Abstract
Background
We recently mitigated a clonal outbreak of hospital-acquired Mycobacterium abscessus complex (MABC), which included a large cluster of adult patients who developed invasive infection after exposure to heater-cooler units during cardiac surgery. Recent studies have detailed Mycobacterium chimaera infections acquired during cardiac surgery; however, little is known about the epidemiology and clinical courses of cardiac surgery patients with invasive MABC infection.
Methods
We retrospectively collected clinical data on all patients who underwent cardiac surgery at our hospital and subsequently had positive cultures for MABC from 2013 through 2016. Patients with ventricular assist devices or heart transplants were excluded. We analyzed patient characteristics, antimicrobial therapy, surgical interventions, and clinical outcomes.
Results
Ten cardiac surgery patients developed invasive, extrapulmonary infection from M. abscessus subspecies abscessus in an outbreak setting. Median time from presumed inoculation in the operating room to first positive culture was 53 days (interquartile range [IQR], 38–139 days). Disseminated infection was common, and the most frequent culture-positive sites were mediastinum (n = 7) and blood (n = 7). Patients received a median of 24 weeks (IQR, 5–33 weeks) of combination antimicrobial therapy that included multiple intravenous agents. Six patients required antibiotic changes due to adverse events attributed to amikacin, linezolid, or tigecycline. Eight patients underwent surgical management, and 6 patients required multiple sternal debridements. Eight patients died within 2 years of diagnosis, including 4 deaths directly attributable to MABC infection.
Conclusions
Despite aggressive medical and surgical management, invasive MABC infection after cardiac surgery caused substantial morbidity and mortality. New treatment strategies are needed, and compliance with infection prevention guidelines remains critical.
Keywords: Mycobacterium abscessus, nontuberculous mycobacteria, hospital outbreak
Cardiac surgery patients with invasive Mycobacterium abscessus complex infection received aggressive and prolonged medical and surgical therapy. However, patients with these infections experienced substantial morbidity and mortality. Novel treatment strategies are needed, and close attention to infection prevention remains paramount.
Nontuberculous mycobacteria (NTM) are emerging pathogens increasingly implicated in healthcare-associated infections and outbreaks [1, 2]. NTM commonly colonize municipal water, and patients can acquire NTM from healthcare facilities after contact with tap water, ice, aerosols, or medical equipment contaminated with NTM [3].
Contamination of heater-cooler units (HCUs) used in cardiac bypass surgery recently instigated a large, global outbreak of > 100 confirmed invasive and often disseminated postoperative infections from Mycobacterium chimaera [4]. Whole genome sequencing of M. chimaera isolates obtained worldwide from cardiac surgery patients and HCUs strongly implicated point-source HCU contamination by a colonized water source at a manufacturing site in Germany [5]. However, other waterborne NTM can also colonize HCUs and generate aerosols in the operating room, leading to invasive infections from other NTM species via the same mechanism [2, 6]. For example, investigators at 2 hospitals showed that cases of Mycobacterium wolinskyi surgical site infection following cardiac surgery were associated with NTM contamination of an HCU and the HCU water supply, respectively [7, 8].
Three other recently reported cardiac surgery outbreaks of invasive mycobacterial infections were caused by the rapidly growing mycobacterium, Mycobacterium abscessus complex (MABC). Each outbreak occurred at hospitals in the southeastern United States and implicated contaminated hospital water systems or HCUs [9–12]. One of these outbreaks occurred at our hospital where investigators mitigated a large, clonal outbreak of MABC linked to colonization of a new hospital addition’s water system [12]. This outbreak included > 20 patients who underwent cardiac surgery from 2013 through 2015 and developed invasive postoperative MABC infection.
Investigation of outbreaks caused by M. chimaera or MABC in cardiac surgery patients have illustrated the importance and complexity of measures required to prevent these infections [12–19]. Furthermore, the global outbreak of invasive M. chimaera infection has also begun to generate valuable data on the epidemiology, clinical management, and outcomes of these often devastating infections [4, 20–23]. However, published clinical data on extrapulmonary, invasive MABC infection remain limited [24–28], especially among cardiac surgery patients [3, 29–33]. Patients who develop extrapulmonary MABC infection typically require months of combination intravenous antimicrobial therapy and surgical debridement, but the optimal clinical management and expected outcomes of cardiac surgery patients with these infections are not known.
This study describes the patient characteristics, medical and surgical management, and clinical outcomes among cardiac surgery patients at our hospital who developed invasive MABC infection.
METHODS
Duke University Hospital (DUH) is a 957-bed tertiary care hospital in central North Carolina. In July 2013, a new hospital addition opened, which included 160 intensive care unit and intermediate beds, as well as 16 operating suites [12].
We identified all patients with growth of MABC from any clinical specimen obtained at our hospital from 2013 through 2016. Our case definition required that a patient had undergone cardiac surgery at DUH prior to diagnosis. We excluded 2 groups of cardiac surgery patients from this analysis: (1) patients who had acquisition of MABC unlikely to be directly related to cardiac surgery at DUH, including patients who had positive cultures from only the respiratory tract; and (2) patients who at time of diagnosis had ventricular assist devices (VADs) in place or had undergone heart transplantation [34, 35]. We defined date of diagnosis to be the date of collection of the first positive culture. We considered the inoculation event to be the cardiac bypass surgery that most immediately preceded diagnosis.
For case patients, we reviewed clinical characteristics, including cardiac history, comorbidities, indication for surgery, and presenting signs and symptoms of infection. We also analyzed clinical data for 2 years following diagnosis, including detailed data on antimicrobial therapy and associated adverse events (AEs). Finally, we evaluated surgical management, burden of hospitalizations, and clinical outcomes, including mortality data.
