Abstract
Background
Data on safety and efficacy of second-line tuberculosis drugs in pregnant women and their infants are severely limited due to exclusion from clinical trials and expanded access programs.
Methods
Pregnant women starting treatment for multidrug/rifampicin-resistant (MDR/RR)-tuberculosis at King Dinuzulu Hospital in KwaZulu-Natal, South Africa, from 1 January 2013 to 31 December 2017, were included. We conducted a record review to describe maternal treatment and pregnancy outcomes, and a clinical assessment to describe infant outcomes.
Results
Of 108 pregnant women treated for MDR/RR-tuberculosis, 88 (81%) were living with human immunodeficiency virus.. Favorable MDR/RR-tuberculosis treatment outcomes were reported in 72 (67%) women. Ninety-nine (91%) of the 109 babies were born alive, but overall, 52 (48%) women had unfavorable pregnancy outcomes. Fifty-eight (54%) women received bedaquiline, and 49 (45%) babies were exposed to bedaquiline in utero. Low birth weight was reported in more babies exposed to bedaquiline compared to babies not exposed (45% vs 26%; P = .034). In multivariate analyses, bedaquiline and levofloxacin, drugs often used in combination, were both independently associated with increased risk of low birth weight. Of the 86 children evaluated at 12 months, 72 (84%) had favorable outcomes; 88% of babies exposed to bedaquiline were thriving and developing normally compared to 82% of the babies not exposed.
Conclusions
MDR/RR-tuberculosis treatment outcomes among pregnant women were comparable to nonpregnant women. Although more babies exposed to bedaquiline were of low birth weight, over 80% had gained weight and were developing normally at 1 year.
Keywords: tuberculosis, pregnancy, drug-resistant, outcome, treatment
Pregnant women with Multidrug/Rifampicin-resistant-tuberculosis can be effectively and safely treated. We observed a relationship between the use of bedaquiline and low birth weight, but the clinical importance of this finding is unclear.
(See the Editorial Commentary by Marais on pages 1169–70.)
Over a half million individuals were estimated to have multidrug/rifampicin-resistant (MDR/RR) tuberculosis globally in 2017, with the highest burden among women of reproductive age (15–45 years) [1]. In women of this age, tuberculosis is the leading cause of death from an infectious agent and a common nonobstetric cause of maternal mortality [1–4]. Worldwide in 2011, an estimated 216 500 pregnant women developed tuberculosis, with the greatest burden in Africa [5]. Not only do biological changes in pregnancy double the risk of pregnant women developing tuberculosis compared to nonpregnant women, but pregnancy complicates the treatment of tuberculosis, and if untreated, pregnancy-associated tuberculosis can be associated with mortality of up to 40% [2]. In women living with tuberculosis and human immunodeficiency virus (HIV), the risk of maternal mortality increases dramatically; neonates born to these women also have increased morbidity and mortality [2, 6].
As the safety of first-line tuberculosis drugs in pregnancy have been established and treatment has been shown to improve maternal and neonatal outcome, the World Health Organization (WHO) recommends that pregnant women with drug-susceptible tuberculosis should be treated in the same way as nonpregnant women [7–9]. The management of MDR/RR-tuberculosis in pregnant women is more complicated as little is known about the use of second-line tuberculosis drugs in pregnancy [10]. Presently, WHO recommends that pregnant women be offered individualized regimens, aiming to include ≥4 effective drugs with an established safety profile and low teratogenic risk [7]. However, as pregnant women have generally been excluded from the studies on which WHO recommendations were based, safety data of most second-line tuberculosis drugs in pregnancy are lacking [7, 10], with fewer than 15 case reports describing the safety and outcomes of second-line tuberculosis treatment during pregnancy. The largest report included 38 women [11]. The tuberculosis treatment landscape has changed with programmatic introduction of 2 novel drugs, delamanid and bedaquiline, and repurposing of older drugs, including linezolid and clofazimine. None of the studies evaluating these drugs have included pregnant women, and currently, due to a paucity of data, the use of new and repurposed drugs during pregnancy and breastfeeding is not widely recommended [7, 12–14].
In South Africa, where the rate of tuberculosis is estimated at 10.3 per 1000 pregnancies [5], pregnant women with MDR/RR-tuberculosis have had access to bedaquiline since 2015. Given the improved treatment success rates among MDR/RR-tuberculosis patients treated with bedaquiline [15], the drug became increasingly used as a substitute for injectable agents. In September 2018, the national Department of Health recommended an injectable-free, modified 9–12-month regimen for all patients diagnosed with MDR/RR-tuberculosis, including pregnant women. We report treatment, pregnancy, and infant outcomes in a cohort of pregnant women with MDR/RR-tuberculosis in KwaZulu-Natal, South Africa, with a focus on women and infants exposed to bedaquiline during pregnancy.
METHODOLOGY
Study Design and Patient Population
In this observational cohort study, medical records of women attending King Dinuzulu Hospital (KDH) for MDR/RR-tuberculosis management between 1 January 2013 and 31 December 2017 were reviewed. KDH is the specialist referral hospital for RR-tuberculosis in KwaZulu-Natal province where all pregnant women with MDR/RR-tuberculosis are referred.
Definitions of Presenting Characteristics
Pulmonary tuberculosis was defined as disease affecting the lungs only. Cavitary/bilateral disease was defined as the presence of either cavities or disease in both left and right lung fields and was considered an indication of extensive disease. Patients on antiretroviral therapy (ART) before MDR/RR-tuberculosis treatment started were defined as those receiving ART for at least 30 days prior to MDR/RR-tuberculosis treatment initiation. Baseline CD4 count was defined as the date recorded in the clinical notes closest to the date of MDR/RR-tuberculosis treatment initiation. Gestational age was recorded from clinical notes; determined by ultrasound, dates, or both.
