase the risk of human infection [23]. Our sequencing of the COX1 gene in this patient is of the European haplotype and, as reflected by its close association with both European and Canadian wildlife isolates phylogenetically, is likely of similar origin as human cases recently reported in Canada [22]. The pathogenicity of the European strain is clear and given the paucity of human cases of disease in the contiguous US to date, it is presumed that the North American strain may be less so.
Echinococcus multilocularis infection has a long latency, becoming symptomatic typically years following infection. It is a severe and chronic infection that acts like a malignancy, with expectations of cure typically requiring complete resection followed by benzimidazole therapy for years. Alternatively, lifelong medical treatment is an option. Even following transplant, there is a high rate of recurrence [24]. Detailed discussion regarding treatment options and long-term management, beyond the scope of this work, can be found in reviews by Brunetti et al [2], Wen et al [4], and Kern et al [25].
Establishment of the European strain of E. multilocularis in human cases in Canada, and now in a patient from the East Coast, may represent the emergence of this disease in the US, and given the morbidity, mortality, and changing epidemiology, it is important for clinicians to consider this diagnosis in patients with tumor-like masses in the liver.
Notes
Acknowledgments. The authors thank Dr Edward T. Ryan (Department of Medicine) and Dr Henning A. Gaissert (Department of Surgery) at the Massachusetts General Hospital, Boston, Massachusetts, for their follow-up care of our patient; Yolanda Aebi from the Inselspital Universitatsspital, Bern, Switzerland, for performing the albendazole drug levels; Dr Beate Gruener of the Department of Medicine, University Hospital and Medical Center, Ulm, Germany, for her expert advice; Dr Alison Krywanczyk (Department of Pathology and Laboratory Medicine) while at the University of Vermont Medical Center, for her assistance with the initial pathologic descriptions of this case; and Dr Jeffrey Klein (Department of Radiology) from the University of Vermont Medical Center for the descriptions and notation of the abdominal and pulmonary imaging.
Financial support. This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Thompson RC. Biology and systematics of Echinococcus. Adv Parasitol 2017; 95:65–109. [DOI] [PubMed] [Google Scholar]
- 2. Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE . Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16. [DOI] [PubMed] [Google Scholar]
- 3. Deplazes P, Rinaldi L, Alvarez Rojas CA, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol 2017; 95:315–493. [DOI] [PubMed] [Google Scholar]
- 4. Wen H, Vuitton L, Tuxun T, et al. Echinococcus: advances in the 21st century. Clin Microbiol Rev 2019; 32:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson JF, Rausch RL. Alveolar hydatid disease. A review of clinical features of 33 indigenous cases of Echinococcus multilocularis infection in Alaskan Eskimos. Am J Trop Med Hyg 1980; 29:1340–55. [PubMed] [Google Scholar]
- 6. Gamble WG, Segal M, Schantz PM, Rausch RL. Alveolar hydatid disease in Minnesota. First human case acquired in the contiguous United States. JAMA 1979; 241:904–7. [DOI] [PubMed] [Google Scholar]
- 7. Deplazes P, Gottstein B. A monoclonal antibody against Echinococcus multilocularis Em2 antigen. Parasitology 1991; 103(Pt 1):41–9. [DOI] [PubMed] [Google Scholar]
- 8. Barth TF, Herrmann TS, Tappe D, et al. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis 2012; 6:e1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottstein B, Lachenmayer A, Beldi G, et al. Diagnostic and followup performance of serological tests for different forms/courses of alveolar echinococcosis. Food Waterborne Parasitology 2019; 16:e00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Müller N, Frei E, Nuñez S, Gottstein B. Improved serodiagnosis of alveolar echinococcosis of humans using an in vitro-produced Echinococcus multilocularis antigen. Parasitology 2007; 134:879–88. [DOI] [PubMed] [Google Scholar]
- 11. Poon RWS, Tam EWT, Lau SKP, et al. Molecular identification of cestodes and nematodes by COX1 gene real-time PCR and sequencing. Diagn Microbiol Infect Dis 2017; 89:185–90. [DOI] [PubMed] [Google Scholar]
- 12. Yamasaki H, Nakao M, Nakaya K, Schantz PM, Ito A. Genetic analysis of Echinococcus multilocularis originating from a patient with alveolar echinococcosis occurring in Minnesota in 1977. Am J Trop Med Hyg 2008; 79:245–7. [PubMed] [Google Scholar]
- 13. Nakao M, Xiao N, Okamoto M, Yanagida T, Sako Y, Ito A. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int 2009; 58:384–9. [DOI] [PubMed] [Google Scholar]
- 14. Craig P. Echinococcus multilocularis. Curr Opin Infect Dis 2003; 16:437–44. [DOI] [PubMed] [Google Scholar]
- 15. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004; 17:107–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hildreth MB, Sriram S, Gottstein B, Wilson M, Schantz PM. Failure to identify alveolar echinococcosis in trappers from South Dakota in spite of high prevalence of Echinococcus multilocularis in wild canids. J Parasitol 2000; 86:75–7. [DOI] [PubMed] [Google Scholar]
- 17. Cerda JR, Buttke DE, Ballweber LR. Echinococcus spp. tapeworms in North America. Emerg Infect Dis 2018; 24:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Massolo A, Liccioli S, Budke C, Klein C. Echinococcus multilocularis in North America: the great unknown. Parasite 2014; 21:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ballard NB. Echinococcus multilocularis in Wisconsin. J Parasitol 1984; 70:844. [PubMed] [Google Scholar]
- 20. Ballard NB, Vande Vusse FJ. Echinococcus multilocularis in Illinois and Nebraska. J Parasitol 1983; 69:790–1. [PubMed] [Google Scholar]
- 21. Klein C, Massolo A. Demonstration that a case of human alveolar echinococcosis in Minnesota in 1977 was caused by the N2 strain. Am J Trop Med Hyg 2015; 92:477–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Massolo A, Klein C, Kowalewska-Grochowska K, et al. European Echinococcus multilocularis identified in patients in Canada. N Engl J Med 2019; 381:384–5. [DOI] [PubMed] [Google Scholar]
- 23. Trotz-Williams LA, Mercer NJ, Walters JM, et al. Public health follow-up of suspected exposure to Echinococcus multilocularis in southwestern Ontario. Zoonoses Public Health 2017; 64:460–7. [DOI] [PubMed] [Google Scholar]
- 24. Koch S, Bresson-Hadni S, Miguet JP, et al. European Collaborating Clinicians . Experience of liver transplantation for incurable alveolar echinococcosis: a 45-case European collaborative report. Transplantation 2003; 75:856–63. [DOI] [PubMed] [Google Scholar]
- 25. Kern P, Menezes da Silva A, Akhan O, et al. The echinococcoses: diagnosis, clinical management and burden of disease. Adv Parasitol 2017; 96:259–369. [DOI] [PubMed] [Google Scholar]