Infectious disease physicians extracted all clinical data from the electronic medical record and adjudicated attribution of antibiotic-related AEs and deaths. AEs with objective definitions included renal toxicity (≥ 50% reduction in creatinine clearance or antibiotic changed), anemia (required blood transfusion or antibiotic changed), leukopenia (required granulocyte-colony stimulating factor or antibiotic changed), thrombocytopenia (required platelet transfusion or antibiotic changed), and development of Clostridioides difficile colitis. AEs defined subjectively included hearing loss; tinnitus; nausea, vomiting, and diarrhea; and peripheral neuropathy. Study physicians recorded these qualitative AEs if they deemed them to be clinically significant. Therapy-limiting AEs were defined as AEs that required changes in antibiotic regimen.
Standard mycobacterial culture methods were utilized and previously described [12]. Isolates from each case patient underwent susceptibility testing using Clinical and Laboratory Standards Institute guidelines, molecular subspecies identification, and molecular fingerprinting at the Mycobacteria/Nocardia Research Laboratory at the University of Texas Health Science Center in Tyler, Texas, as previously described [12].
Calculations were performed in SAS software, version 9.4 (SAS Institute, Cary, North Carolina). The institutional review boards at Duke University and the University of Texas Health Science Center approved this investigation and research.
RESULTS
Patient Characteristics and Presentation of Infection
Over the 4-year study, 38 patients with a history of cardiac surgery at DUH had subsequent positive cultures for MABC. We excluded 28 of these patients due to presence of VADs or heart transplants at time of diagnosis (n = 20) [34, 35], positive respiratory cultures in the absence of extrapulmonary infection (n = 6), and invasive MABC infections unlikely related to cardiac surgery performed at DUH (n = 2).
The 10 patients who met the case definition for this study were adults without notable immunosuppression and relatively few noncardiac comorbidities (Tables 1 and 2). However, most patients had severe cardiac illnesses preceding their cardiac surgery and underwent complicated and prolonged surgical procedures. Surgeries for all patients required cardiopulmonary bypass and use of HCUs (Supplementary Table). Seven patients underwent cardiac valve surgery, and 9 patients had cardiothoracic prosthetic material in place after surgery. All patients had infections linked to cardiac surgeries performed after the opening of the new hospital addition’s operating suites in July 2013 and before the May 2015 institution of a new HCU disinfection and maintenance protocol [12].
Table 1.
Characteristics of 10 Patients Who Developed Extrapulmonary Mycobacterium abscessus Subspecies abscessus Infection After Cardiac Surgery
| Characteristic | No. | (%) |
|---|---|---|
| Age at diagnosis, y, median (IQR) | 66 | (51–76) |
| Male sex | 7 | 70 |
| Race/ethnicity | ||
| White | 9 | 90 |
| Black | 1 | 10 |
| Hispanic | 0 | 0 |
| Preoperative noncardiac comorbidities | ||
| CKD III, IV, or V | 4 | 40 |
| Obesity (BMI > 30 kg/m2) | 3 | 30 |
| Diabetes mellitus | 1 | 10 |
| Intravenous drug use | 1 | 10 |
| Indications for cardiac surgery | ||
| Valvular disease | 5 | 50 |
| Coronary artery disease | 4 | 40 |
| Cardiac or vascular infection | 3 | 30 |
| Types of cardiac surgeries | ||
| Valve replacement or repair | 7 | 70 |
| Vascular graft placement | 5 | 50 |
| Coronary artery bypass grafting | 4 | 40 |
| Presenting symptoms of infection | ||
| Fever | 6 | 60 |
| Sternal wound drainage | 6 | 60 |
| Weight loss | 3 | 30 |
| Laboratory abnormalities at time of diagnosis | ||
| Anemia (hemoglobin < 10 g/dL) | 7 | 70 |
| Leukocytosis (WBC count ≥ 10 × 109/L) | 3 | 30 |
| Elevated LFTs (any liver enzyme or total bilirubin ≥ 2 times ULN) | 2 | 20 |
| Thrombocytopenia (platelet count < 100 × 109/L) | 1 | 10 |
| First site of culture-proven infection | ||
| Bloodstream | 5 | 50 |
| Mediastinum | 4 | 40 |
| Sternal wound | 1 | 10 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; IQR, interquartile range; LFT, liver function test; ULN, upper limit of normal; WBC, white blood cell.
Table 2.