Drug Resistance Definitions
MDR/RR-tuberculosis was classified as tuberculosis caused by Mycobacterium tuberculosis with genotypic or phenotypic resistance to rifampicin. It included MDR-tuberculosis (resistance to both isoniazid and rifampicin), rifampicin monoresistant tuberculosis (susceptibility to isoniazid), and forms of disease where rifampicin resistance has been identified, but no result for isoniazid has been returned (increasingly common when testing with the GeneXpert MTB/RIF molecular assay). Extensively drug-resistant (XDR) tuberculosis was classified as MDR-tuberculosis with additional resistance to a fluoroquinolone and a second-line injectable agent. Pre-XDR-tuberculosis is MDR-tuberculosis with additional resistance to either a fluoroquinolone or an injectable agent but not both.
Diagnosis and Treatment
Drug susceptibility testing (DST) was performed on all sputum cultures positive for M. tuberculosis. From 2013 to 2015 DST included isoniazid, rifampicin, streptomycin, kanamycin, and ofloxacin. From 2015 to 2018 moxifloxacin and capreomycin were added. Given the complexity of treating pregnant women with MDR/RR-tuberculosis, most women were initially hospitalized and received individualized treatment. Women who had started a standardized regimen for MDR/RR-tuberculosis prior to becoming pregnant may have subsequently had treatment modified, depending on the stage of treatment. Between 2013 and 2015, national MDR/RR-tuberculosis treatment guidelines recommended a standardized 18–24 month regimen, unless resistance or intolerance to specific drugs required regimen modification, as per WHO 2011 treatment guidelines [16, 17]. The injectable phase of treatment (6–8 months) included kanamycin, moxifloxacin, ethionamide, terizidone, and pyrazinamide, followed by the continuation phase (12–18 months) comprising moxifloxacin, ethionamide, terizidone, and pyrazinamide [17]. In some cases ethambutol and higher doses of isoniazid were added. Standard treatment changed in early 2017 when the WHO 9–12 month regimen was introduced across South Africa, and, like other adults with MDR/RR-tuberculosis, pregnant women benefitted from the shorter regimen [7]. However, kanamycin/amikacin and ethionamide are contraindicated in pregnancy. In most pregnant women receiving MDR/RR-tuberculosis treatment, these drugs were either omitted, stopped, or substituted with alternative agents, such as capreomycin or para-aminosalicylic acid prior to 2015, or linezolid or bedaquiline as access to these newer agents increased [18]. In line with local guidelines, specific drugs in the treatment regimens were stopped or substituted if drug resistance patterns changed, toxicity developed, or drug: drug interactions occurred.
During the study period it was standard of care for pregnant women living with HIV to receive antiretroviral therapy (ART). Patients who were ART-naive started on ART after 2 weeks of MDR/RR-TB treatment During the study period first-line ART comprised tenofovir, emtricitabine, and efavirenz. ART regimen changes were not comprehensively documented, but pregnant women receiving efavirenz switched to nevirapine prior to bedaquiline initiation.
Outcome Definitions
We defined 3 outcomes: maternal treatment outcomes, pregnancy outcomes, and infant outcomes. Each were considered favorable or unfavorable and are defined in Table 1.
Table 1.
Definition of Terms
| Treatment Outcomes [19, 20] | |
|---|---|
| Favorable treatment outcomes | |
| Cured | Treatment completed as recommended by the national policy without evidence of failure, AND ≥3 consecutive cultures taken at least 30 days apart are negative after the intensive phase. |
| Treatment completed | Treatment completed as recommended by the national policy without evidence of failure, BUT no record that ≥3 consecutive cultures taken at least 30 days apart are negative after the intensive phase. |
| Treatment success | The sum of cured and treatment completed. |
| Unfavorable treatment outcomes | |
| Treatment failed | Treatment terminated or need for permanent regimen change of at least 2 antituberculosis drugs because of a lack of culture conversion by the end of the intensive phase; bacteriological culture reversion in the continuation phase after conversion to negative; evidence of additional acquired resistance to fluoroquinolones or second-line injectable drugs; ADRs. |
| Died | A patient who dies for any reason during treatment. |
| Lost to follow-up | A patient whose treatment was interrupted for ≥2 consecutive months. |
| Treatment unsuccessful | The sum of failed, died, lost to follow-up, and not evaluated. |
| Pregnancy outcomes | |
| Favorable pregnancy outcomes—all the below features are required to classify a pregnancy outcome as favorable | |
| Full term | Babies born ≥37 weeks of pregnancy. |
| Normal birth weight | Birth weight of ≥2500 grams according to the World Health Organization (WHO) [21]. |
| Alive | A baby born alive who lives for >28 days. |
| Unfavorable pregnancy outcomes—any of the following classify a pregnancy as having an unfavourable outcome | |
| Preterm birth | Babies born <37 weeks of pregnancy. |
| Miscarriage | Spontaneous loss of a pregnancy before the fetus has reached viability at 24 weeks. This includes all pregnancy losses from the time of conception until 23 completed weeks of gestation [22]. |
| Stillbirth | In South Africa, the legal definition of stillbirth is an infant born dead after “6 months of intrauterine life” (ie, 28 weeks since the start of the last period or 26 weeks since conception). If the gestational age is not known, a weight of 1000 g is used to legally define a stillbirth. Infants that are born dead before this time are legally regarded as miscarriages. |
| Termination of pregnancy | Termination of pregnancy is when a woman decides to end her pregnancy before the full term by medical means. The woman must be <13 weeks pregnant to end the pregnancy without giving reasons. If she is between 13 and 20 weeks pregnant, the pregnancy may be terminated only under specific conditions. If she is >20 weeks pregnant, it will be done only if her life or the fetus’ life is in danger, or there are likely to be serious birth defects [23]. |
| Low birth weight | A birth weight of <2500 g (up to and including 2499 g), as per the World Health Organization (WHO) [21]. |
| Infant outcomes | |
| Development | Child development refers to how a child becomes able to do more complex things as they get older. Developmental milestones are a set of functional skills or age-specific tasks that most children can do at a certain age range [24]. These skills include gross and fine motor, language, cognitive, and social skills. |
| Lost to follow-up | It was not possible to verify the status of the child at 12 months. |
| Favorable infant outcomes—both of the following are required to state that an infant has a favorable outcome | |
| Thrive normally | If a child gains weight following the normal trajectory according to the growth chart, the child is said to be thriving normally. |
| Normal development | A child is described as having normal development if they achieve the developmental milestones timeously. |
| Unfavorable infant outcomes—any of the following classify the child as having an unfavorable outcome | |
| Failure to thrive | The infant fails to maintain an established pattern of growth [25]. |
| Delayed development | The child reaches developmental milestones later than the average child. |
| TB diagnosis | Diagnosed with TB or RR-TB before 12 months of age. |
| Neonatal death | Death of a live born infant in the first 28 days of life. An early neonatal death is a death that occurs in the first week of life [26]. |
| Infant death | Infant dies before 12 months. |
Abbreviations: ADR, adverse drug reactions; RR-TB, rifampicin-resistant TB; TB, tuberculosis.