Characteristics, Clinical Management, and Survival of 10 Patients Who Developed Mycobacterium abscessus Subspecies abscessus Infection After Cardiac Surgery
| Patient No. | Age, ya | Sex | Preoperative Noncardiac Comorbidities | Indication for Surgery | Type of Surgery | Sites of Culture- proven Infection | Majority Antibiotic Regimen (Total Duration, wk)b | Procedural Interventions | Time Alive After Diagnosis, wk (Death Attribution) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60–70 | F | CKD IV, obesity | RV infarct, RV failure, and tricuspid regurgitation after CABG | Tricuspid valve replacement | Bloodstream, pleural fluid | Clarithromycin, cefoxitin, imipenem | (2) | Chest tube placement; VATS with decortication | 4 (attributable) |
| 2 | 80–90 | M | None | Mitral valve dysfunction, atrial fibrillation | MV repair, ablation of atrial fibrillation, LAA clip | Bloodstream | Amikacin, imipenem, tigecycline | (3) | None | 4 (attributable) |
| 3 | 60–70 | F | None | Abdominal aortic aneurysm, CAD | CABG, aortic grafting, AVR | Bloodstream, mediastinum | Imipenem, tedizolid, tigecycline | (27) | Sternal debridement (×3) | 27 (attributable) |
| 4 | 40–50 | M | CKD IV | Aortic graft infection, CAD | CABG, redo aortic grafting, AVR, and temporary RVAD | Bloodstream, mediastinum, pacemaker pocket | Amikacin, imipenem, tigecycline | (45) | Sternal debridement (×2), omental flap | 46 (attributable) |
| 5 | 60–70 | M | Hemodialysis | Aortic valve endocarditis with aortic root abscess | Replacement of aortic root, coronary reconstruction | Bloodstream | Amikacin, imipenem, tigecycline | (5) | None | 12 (not directly attributable) |
| 6 | 80–90 | M | CKD IV, obesity | Aortic stenosis and pseudoaneurysm, CAD, tricuspid regurgitation | AVR, tricuspid valve repair, CABG, aortic pseudoaneurysm repair | Bloodstream, mediastinum | Amikacin, imipenem, tigecycline | (11) | Sternal debridement (×2), myocutaneous flap | 44 (not directly attributable) |
| 7 | 60–70 | M | None | Right ventricular rupture after CABG | RV graft placement | Bloodstream, mediastinum, RV outflow tract | Amikacin, imipenem, tigecycline | (32) | Sternal debridement, removal of RV outflow tract graft and vegetation, omental flap | 63 (not directly attributable) |
| 8 | 30–40 | M | IV drug use | Tricuspid valve endocarditis | Tricuspid valve replacement | Abdominal wall, mediastinum | Amikacin, imipenem, tigecycline | (33) | Sternal debridement (×2), abdominal wall debridement, removal of epicardial pacing lead | 82 (not directly attributable) |
| 9 | 50–60 | M | Diabetes, obesity | LVAD no longer required | LVAD explant with retention of LV apical plug | Mediastinum, pericardium | Amikacin, azithromycin, imipenem, tedizolid | (85) | Sternal drain placement; Sternal debridement (×3), LV aneurysm repair with removal of LV apical plug, omental flap | >104 (NA) |
| 10 | 70–80 | F | None | CAD | CABG | Mediastinum | Amikacin, imipenem, tigecycline | (20) | Sternal debridement (×2), myocutaneous flap | >104 (NA) |
Abbreviations: AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; IV, intravenous; LAA, left atrial appendage; LV, left ventricle; LVAD, left ventricular assist device; MV, mitral valve; NA, not applicable; RV, right ventricle; RVAD, right ventricular assist device; VATS, video-assisted thorascopic surgery.
aAges are given in ranges to protect identities of individual patients.
bMajority antibiotic regimen is the combination of antibiotics that was used for the longest duration during the treatment course. Total duration includes all treatment time periods, regardless of antibiotic combination. Azithromycin, clarithromycin, and tedizolid were administered orally unless a patient could not take oral medications. Other listed antibiotics were administered intravenously.
Four patients were diagnosed with MABC infection during index cardiac surgery hospitalizations. These patients had prolonged postoperative hospitalizations and multiple associated complications that preceded the diagnosis of MABC disease, and their initial site of infection was the bloodstream (Supplementary Table). The remaining 6 patients were discharged from cardiac surgery hospitalizations without suspected mycobacterial infection but were readmitted with subacute signs and symptoms of MABC infection, including 5 patients readmitted with sternal wound drainage (Figure 1). In addition to sternal wound drainage (n = 6), the other most common presenting symptom of MABC infection was fever (n = 6) (Table 1). Laboratory abnormalities were variable and nonspecific; anemia was common in this cohort of postoperative patients.
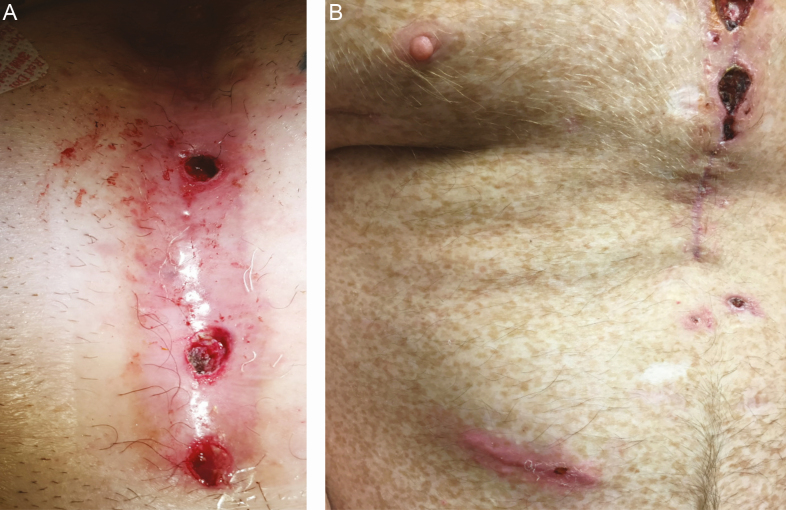
Figure 1.
Clinical photographs of Mycobacterium abscessus subsp abscessus infections following cardiac surgery. A, Sternal wound at time of diagnosis of M. abscessus subsp abscessus mediastinal infection. B, Sternal wound and abdominal wall abscess in patient with chronic M. abscessus subsp abscessus mediastinal infection and infected epicardial pacing lead at abdominal wall. In this cohort, disseminated infection commonly presented with mild, nonpurulent drainage from sternal wounds.
Median time from cardiac surgery to first positive culture was 53 days (interquartile range [IQR], 38–139 days [range, 2–324 days]; Figure 2). The first positive culture was obtained from either blood (n = 5; median time from surgery to positive culture, 38 days) or sternal wound/mediastinum (n = 5; median time from surgery to positive culture, 107 days) (Table 1). Six patients ultimately developed disseminated disease with culture-proven infection at 2 or more noncontiguous sites (Table 2). Four patients developed culture-proven infections of cardiothoracic prosthetic material, and additional patients likely had unproven involvement of cardiothoracic hardware (Supplementary Table). No patients had proven or suspected mycobacterial ocular disease, bone marrow infiltration, or hepatitis.