Data Variables and Collection
We reviewed maternal medical records to collect demographic, clinical, and laboratory data. Response to treatment was determined from medical records and the laboratory database. Pregnancy outcomes, birth weight, APGAR scores, and postdelivery screening were extracted from patient-held notes and road-to-health cards. Infants were seen at KDH 6–8 weeks after delivery and assessed clinically to determine if they were thriving and screened for symptoms and signs of tuberculosis disease. Infants were assessed again at 6 and 12 months. If any problems were detected, the child was referred to routine care for further evaluation and investigation.
Data Management and Statistical Analyses
Data were captured using an Excel spreadsheet and imported into STATA/SE version 15.0. for analysis. Baseline clinical characteristics and maternal treatment, pregnancy, and infant outcomes were presented using descriptive statistics. Missing data were noted, and each analysis reflects the sample size used. All clinical characteristics, individual tuberculosis drugs, and ART drugs were assessed as potential predictors of outcome using univariable logistic regression. Any drug use during the MDR/RR-tuberculosis treatment episode was included in evaluation of treatment outcome, but only drug use ≥14 days during pregnancy was included for the evaluations of pregnancy and infant outcomes, reflecting in utero exposure. Clinical characteristics, other tuberculosis drugs, ART drugs, and all 3 outcomes were compared between mothers receiving regimens with bedaquiline and without bedaquiline using the χ 2 or Fisher exact test for categorical variables, and the t test or Wilcoxon rank sum test for continuous variables. After observing an effect of bedaquiline on birth weight, further multivariable analysis was conducted. Due to the collinearity between individual tuberculosis drugs, separate multivariable regression models were fitted for each tuberculosis drug noted to have a significant association with birth weight. All clinical characteristics were considered as potential explanatory variables and confounders and included in the final models if their inclusion resulted in a ≥10% change in the model coefficient associated with the tuberculosis drug of interest in bivariate analyses. Multiple imputation was used to conduct sensitivity analysis for the effect of bedaquiline on infant outcomes for all live births. The mother’s age, drug resistance category, body mass index, HIV status, and the infants’ exposure to bedaquiline in utero were used in the imputation model for infant outcome.
Ethics
This study was approved by the South African Medical Research Council (SAMRC) Ethics Review Committee (EC017-6/2016) and the KwaZulu-Natal Health Research Committee.
RESULTS
Presenting Characteristics
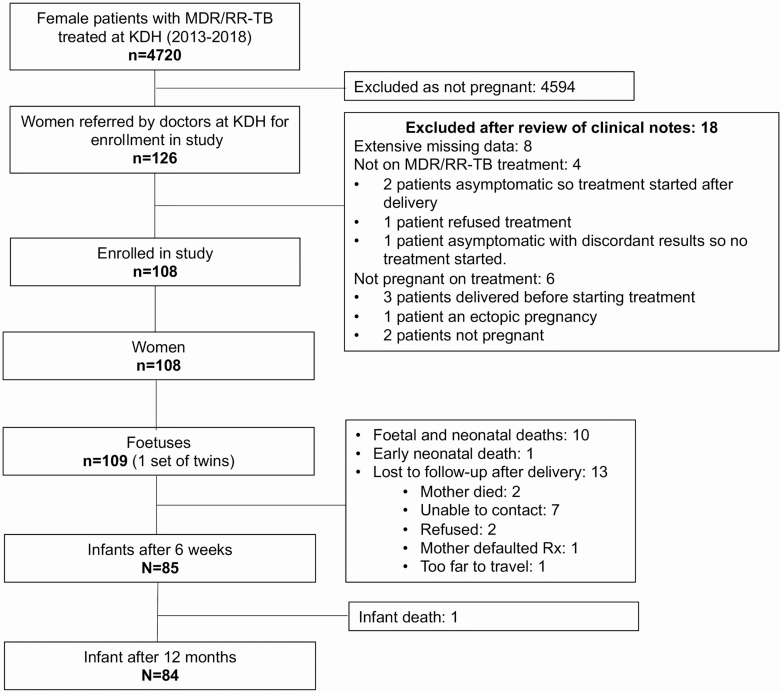
Between 1 January 2013 and 30 December 2017, 10 042 patients were treated for MDR/RR-tuberculosis at KDH hospital; 4720 (47%) of these were women, and 126 were reported to have been pregnant; 108 women exposed to second-line tuberculosis treatment for at least 2 weeks while pregnant were included in the study. Figure 1 details study enrollment and attrition during the study. Of the 108 women in the study cohort, 20 (18%) started MDR/RR-TB treatment prior to becoming pregnant, 19 (18%) during the first trimester and 42 (39%) and 28 (26%) in the second and third trimesters, respectively. This is represented in Supplementary Figure 1 in which women are divided into 2 groups: those starting MDR/RR-TB treatment before pregnancy and in the first trimester and those starting treatment after the first trimester.
Figure 1.
Schema of enrollment and attrition (1 January 2013–31 December 2018). Abbreviations: KDH, King Dinuzulu Hospital; MDR/RR-TB, multidrug/rifampicin-resistant tuberculosis.
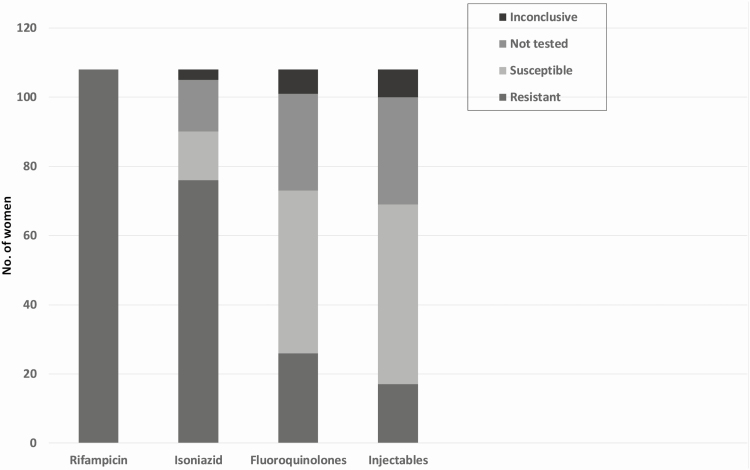
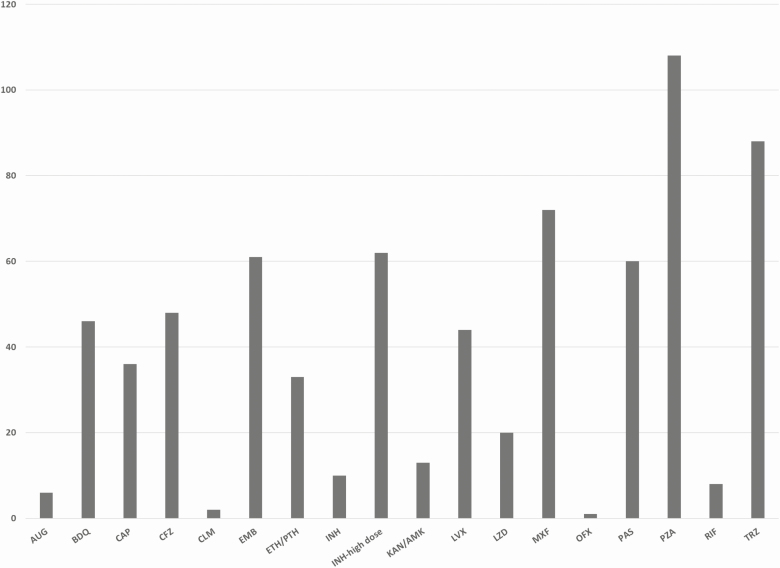
Baseline characteristics of women in the study are shown in Table 2. The frequency of tuberculosis resistance to individual drugs is shown in Figure 2. Only 76 (70%) women had tuberculosis samples tested for fluoroquinolone resistance. Figure 3 demonstrates the second-line tuberculosis drugs to which fetuses were exposed in utero for at least 14 days.
Table 2.
Baseline Clinical Characteristics of Pregnant Women With Multidrug/Rifampicin-Resistant Tuberculosis (n = 108)
| Clinical Characteristics | No. (%) |
|---|---|
| Age: years, mean; SD | 28.0; 6.13 |
| Hb, g/dl: mean; SD (n = 102) | 10.4; 1.59 |
| BMI, kg/m2: mean; SD (n = 106) | 24.0; 4.85 |
| TB characteristics | |
| Culture positive at treatment initiation | 73 (68%) |
| Previous TB or MDR/RR-TB | 38/82 (46%) |
| Site of TB: Pulmonary | 108 (100%) |
| Extensive disease pattern on chest radiograph | 45/97 (46%) |
| Resistance pattern | |
| RR-TB/Rif-mono/MDR-TB | 83 (77%) |
| Pre-XDR-TB/XDR-TB | 25 (23%) |
| HIV-characteristics | |
| HIV-positive | 88 (81%) |
| HIV-positive patients on ART before MDR/RR-TB treatment started (n = 88): | 74 (83%) |
| Baseline CD4 count, median cells/mm3 [IQR] (n = 88) | 353 [165–511] |
| Pregnancy characteristics | |
| Pregnant before MDR/RR-TB treatment started | 89 (82%) |
| Gestational age at treatment start: weeks, median [IQR] | 22 [14–28] |
| Foetal exposure to any second-line drugs: Days, median [IQR] | 118 [70–208] |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; Hb, hemoglobin; HIV, human immunodeficiency virus; IQR, interquartile range; MDR/RR-TB, multidrug/rifampicin-resistant TB; Pre-XDR-TB, pre-extensively drug-resistant TB; Rif-mono, rifampicin monoresistant; RR-TB, rifampicin-resistant TB; SD, standard deviation; TB, tuberculosis; XDR-TB, extensively drug-resistant TB.
aExtensive disease was classified as bilateral disease and/or cavities on chest radiograph.
Figure 2.
Mycobacterial drug susceptibility test pattern for pregnant women treated for multidrug/rifampicin-resistant tuberculosis. Fluoroquinolones: Resistance to any fluoroquinolone. (Some isolates were tested for resistance to levofloxacin, some to moxifloxacin, and in some, a genotypic result was provided that stated fluoroquinolone resistance without specifying individual drug). Injectables: Resistance to any second-line injectable drug. (Some isolates were tested for resistance to amikacin, some to kanamycin, some to capreomycin, and in some, a genotypic result was provided that stated injectable resistance without specifying individual drug). During the study period, no drug susceptibility testing was done for ethambutol, ethionamide, pyrazinamide, para-aminosalicylic acid, or terizidone.
Figure 3.
Individual drugs to which fetuses were exposed in utero. Abbreviations: AMK, amikacin; AUG, augmentin; BDQ, bedaquiline; CAP, capreomycin; CFZ, clofazimine; CLM, clarithromycin; EMB, ethambutol; ETH, ethionamide; INH, isoniazid; KAN, kanamycin; LVX, levofloxacin; LZD, linezolid; MXF, moxifloxacin; OFX, ofloxacin; PAS, para-aminosalicylic acid; PTH, prothionamide; PZA, pyrazinamide; RIF, rifampin; TRD, terizidone.