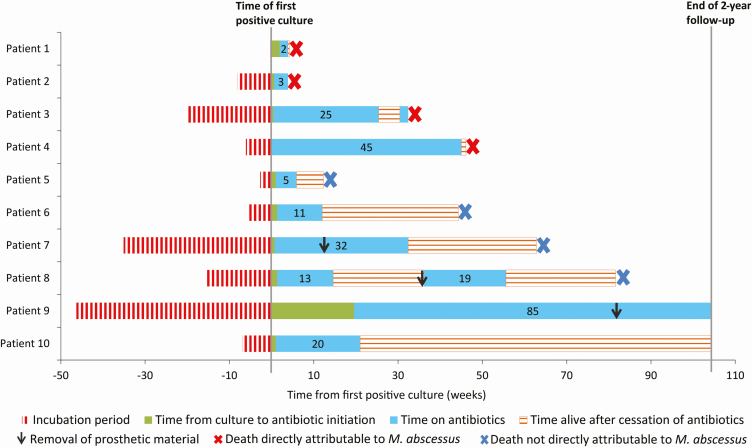
Figure 2.
Clinical courses of 10 patients who developed invasive Mycobacterium abscessus subsp abscessus infection after cardiac surgery. Incubation period is given from time of presumed inoculation in operating room to time that the first positive culture was obtained. Time periods of antibiotic therapy are given in weeks.
Microbiology Data
In addition to positive mycobacterial-specific cultures, 5 of 7 patients with mycobacteremia had at least 1 positive blood culture performed on routine media. However, diagnosis of infection outside of the bloodstream occurred in 8 patients and uniformly required mycobacterial-specific tissue or fluid cultures.
Susceptibility testing demonstrated that isolates obtained from all 10 patients were multidrug resistant with nearly identical susceptibility profiles, including erm gene–mediated resistance to clarithromycin (Table 3). Molecular fingerprinting, including multilocus sequence typing and pulsed-field gel electrophoresis (PFGE), confirmed a clonal outbreak of M. abscessus subspecies (subsp) abscessus: isolates had the same unique erm and rpoβ gene combination and were clonal via PFGE [12].
Table 3.
Antibiotic Susceptibility Testing, Use, and Associated Adverse Events Among 10 Patients Who Developed Mycobacterium abscessus Subspecies abscessus Infection After Cardiac Surgery
| Antibiotica | Susceptibility Data | Used During Treatment Course | Component of Majority Regimen | Duration, wk, Median (range) | Patients With Attributable AEs (No. of Therapy-limiting AEs)b |
|---|---|---|---|---|---|
| Amikacin | S: 10/10 | 10/10 | 8/10 | 11 (1–36) | Renal injury: 4/10 (3) |
| Hearing loss and tinnitus: 2/10 (1) | |||||
| Azithromycin/clarithromycinc | R: 10/10 | 5/10 | 2/10 | 7 (3–85) | 0/5 |
| Cefoxitin | I: 10/10 | 1/10 | 1/10 | 2 (2) | 0/1 |
| Imipenem | I: 9/10 | 10/10 | 10/10 | 20 (1–45) | 0/10 |
| R: 1/10 | |||||
| Linezolid | I: 9/10 | 5/10 | 1/10 | 7 (1–18) | Peripheral neuropathy: 2/5 (2) |
| R: 1/10 | Gastrointestinal: 1/5 (1) | ||||
| Thrombocytopenia: 1/5 (1) | |||||
| Moxifloxacin | R: 10/10 | 1/10 | 0/10 | 7 (7) | 0/1 |
| Tedizolid | Not tested | 2/10 | 1/10 | 50 (15–84) | 0/2 |
| Tigecycline | MIC ≤ 0.25: 9/10 | 9/10 | 8/10 | 11 (3–45) | Gastrointestinal: 4/9 (1) |
| MIC = 0.5: 1/10 | |||||
| Ciprofloxacin | R: 10/10 | 0/10 | 0/10 | NA | NA |
| Doxycycline | R: 10/10 | 0/10 | 0/10 | NA | NA |
| Minocycline | R: 10/10 | 0/10 | 0/10 | NA | NA |
| TMP-SMX | R: 10/10 | 0/10 | 0/10 | NA | NA |
Abbreviations: AE, adverse events; I, intermediate; MIC, minimum inhibitory concentration; NA, not applicable; R, resistant; S, susceptible; TMP-SMX, trimethoprim-sulfamethoxazole.
aAzithromycin, clarithromycin, linezolid, moxifloxacin, and tedizolid were administered orally unless a patient could not take oral medications. Other antibiotics were administered intravenously.
bAttributable AEs are given as a proportion of the number of patients who developed the attributable AE out of the total number of patients who received the antibiotic. Therapy-limiting AEs required changes in antibiotic regimen. Gastrointestinal AEs consisted of clinically significant nausea, vomiting, or diarrhea.
cSusceptibility data are given for clarithromycin but do not have interpretable criteria for azithromycin. Other data elements for azithromycin (received by 3 patients) and clarithromycin (received by 2 patients) are combined.
Clinical Management and Outcomes
All patients received combination antimicrobial therapy, initially with long courses of induction therapy consisting of at least 3 agents (Table 2). However, patients completed a median of only 8 weeks (IQR, 5–11 weeks) of initial antibiotic regimens because therapy frequently resulted in antibiotic-related AEs requiring changes in therapy. The median total duration of therapy was 24 weeks (IQR, 5–33 weeks), including brief courses of therapy for 2 patients who died from MABC disease within 5 weeks of diagnosis. The most common antibiotic regimen consisted of amikacin, imipenem, and tigecycline (n = 7), but most patients received numerous additional antibiotics during their complicated treatment courses (Table 3).
Multiple strategies were implemented to limit anticipated AEs associated with high-risk antibiotics. For example, patients receiving amikacin typically underwent baseline and surveillance audiology assessments [36], took concomitant N-acetylcysteine to decrease risk of ototoxicity [37], underwent close laboratory monitoring of renal function and drug levels, and transitioned to aminoglycoside-sparing regimens after completing many weeks of initial induction therapy. Linezolid was usually dosed at 600 mg once daily rather than the twice-daily dosing regimen commonly used for bacterial infections [38]. Two patients received tedizolid and tolerated 200-mg once-daily dosing, including 1 patient who tolerated long-term suppression with tedizolid for > 80 weeks [39]. Finally, 2 patients developed gastrointestinal toxicity from tigecycline but were able to continue this agent after the dosing frequency was decreased from the standard 50-mg twice-daily dose to once-daily dosing [40].
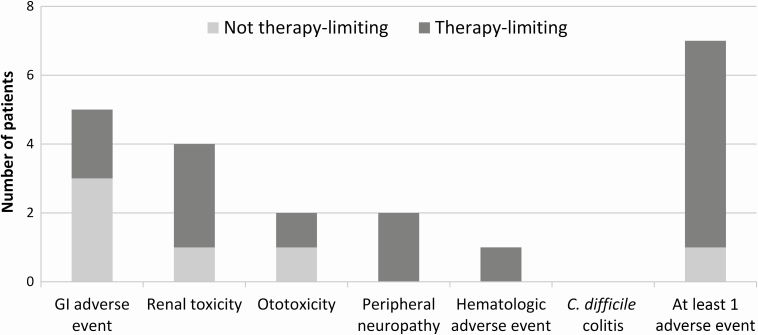
Despite strategies designed to decrease risk of AEs, 7 patients developed a total of 15 antibiotic-associated AEs, including 5 patients with gastrointestinal toxicity and 4 patients with acute kidney injury (Figure 3). All 6 patients who received at least 20 weeks of therapy developed antibiotic-related AEs, and 5 of these patients required changes in antibiotic regimen. All but 1 AE was attributed to either amikacin (n = 6), linezolid (n = 4), or tigecycline (n = 4) (Table 3).
Figure 3.
Antibiotic-associated adverse events experienced by 10 cardiac surgery patients treated for invasive Mycobacterium abscessus subsp abscessus infection. Therapy-limiting adverse events required changes in antibiotic regimen. Gastrointestinal (GI) adverse events consisted of nausea, vomiting, or diarrhea. Both patients with ototoxicity developed hearing loss and tinnitus. The single hematologic adverse event was thrombocytopenia. One patient experienced 2 distinct GI adverse events attributed to different antibiotics; this patient’s GI symptoms are represented by a single bar on the figure. Abbreviation: C. difficile, Clostridioides difficile.
Except for 2 patients with mycobacteremia in the absence of known local infection, all patients underwent surgical management for source control (Table 2). Seven patients had confirmed mediastinal infection requiring a total of 15 sternal debridements (median, 2 debridements); 5 patients also underwent flap coverage for sternal defects. Three of 4 patients with confirmed infection of prosthetic material (patients 7, 8, and 9) eventually underwent hardware removal and did not subsequently experience relapse of infection (Figure 2 and Supplementary Table). The remaining patient (patient 4) was not a candidate for removal of an infected aortic graft. This patient died from progressive, disseminated MABC infection despite sternal debridement, flap coverage of the graft, and months of antibiotic therapy. Three additional patients whose deaths were directly attributable to MABC infection (patients 1, 2, and 3) may also have had infection of prosthetic material but were too ill to undergo additional diagnostic studies or removal of their implanted hardware.
During the first 12 months after diagnosis, the 10 patients required a total of 26 hospitalizations (median, 3 [range, 1–6]) and accumulated 423 days of hospitalization (median, 42 days [IQR, 28–55 days]). Eight patients died within 2 years after diagnosis (median, 38 weeks [IQR, 8–55 weeks]) (Figure 2). Four deaths were considered to be directly attributable to MABC infection; these deaths occurred a median of 18 weeks (range, 4–46 weeks) after diagnosis. Five patients completed antimicrobial therapy with presumed clinical cure and did not have evidence of disease relapse off of therapy; however, 4 of these 5 patients also died during study follow-up, a median of 54 weeks (range, 12–82 weeks) after diagnosis and 28 weeks (range, 6–32 weeks) after completion of antibiotics (Supplementary Table). Only 2 patients were alive at the conclusion of the study, and neither patient had remaining cardiothoracic prosthetic material: patient 9 continued to receive chronic suppressive therapy with azithromycin and tedizolid without evidence of active infection, and patient 10 remained well in the outpatient setting for > 18 months after completing antimicrobial therapy.
DISCUSSION
We described the epidemiology, clinical management, and outcomes of 10 cardiac surgery patients who developed invasive MABC infection at a single hospital. All patients were presumed to be infected in the operating room via aerosolization of MABC from HCUs used for cardiopulmonary bypass; incubation periods ranged from 2 days to nearly 1 year. Nearly all patients underwent complex index cardiac surgeries, but preoperative noncardiac comorbidities were uncommon, and no patients were considered to be immunosuppressed.