RR-Tuberculosis Treatment Outcomes
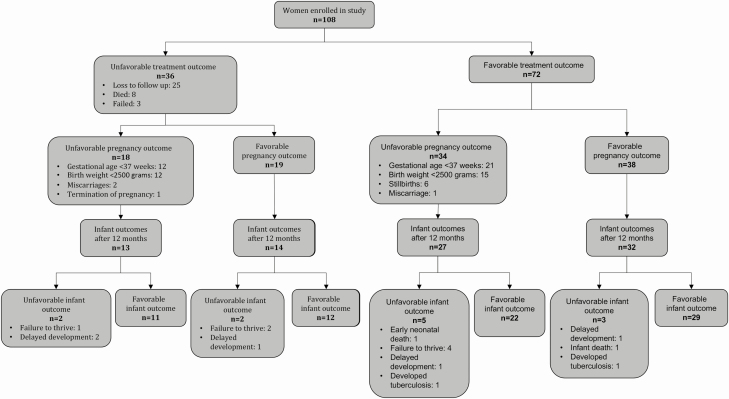
Favorable treatment outcomes were reported in 72 (67%) women (Table 3; Figure 4). Multivariate analyses identified lower maternal hemoglobin at baseline as a predictor of an unfavorable maternal treatment outcome (unadjusted hazard ratio [uHR] 0.67, P = .006; see Supplementary Table 1). Eight women died a median of 67 days (interquartile range [IQR]: 32–299) after childbirth, but all 8 infants survived. Four maternal deaths were related to tuberculosis disease: 1 woman with XDR-tuberculosis, and 1 with pre-XDR-tuberculosis succumbed despite good adherence to treatment, but 2 women (1 with XDR-tuberculosis and 1 with MDR/RR-tuberculosis) had been repeatedly interrupting treatment. Two maternal deaths were related to childbirth and occurred 2 days and 5 days after delivery. One woman died from a cerebrovascular accident a year after completion of MDR/RR-tuberculosis therapy when her child was 5 months old. The cause of the remaining death was unknown; the woman died in a rural district hospital 3 months after the birth of her child. No maternal deaths were considered related to tuberculosis medication.
Table 3.
Maternal Treatment, Pregnancy, and Infant Outcomes
| Study Outcomes | No. (%) |
|---|---|
| Maternal MDR/RR-tuberculosis treatment outcomes (n = 108) | |
| Favorable treatment outcomes | 72 (67%) |
| Cured | 58 (54%) |
| Treatment completion | 14 (13%) |
| Unfavorable treatment outcomes | 36 (33%) |
| Died | 8 (7%) |
| Treatment failed | 3 (3%) |
| Lost to follow-up | 25 (23%) |
| Pregnancy outcomes (n = 108 women pregnant with n = 109 fetuses, including a set of twins) | |
| Newborn characteristics | |
| Live births | 99 (91%) |
| Gestational age at delivery: weeks, mean; SDa | 37.76; SD 3.10 |
| Birth weight, grams, median [IQR]b | 2800 [2430–3200] |
| Fetal and neonatal deaths | 10 (9%) |
| Stillbirth | 6 (6%) |
| Miscarriagec | 3 (3%) |
| Termination of pregnancy | 1 (1%) |
| Favorable pregnancy outcomes (out of 109 fetuses) | 57 (52%) |
| ≥37 weeksa | 71 (72%) |
| Birthweight ≥2500 gb | 61 (65%) |
| Unfavorable pregnancy outcomes (out of 109 fetuses) | 52 (48%) |
| Fetal and neonatal deaths | 10 (9%) |
| Preterm <37 weeksa | 28 (28%) |
| Low birth weight <2500 gb | 33 (35%) |
| Infant outcomes (n = 109) | |
| No infant outcomes at 12 months (n = 23) | |
| Fetal and neonatal deaths | 10 (9%) |
| Lost to follow-up after birth | 13 (12%) |
| Infant outcomes at 12 months (n = 86) | |
| Favorable infant outcomes | 72 (84%) |
| Thriving normally | 73 (85%) |
| Normal development | 77 (89%) |
| Unfavorable infant outcomes | 14 (16%) |
| Failure to thrive | 9 (10%) |
| Delayed development | 5 (6%) |
| Early neonatal death | 1 (1%) |
| Infant death | 1 (1%) |
| Diagnosed with tuberculosis disease in the 1st year of life | 2 (2%) |
Abbreviations: IQR, interquartile range; MDR/RR, multidrug/rifampicin-resistant.
aLive births only (n = 99).
bLive births only and missing data for 5 neonates (n = 94).
cOne miscarriage was a set of twins.
Figure 4.
Primary outcomes.
Pregnancy Outcomes
Ninety-nine (91%) of the 109 fetuses, including a set of twins, were born alive, with a mean gestational age of 38 weeks (standard deviation [SD] 3.10) and median birth weight of 2800 grams (IQR 2430–3200) (Table 3). Only 57 (52%) pregnancies had a favorable pregnancy outcome when applying our study criteria (Table 1). Women living with HIV had a higher risk of an unfavorable pregnancy outcome (uHR 3.35; P = .030). Four infants born alive had congenital anomalies; an umbilical hernia, a ventral septal defect, kyphoscoliosis, and 1 infant had Ehlers-Danlos syndrome. Four of the 109 fetuses were lost early in pregnancy, and of the 6 stillborn babies, 5 were delivered at a gestational age <37 weeks. In 9 of the 10 fetal deaths the mother was living with HIV. All HIV-exposed babies were given nevirapine at birth for 6 weeks, and all tested HIV-negative at 6 weeks.
Infant Outcomes
We report infant outcomes after 12 months for 86 of the 99 live infants (Table 3). In addition to the 10 pregnancies that did not result in a live birth, we were unable to follow-up 13 of the infants after birth (Figure 1) Favorable infant outcomes were documented in 72 (84%) of the liveborn infants (Table 3). No baseline maternal characteristics, tuberculosis drugs, or ART were associated with unfavorable infant outcomes, even after reanalysis using multiple imputation for the 13 missing infant outcomes. There was 1 early neonatal death, 7 days after birth, and 1 infant death at 3 months. In their first year of life, 11 infants developed signs and symptoms of tuberculosis disease:” weight loss, cough, or infiltrates on chest radiograph. Two of these 11 infants were diagnosed and treated for MDR/RR-tuberculosis. Four had microbiological investigations, which were culture-negative for M. tuberculosis. The remaining 5 infants were never treated for tuberculosis and at a subsequent visit were well.