Clinical recognition and diagnosis of MABC infection was challenging in this cohort of patients. Several patients had serious postoperative complications during index cardiac surgery hospitalizations that were thought to be independent of MABC infection and may have delayed recognition of more subtle presentations of MABC disease. In addition, patients who presented as outpatients with sternal wound infections were often treated with empiric antibiotics for several weeks or months before a diagnosis of MABC was made. Subsequent evaluation of patients who initially had relatively benign-appearing incisional surgical site infections often revealed that these patients actually had disseminated infection with mediastinitis and mycobacteremia. Surgical exploration of the sternum revealed nonpurulent wounds that appeared chronically infected, in contrast to the purulent wounds often seen with infections due to typical bacterial pathogens.
Despite long courses of antimicrobial therapy and aggressive surgical debridement, clinical outcomes in most patients were extremely poor. Patients who did not die shortly after diagnosis typically received > 6 months of combination antimicrobial therapy, and most patients required changes in antibiotic regimens due to AEs from amikacin, linezolid, or tigecycline. Eight of 10 patients, including all 7 patients with mycobacteremia, died < 2 years after diagnosis.
Not surprisingly, our experience in managing this cohort of patients suggests that removal of cardiothoracic prosthetic material contaminated with MABC, when feasible, improves odds of successful treatment. One patient who was able to undergo removal of cardiac prosthetic material survived 2-year follow-up; the other surviving patient did not have endovascular hardware.
These cases of MABC infection following cardiac surgery share some similarities with reported cases of HCU-related M. chimaera infection [4, 20, 21]. Patients described in both cohorts often presented with nonspecific, subacute symptoms. However, many patients in both cohorts were ultimately found to have disseminated infections involving cardiothoracic prosthetic material, required long courses of combined medical and surgical therapy, and had high mortality rates.
Patients with MABC infection also exhibited notable clinical features that differed from the clinical characteristics of patients with M. chimaera infection. First, the interval between cardiac bypass surgery and disease onset was typically several weeks to several months for infections from the rapidly growing MABC, compared to a median of > 1 year for infections due to the slow-growing M. chimaera [4, 20, 21]. Also, patients with MABC infection more commonly had sternal wound infections, and, unlike patients with M. chimaera infection, did not have evidence of ocular, bone marrow, or liver involvement. Finally, patients with MABC infection usually required induction therapy with 3 intravenous antibiotics because few oral agents were active against the infecting strain of MABC; prolonged use of combination intravenous antibiotic therapy may have increased risk of antibiotic-associated AEs. In contrast, patients with M. chimaera infection often received long courses of combination oral therapy, at times with the temporary inclusion of intravenous amikacin for induction therapy.
Our experience highlights important treatment considerations for invasive MABC disease that merit further study. For example, all isolates in this study represented M. abscessus subsp abscessus with in vitro erm gene–mediated macrolide resistance; however, a number of patients were nonetheless treated with macrolides, in particular oral azithromycin, given poor tolerability of other agents and potential for less erm induction by azithromycin compared to clarithromycin [41, 42]. In addition, use of tedizolid to treat or suppress NTM disease has rarely been described, but this agent may have a lower risk of AEs than long-term treatment with linezolid [39]. Similarly, tigecycline may have less gastrointestinal toxicity without decreased effectiveness when given as a once-daily 50-mg dose [40]. While patients in this cohort did not receive clofazimine, bedaquiline, eravacycline, or omadacycline, other clinicians have recently reported success with off-label use of these novel agents when treating refractory NTM infections, including MABC [43–46]. Finally, a recent case study reported successful use of bacteriophage therapy targeting disseminated MABC [47]. If supported by additional clinical data, phage therapy could become a viable treatment option for invasive MABC infection.
To our knowledge, this study represents the largest published cohort of cardiac surgery patients with MABC infection in > 30 years, regardless of mechanism of infection [29]. However, other cardiac surgery outbreaks of invasive MABC have recently occurred, and we suspect that many additional hospitals have also had cases that are not yet reported due to long incubation periods, difficulty in confirming diagnosis, and hesitancy to publish outbreaks. We believe that the clinical analysis provided in this study will provide unique reference to clinicians who investigate potential cases of NTM infection after cardiac surgery, treat confirmed postoperative cases, and manage extrapulmonary invasive MABC disease that occurs in other clinical contexts [24–27]. In particular, our experience should encourage clinicians to consider a diagnosis of invasive NTM infection in postoperative cardiac surgery patients, especially if patients have unexplained fever or sternal wound drainage, and to have a low threshold for performing mycobacterial-specific cultures.
We previously described the clinical characteristics and outcomes of MABC infections in patients with VADs or heart transplants at the time of MABC diagnosis [34, 35]. Clinical features and management strategies used for these patients were unique and distinct from those associated with the cardiac surgery patients described in this cohort. For example, patients with VADs underwent specialized surgical management, including assessment of candidacy for either surgical exposure of the infected VAD pump pocket or heart transplantation. Among heart transplant recipients, prolonged incubation periods of > 300 days were common. Furthermore, clinical management after transplant required careful attention to immunosuppression strategies, including potential for cumulative toxicities related to concomitant exposure to MABC therapy and immunosuppression regimens.
The primary limitations of this study were related to the fact that all patients developed infection in an outbreak setting from the same macrolide-resistant clone of M. abscessus subsp abscessus. This subspecies is the most common MABC subspecies to cause human disease and typically exhibits erm gene–mediated macrolide resistance [42, 48]; nonetheless, patients with invasive infection from other MABC subspecies, or even other strains of M. abscessus subsp abscessus, may have different presentations and responses to therapy (eg, macrolides may be more effective in treating other MABC isolates). Also, clinicians in our health system had an increased index of suspicion for invasive MABC infection in cardiac surgery patients. Reported incubation time from cardiac surgery to diagnosis may have been longer in a non-outbreak setting.