Bedaquiline
As bedaquiline is central to most novel treatment regimens being studied in clinical trials, we evaluated the impact of bedaquiline on all outcomes. No significant differences in baseline characteristics were identified between women treated with bedaquiline, compared to those who were not treated with bedaquiline (Table 4). Table 5 shows study outcomes stratified by bedaquiline exposure. Of the 58 women who received bedaquiline, 41 (71%) had a favorable treatment outcome, compared to 31 (62%) of those who received treatment without bedaquiline (P = .349). There was no difference in pregnancy outcomes between women whose fetuses were exposed to bedaquiline in utero (49% favorable pregnancy outcome) compared to those unexposed (57% favorable outcome, P = .312). However, a higher proportion of newborns exposed to bedaquiline in utero had a birth weight <2500 grams (45% vs 24%; P = .034; Table 5). Of infants exposed to bedaquiline, 36 (88%) had a favorable infant outcome, compared to 36 (80%) infants not exposed (P = .136). This result remained after multiple imputation for the 13 missing infant outcomes (P = .160).
Table 4.
Baseline Clinical Characteristics of Pregnant Women With Multidrug/Rifampicin-Resistant Tuberculosis, Stratified by Bedaquiline Exposure
| Clinical Characteristics | Bedaquiline in Regimen n = 58 No. (%) | No Bedaquiline in Regimen n = 50 No. (%) | P Value |
|---|---|---|---|
| Age: years, mean; SD | 28.7; 6.08 | 27.0; 6.01 | .150 |
| Hb, g/dl: mean; SD | 10.4; 1.55 | N = 46; 10.4; 1.65 | .928 |
| Body Mass Index (BMI): kg/m2 mean; SD | 23.7; 4.70 | N = 48; 24.2; 5.08 | .543 |
| TB characteristics | |||
| Culture positive at treatment initiation | 42 (72%) | N = 31; (63%) | .405 |
| Previous tuberculosis or MDR/ RR-tuberculosis | N = 35; 19 (54%) | N = 47; 19 (40%) | .265 |
| Site of tuberculosis: pulmonary | 57 (100%) | 51 (100%) | NA |
| Chest radiograph | .923 | ||
| Extensive disease | N = 51; 23 (45%) | N = 46; 22 (48%) | |
| Resistance pattern: no (%) | 1.000 | ||
| RR-/Rif-mono/MDR-tuberculosis | 45 (78%) | 38 (76%) | |
| Pre-XDR-/XDR-tuberculosis | 13 (22%) | 12 (24%) | |
| HIV characteristics | |||
| HIV-positive, no. (%) | 48 (83%) | 40 (80%) | .806 |
| HIV-positive patients on ART before MDR/RR-tuberculosis treatment started | N = 48; 37 (77%) | N = 40; 37 (90%) | .155 |
| Baseline CD4 count, median cells/mm3 [IQR] | N = 45; 335 [138–500] | N = 36; 395 [219–540] | .352 |
| Pregnancy characteristics | |||
| Pregnant before MDR/RR-tuberculosis treatment started | 47 (81%) | 42 (84%) | .802 |
| Gestational age at treatment start: weeks, median [IQR] | 23 [13–28] | 20.5 [15–28] | .905 |
| Foetal exposure to any second-line drugs: days, median [IQR] | 110 [66–203] | 141 [70–213] | .562 |
| Fetal exposure to bedaquiline: days, median [IQR] | 77 [28–140] | NA | NA |
Abbreviations: ART, antiretroviral therapy; Hb, hemoglobin; HIV, human immunodeficiency virus; IQR, interquartile range; MDR/RR-TB, multidrug/rifampicin-resistant TB; NA, not applicable; Pre-XDR, pre-extensively drug-resistant; Rif-mono, rifampicin monoresistant; SD, standard deviation; TB, tuberculosis; XDR, extensively drug-resistant.
aExtensive disease was classified as bilateral disease and/or cavities on chest radiograph.
Table 5.
Maternal Treatment, Pregnancy, and Infant Outcomes Stratified by Bedaquiline Exposure
| Maternal RR-TB Treatment Outcomes | Bedaquiline in Regimen n = 58 No. (%) | No Bedaquiline in Regimen n = 50 No. (%) | P Value |
|---|---|---|---|
| Maternal MDR/RR-TB treatment outcomes | .349 | ||
| Favorable treatment outcomes | 41 (71%) | 31 (62%) | |
| Cured | 34 (59%) | 24 (48%) | |
| Treatment completion | 7 (12%) | 7 (14%) | |
| Unfavorable treatment outcomes | 17 (29%) | 19 (38%) | |
| Died | 4 (7%) | 4 (8%) | |
| Treatment failed | 2 (4%) | 1 (2%) | |
| Loss to follow-up | 11 (19%) | 14 (28%) | |
| Pregnancy outcomes | Bedaquiline exposure in utero n = 49 | No bedaquiline exposure in utero n = 60 | |
| Newborn characteristics | |||
| Birth outcomes | .741 | ||
| Live births | 45 (92%) | 54 (90%) | |
| Gestational age at delivery: weeks, mean; SDa | 37·68; SD 2·93 | 37·82; SD 3·25 | .830 |
| Birth weight, grams, median [IQR]b | 2690 [2380–3095] | 2900 [2550–3270] | .179 |
| Fetal and neonatal deaths | 4 (8%) | 6 (10%) | |
| Stillbirth | 3 (5%) | 3 (6%) | |
| Miscarriage | 0 | 3 (6%)c | |
| Termination of pregnancy | 1 (2%) | 0 | |
| Pregnancy outcomes | .312 | ||
| Favorable pregnancy outcomes (out of 109 fetuses) | 24 (49%) | 34 (57%) | |
| ≥37 weeksa | 32 (71%) | 39 (72%) | |
| Birth weight ≥2500 gb | 24 (55%) | 37 (74%) | |
| Unfavourable pregnancy outcomes (out of 109 fetuses) | 25 (51%) | 26 (43%) | |
| Fetal and neonatal deaths | 4 (8%) | 6 (10%) | |
| Preterm <37 weeksa | 13 (29%) | 15 (28%) | .903 |
| Low birth weight <2500 gb | 20 (45%) | 13 (26%) | .034 |
| Infant outcomes | Bedaquiline exposure in utero n = 49 | No bedaquiline exposure in utero n = 60 | |
| No infant outcomes at 12 months | 8 (16%) | 15 (25%) | |
| Fetal and neonatal deaths | 4 (8%) | 6 (10%) | |
| Lost to follow-up after birth | 4 (8%) | 9 (15%) | |
| Infant outcomes | n = 41 | n = 45 | .136 |
| Favorable infant outcomes at 12 months | 36 (88%) | 36 (80%) | |
| Thriving normally | 36 (88%) | 37 (82%) | .914 |
| Normal development | 38 (93%) | 39 (86%) | .705 |
| Unfavorable infant outcomes at 12 months | 5 (12%) | 9 (20%) | |
| Failure to thrive | 4 (10%) | 5 (11%) | |
| Delayed development | 2 (5%) | 3 (7%) | |
| Early neonatal death | 0 | 1 (2%) | |
| Infant death | 1 (2%) | 0 | |
| Developed TB in the 1st year of life | 0 | 2 (4%) | .186 |
Abbreviations: IQR, interquartile range; MDR/RR-TB, multidrug/rifampicin-resistant TB; SD, standard deviation; TB, tuberculosis.
aLive births only (n = 99).
bLive births only and missing data for 5 neonates (n = 94).
cOne miscarriage was a set of twins.
We evaluated whether exposure to bedaquiline was associated with exposure to any other tuberculosis drug or ART. In Supplementary Table 2 we describe the use of all tuberculosis and ART drugs in pregnancy, and in Supplementary Table 3 we explore the relationship between in utero exposure to bedaquiline and other drugs. We observed a relationship between bedaquiline use and more frequent use of clofazimine, levofloxacin, and linezolid. We evaluated risk factors for low birth weight among women and newborns. In univariate analysis no baseline maternal characteristics were associated with low birth weight (Supplementary Table 4). Exposure to bedaquiline, clofazimine, and levofloxacin in utero were all associated with an increased risk of low birth weight. In a multivariate model, bedaquiline and levofloxacin remained significant predictors of low birth weight.
DISCUSSION
In this cohort of pregnant women treated for MDR/RR-tuberculosis in KwaZulu-Natal, South Africa, favorable treatment outcomes were reported for two thirds of the women. Twenty-three percent of the women in our cohort had MDR/RR-tuberculosis with known resistance to the fluoroquinolones, injectables or both, and a large, additional proportion had RR-tuberculosis in which the isolate was not tested for susceptibility to these second-line drugs. The favorable treatment outcomes we report are better than the 55–60% reported for nonpregnant women in our setting [27, 28], and the 61% in the cohort of 38 pregnant women treated for MDR/RR-tuberculosis in Peru between 1996 and 2005 [11]. Notably, only 8% of the Peruvian cohort were living with HIV, and rates of second-line drug resistance were low.
Favorable pregnancy outcomes were reported in 52% of the cohort with 28% of the live births born preterm and 35% with documented measurements having low birth weight. These proportions are higher than the preterm delivery rate of 12% and low birth weight rate of 15% reported for South Africa [29, 30], and this is likely to, at least in some part, be due to the high HIV prevalence with associated ART use in our study. There is growing evidence of adverse pregnancy outcomes, including preterm birth and stillbirth, in women living with HIV [31, 32]. Given that drug-susceptible tuberculosis in pregnant women living with HIV is known to increase unfavorable pregnancy and infant outcomes [2, 33, 34], it is not surprising that in our study of women with MDR/RR-tuberculosis, most of whom were living with HIV, the rate of unfavorable pregnancy outcomes is higher than that of the average population. We used a conservative definition of unfavorable pregnancy outcome, as many babies of moderate prematurity and/or marginally low birth weight were considered as unfavorable outcomes, even if otherwise well.
Pregnant women have been treated with bedaquiline in South Africa since 2015. In our study, over 90% of the fetuses exposed to bedaquiline in utero were born alive, with similar fetal and neonatal deaths compared to those not exposed. Although no difference in the proportion of babies born prematurely in those exposed to bedaquiline, compared to those not exposed, more of the babies exposed to bedaquiline had a low birth weight. We analyzed our data for other predictors of low birth weight. However, given the extensive collinearity between drugs used to treat MDR/RR-tuberculosis, it was not possible to exclude other second-line tuberculosis drugs and ART either individually or in combination as being implicated.
Healthy children with normal growth and development were reported in over 80% of the infants that were followed up for 12 months. Although bedaquiline and levofloxacin use was associated with low birth weight, 88% of the infants exposed to bedaquiline and levofloxacin in utero, who were born alive and followed up for 12 months, had a favorable infant outcome, with 88% thriving and 93% developing normally.
Although this is the largest study to our knowledge to date documenting treatment, pregnancy, and infant outcomes in a cohort of women treated for MDR/RR-tuberculosis during pregnancy, it remains limited by its small size. It was a pragmatic observational study conducted in the public sector with limited resources, using often incomplete data routinely collected by health workers. The poor quality of data on adverse events, drug: drug interactions, and reasons for changes in both drugs and drug dosages limited our analyses and findings.