The worldwide cardiac surgery outbreak of M. chimaera has begun to produce important clinical data on extrapulmonary M. chimaera infections. However, several major cardiac surgery outbreaks of MABC have also recently occurred, and detailed clinical data have not previously been published. Invasive MABC infections cause substantial morbidity and mortality, and they have clinical presentations and treatment strategies that differ from invasive M. chimaera infections. Poor clinical outcomes related to invasive MABC infections emphasize the emerging need for new treatment strategies, including further study of novel antibiotics, and importance of adherence to infection prevention guidelines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Micah McClain, MD, PhD, for abstraction of clinical data.
Financial support. A. W. B. was supported in part by the Transplant Infectious Disease Interdisciplinary Research Training Grant of the National Institutes of Health (grant number 5T32AI100851-02).
Potential conflicts of interest. B. A. reports research grants to Duke University from Lediant Pharmaceuticals, Astellas Pharmaceuticals, and Scynexis Pharmaceuticals, and personal fees from Lediant, Astellas, Scynexis, Shire, Shionogi, F2G Pharmaceuticals, and Cidara Pharmaceuticals, outside the submitted work. R. W. reports that the Mycobacteria/Nocardia Research Laboratory at the University of Texas Health Science Center in Tyler, Texas, was reimbursed by Duke University for molecular fingerprinting and susceptibility testing of Mycobacterium abscessus isolates during the initial 2014–2016 outbreak investigation; R. W. personally received no monies or reimbursement. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek, San Diego, California, 6 October 2017. Abstract 999.
References
- 1. Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis 2016; 62:1423–35. [DOI] [PubMed] [Google Scholar]
- 2. Crist MB, Perz JF. Modern healthcare versus nontuberculous mycobacteria: who will have the upper hand? Clin Infect Dis 2017; 64:912–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace RJ Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 1998; 52:453–90. [DOI] [PubMed] [Google Scholar]
- 4. Kasperbauer SH, Daley CL. Mycobacterium chimaera infections related to the heater-cooler unit outbreak: a guide to diagnosis and management. Clin Infect Dis 2019;68:1244– 50. [DOI] [PubMed] [Google Scholar]
- 5. van Ingen J, Kohl TA, Kranzer K, et al. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis 2017; 17:1033–41. [DOI] [PubMed] [Google Scholar]
- 6. Allen KB, Yuh DD, Schwartz SB, et al. Nontuberculous mycobacterium infections associated with heater-cooler devices. Ann Thorac Surg 2017; 104:1237–42. [DOI] [PubMed] [Google Scholar]
- 7. Dupont C, Terru D, Aguilhon S, et al. Source-case investigation of Mycobacterium wolinskyi cardiac surgical site infection. J Hosp Infect 2016; 93:235–9. [DOI] [PubMed] [Google Scholar]
- 8. Nagpal A, Wentink JE, Berbari EF, et al. A cluster of Mycobacterium wolinskyi surgical site infections at an academic medical center. Infect Control Hosp Epidemiol 2014; 35:1169–75. [DOI] [PubMed] [Google Scholar]
- 9. Greenville Health System. GHS releases preliminary results of investigation. Available at: https://www.ghs.org/healthcenter/ghsblog/ghs-releases-preliminary-results-of-investigation/. Accessed 16 September 2019.
- 10. Children’s Hospital New Orleans. Statement regarding mycobacterial infection investigation. Available at: https://www.chnola.org/blog/2017/september/statement-regarding-mycobacterial-infection-inve. Accessed 5 August 2019.
- 11. Brasted C. At least 12 patients contract rare infection after Children’s Hospital heart surgeries: report. Available at: https://www.nola.com/health/article_4c596d4c-04ab-5d60-89f5-79ecf73f195a.html. Accessed 18 March 2020.
- 12. Baker AW, Lewis SS, Alexander BD, et al. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 2017; 64:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marra AR, Diekema DJ, Edmond MB. Mycobacterium chimaera infections associated with contaminated heater-cooler devices for cardiac surgery: outbreak management. Clin Infect Dis 2017; 65:669–74. [DOI] [PubMed] [Google Scholar]
- 14. Kanamori H, Weber DJ, Rutala WA. Healthcare-associated Mycobacterium chimaera transmission and infection prevention challenges: role of heater-cooler units as a water source in cardiac surgery. Clin Infect Dis 2017; 64:343–6. [DOI] [PubMed] [Google Scholar]
- 15. Chand M, Lamagni T, Kranzer K, et al. Insidious risk of severe Mycobacterium chimaera infection in cardiac surgery patients. Clin Infect Dis 2017; 64:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haller S, Holler C, Jacobshagen A, et al. Contamination during production of heater-cooler units by Mycobacterium chimaera potential cause for invasive cardiovascular infections: results of an outbreak investigation in Germany, April 2015 to February 2016. Euro Surveill 2016; 21. doi:10.2807/1560-7917.ES.2016.21.17.30215. [DOI] [PubMed] [Google Scholar]
- 17. Schreiber PW, Sax H. Mycobacterium chimaera infections associated with heater-cooler units in cardiac surgery. Curr Opin Infect Dis 2017; 30:388–94. [DOI] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration. Medical devices. Heater-cooler devices. Information for health care providers and staff at health care facilities. Available at: https://www.fda.gov/medicaldevices/productsandmedicalprocedures/cardiovasculardevices/heater-coolerdevices/ucm492583.htm. Accessed 5 August 2019.
- 19. Centers for Disease Control and Prevention. Contaminated heater-cooler devices. Available at: https://www.cdc.gov/hai/outbreaks/heater-cooler.html. Accessed 5 Auguest 2019.