In this study we noted an association between bedaquiline use and low birth weight, but it is not possible to conclusively ascribe this effect to bedaquiline, and more investigation is required to explore this relationship. Our data suggest that the use of bedaquiline is safe in pregnant women and is associated with good treatment, pregnancy, and infant outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. M. L., B. S., I. M., S. C., N. G., and J. S. conceptualized and led the study. M. L., B. S., I. M., S. C., N. G., S. H., and J. S. contributed to the development of the methods. M. L., B. S., I. M., S. C., N. G., and S. H. collected the data. M. L., T. R., J. H., and J. S. analyzed the data and drafted the initial manuscript. All authors reviewed and contributed to the interpretation and approved the final manuscript.
Acknowledgments. The authors thank Dr Norbet Ndjeka and Ms Jacqueline Ngozo for the support of the national and provincial tuberculosis directorates respectively.
Financial support. This work was supported by the South African Medical Research Council. The funder had no role in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication. All researchers were independent of funders and sponsors.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Global tuberculosis report 2019 WHO/CDS/TB/2019.15. Geneva: World Health Organization, 2019. [Google Scholar]
- 2. Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012; 55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mnyani CN, McIntyre JA. Tuberculosis in pregnancy. BJOG 2011; 118:226–31. [DOI] [PubMed] [Google Scholar]
- 4. Grange J, Adhikari M, Ahmed Y, et al. Tuberculosis in association with HIV/AIDS emerges as a major nonobstetric cause of maternal mortality in Sub-Saharan Africa. Int J Gynaecol Obstet 2010; 108:181–3. [DOI] [PubMed] [Google Scholar]
- 5. Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Glob Health 2014; 2:e710–6. [DOI] [PubMed] [Google Scholar]
- 6. Ribeiro PS, Jacobsen KH, Mathers CD, Garcia-Moreno C. Priorities for women’s health from the Global Burden of Disease study. Int J Gynaecol Obstet 2008; 102:82–90. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. WHO/HTM/TB/2016.04. Geneva: World Health Organization, 2016. [Google Scholar]
- 8. Efferen LS. Tuberculosis and pregnancy. Curr Opin Pulm Med 2007; 13:205–11. [DOI] [PubMed] [Google Scholar]
- 9. Tripathy SN, Tripathy SN. Tuberculosis and pregnancy. Int J Gynaecol Obstet 2003; 80:247–53. [DOI] [PubMed] [Google Scholar]
- 10. Gupta A, Hughes MD, Garcia-Prats AJ, McIntire K, Hesseling AC. Inclusion of key populations in clinical trials of new antituberculosis treatments: current barriers and recommendations for pregnant and lactating women, children, and HIV-infected persons. PLoS Med 2019; 16:e1002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palacios E, Dallman R, Muñoz M, et al. Drug-resistant tuberculosis and pregnancy: treatment outcomes of 38 cases in Lima, Peru. Clin Infect Dis 2009; 48:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. WHO treatment guidelines for multidrug- and rifampicin-resistant tuberculosis, 2018 update. Geneva: World Health Organization, 2019. [Google Scholar]
- 13. World Health Organization. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: interim policy guidance. WHO/HTM/TB/2013.6. Geneva: World Health Organization, 2013. [PubMed] [Google Scholar]
- 14. World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis: interim policy guidance. WHO/HTM/TB/2014.23. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 15. Schnippel K, Ndjeka N, Maartens G, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 2018; 6:699–706. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis - 2011 update. WHO/HTM/TB/2011.6. Geneva: World Health Organization, 2011. [PubMed] [Google Scholar]
- 17. South African Department of Health. Management of drug-resistant tuberculosis. Policy guidelines (updated - January 2013). Pretoria: Department of Health, 2013. [Google Scholar]
- 18. South African National Department of Health. Introduction of new drugs and drug regimens for the management of DR-TB in South Africa: policy framework. Pretoria: National Department of Health, 2015. [Google Scholar]
- 19. World Health Organization. Definitions and reporting framework for tuberculosis-2013 revision. Geneva: World Health Organization, 2013. [Google Scholar]
- 20. World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2014.11. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 21. World Health Organization, UNICEF. Low birthweight: country, regional and global estimates. Geneva: World Health Organization, 2004. [Google Scholar]
- 22. Van Niekerk E, Siebert I, Kruger T. An evidence-based approach to recurrent pregnancy loss. S Afr J OG 2013; 19:61–5. [Google Scholar]
- 23. South African National Department of Health. Choice on termination of pregnancy act. Pretoria, 1996. [Google Scholar]
- 24. Berk LE. Young children: prenatal through middle childhood. New York: Pearson, 2012. ISBN 0205011098. [Google Scholar]
- 25. The Free Medical Dictionary. Available at: https://medical-dictionary.thefreedictionary.com/failure+to+thrive. Accessed 29 November 2017. [Google Scholar]
- 26. Stats South Africa. Perinatal deaths in South Africa 2014. Pretoria, South Africa: Statistics South Africa, 2016. [Google Scholar]
- 27. Loveday M, Wallengren K, Brust J, et al. Community-based care vs. centralised hospitalisation for MDR-TB patients, KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis 2015; 19:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. VanLeeuw L, Mzobe Y, Loveday M. Tuberculosis in district health barometer 2016/17. In: Massyn N, Nazia P, English R, Padarath A, Barron P, Day C, eds. District health barometer. Durban: Health Systems Trust, 2017. [Google Scholar]
- 29. Pattinson R, Rhoda N.. Saving babies 2012–13: ninth report on perinatal care in South Africa. Pretoria: Tshepesa Press, 2014. [Google Scholar]
- 30. Chawanpaiboon S, Vogel J, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019; 7:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stringer EM, Kendall MA, Lockman S, et al. Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS One 2018; 13:e0199555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fowler MG, Qin M, Fiscus SA, et al. ; IMPAACT 1077BF/1077FF PROMISE Study Team . Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta A, Bhosale R, Kinikar A, et al. ; Six Week Extended-Dose Nevirapine (SWEN) India Study Team . Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis 2011; 203:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salazar-Austin N, Hoffmann J, Cohn S, et al. ; TSHEPISO Study Team . Poor obstetric and infant outcomes in human immunodeficiency virus-infected pregnant women with tuberculosis in South Africa: The Tshepiso Study. Clin Infect Dis 2018; 66:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.