- 20. Scriven JE, Scobie A, Verlander NQ, et al. Mycobacterium chimaera infection following cardiac surgery in the United Kingdom: clinical features and outcome of the first 30 cases. Clin Microbiol Infect 2018; 24:1164–70. [DOI] [PubMed] [Google Scholar]
- 21. Kohler P, Kuster SP, Bloemberg G, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J 2015; 36:2745–53. [DOI] [PubMed] [Google Scholar]
- 22. Tan N, Sampath R, Abu Saleh OM, et al. Disseminated Mycobacterium chimaera infection after cardiothoracic surgery. Open Forum Infect Dis 2016; 3:ofw131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamad R, Noly PE, Perrault LP, Pellerin M, Demers P. Mycobacterium chimaera infection after cardiac surgery: first Canadian outbreak. Ann Thorac Surg 2017; 104:e43–5. [DOI] [PubMed] [Google Scholar]
- 24. Novosad SA, Beekmann SE, Polgreen PM, Mackey K, Winthrop KL; M. abscessus Study Team. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis 2016; 22:511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 2015; 21:1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El Helou G, Viola GM, Hachem R, Han XY, Raad II. Rapidly growing mycobacterial bloodstream infections. Lancet Infect Dis 2013; 13:166–74. [DOI] [PubMed] [Google Scholar]
- 27. El Helou G, Hachem R, Viola GM, et al. Management of rapidly growing mycobacterial bacteremia in cancer patients. Clin Infect Dis 2013; 56:843–6. [DOI] [PubMed] [Google Scholar]
- 28. Tejura N, Bontempo G, Chew D. Disseminated Mycobacterium abscessus infection secondary to an infected vascular stent: case report and review of the literature. Open Forum Infect Dis 2018; 5:ofy207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoffman PC, Fraser DW, Robicsek F, O’Bar PR, Mauney CU. Two outbreaks of sternal wound infection due to organisms of the Mycobacterium fortuitum complex. J Infect Dis 1981; 143:533–42. [DOI] [PubMed] [Google Scholar]
- 30. Kuritsky JN, Bullen MG, Broome CV, Silcox VA, Good RC, Wallace RJ Jr. Sternal wound infections and endocarditis due to organisms of the Mycobacterium fortuitum complex. Ann Intern Med 1983; 98:938–9. [DOI] [PubMed] [Google Scholar]
- 31. Garcia DC, Nascimento R, Soto V, Mendoza CE. A rare native mitral valve endocarditis successfully treated after surgical correction. BMJ Case Rep 2014; 2014. doi:10.1136/bcr-2013-202610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richey LE, Bahadorani J, Mushatt D. Endovascular Mycobacterium abscessus infection in a heart transplant recipient: a case report and review of the literature. Transpl Infect Dis 2013; 15:208–13. [DOI] [PubMed] [Google Scholar]
- 33. Huth RG, Douglass E, Mondy K, Vasireddy S, Wallace RJ Jr. Treatment of Mycobacterium abscessus subsp. massiliense tricuspid valve endocarditis. Emerg Infect Dis 2015; 21:535–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolfe CR, Baker AW, Patel CB, et al. Mycobacterium abscessus infection in patients with ventricular assist devices. J Heart Lung Transpl 2017; 36(Suppl 4):S257–8. [Google Scholar]
- 35. Maziarz EK, Baker AW, Patel CB, et al. Invasive Mycobacterium abscessus infection in heart transplant recipients. J Heart Lung Transpl 2017; 36(Suppl 4):S25. [Google Scholar]
- 36. Vasconcelos KA, Frota SMMC, Ruffino-Netto A, Kritski AL. The importance of audiometric monitoring in patients with multidrug-resistant tuberculosis. Rev Soc Bras Med Trop 2017; 50:646–51. [DOI] [PubMed] [Google Scholar]
- 37. Kranzer K, Elamin WF, Cox H, Seddon JA, Ford N, Drobniewski F. A systematic review and meta-analysis of the efficacy and safety of N-acetylcysteine in preventing aminoglycoside-induced ototoxicity: implications for the treatment of multidrug-resistant TB. Thorax 2015; 70:1070–7. [DOI] [PubMed] [Google Scholar]
- 38. Agyeman AA, Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 2016; 15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuste JR, Bertó J, Del Pozo JL, Leiva J. Prolonged use of tedizolid in a pulmonary non-tuberculous mycobacterial infection after linezolid-induced toxicity. J Antimicrob Chemother 2017; 72:625–8. [DOI] [PubMed] [Google Scholar]
- 40. Wallace RJ Jr, Dukart G, Brown-Elliott BA, Griffith DE, Scerpella EG, Marshall B. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother 2014; 69:1945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi GE, Shin SJ, Won CJ, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med 2012; 186:917–25. [DOI] [PubMed] [Google Scholar]
- 42. Stout JE, Floto RA. Treatment of Mycobacterium abscessus: all macrolides are equal, but perhaps some are more equal than others. Am J Respir Crit Care Med 2012; 186:822–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Philley JV, Wallace RJ Jr, Benwill JL, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 2015; 148:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGuffin SA, Pottinger PS, Harnisch JP. Clofazimine in nontuberculous mycobacterial infections: a growing niche. Open Forum Infect Dis 2017; 4:ofx147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaushik A, Ammerman NC, Martins O, Parrish NM, Nuermberger EL. In vitro activity of new tetracycline analogs omadacycline and eravacycline against drug-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 2019; 63. doi:10.1128/AAC.00470-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bax HI, de Vogel CP, Mouton JW, de Steenwinkel JEM. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J Antimicrob Chemother 2019; 74:2930–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019; 25:730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi H, Kim SY, Kim DH, et al. Clinical characteristics and treatment outcomes of patients with acquired macrolide-resistant Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 2017; 61. doi:10.1128/AAC.01146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.