Abstract
Background
Since 2013, quadrivalent influenza vaccines containing 2 B viruses gradually replaced trivalent vaccines in the United States. We compared the vaccine effectiveness of quadrivalent to trivalent inactivated vaccines (IIV4 to IIV3, respectively) against illness due to influenza B during the transition, when IIV4 use increased rapidly.
Methods
The US Influenza Vaccine Effectiveness (Flu VE) Network analyzed 25 019 of 42 600 outpatients aged ≥6 months who enrolled within 7 days of illness onset during 6 seasons from 2011–2012. Upper respiratory specimens were tested for the influenza virus type and B lineage. Using logistic regression, we estimated IIV4 or IIV3 effectiveness by comparing the odds of an influenza B infection overall and the odds of B lineage among vaccinated versus unvaccinated participants. Over 4 seasons from 2013–2014, we compared the relative odds of an influenza B infection among IIV4 versus IIV3 recipients.
Results
Trivalent vaccines included the predominantly circulating B lineage in 4 of 6 seasons. During 4 influenza seasons when both IIV4 and IIV3 were widely used, the overall effectiveness against any influenza B was 53% (95% confidence interval [CI], 45–59) for IIV4 versus 45% (95% CI, 34–54) for IIV3. IIV4 was more effective than IIV3 against the B lineage not included in IIV3, but comparative effectiveness against illnesses related to any influenza B favored neither vaccine valency.
Conclusions
The uptake of quadrivalent inactivated influenza vaccines was not associated with increased protection against any influenza B illness, despite the higher effectiveness of quadrivalent vaccines against the added B virus lineage. Public health impact and cost-benefit analyses are needed globally.
Keywords: quadrivalent, trivalent, inactivated influenza vaccine, effectiveness, influenza B lineage
During the 4 influenza seasons beginning in 2013–2014, trivalent and quadrivalent inactivated influenza vaccines provided equivalent protection against any influenza B illness in the United States, while quadrivalent vaccines provided better protection against the added B virus lineage.
First detected in 1940, type B viruses contribute substantially to the annual global disease burden of influenza, including hospitalization and mortality in children and adults [1]. There are 2 distinct influenza B lineages (Victoria and Yamagata) identified based on antigenic relatedness to hemagglutinin proteins of reference strains B/Victoria/02/87 and B/Yamagata/16/88, respectively. Both lineages have cocirculated worldwide over the last 3 decades [1–3]. Trivalent vaccines include 1 B lineage, along with A(H1N1) and A(H3N2) antigens. The selection of the B lineage included in trivalent vaccines occurs twice annually: once each for Northern and Southern hemisphere vaccine formulations [4]. The reassortment of gene segments and genetic mutations in the surface glycoproteins give rise to diverse B virus genotypes, which may require vaccine strain updates [5, 6]. B/Victoria viruses commonly infect naive children and adolescents, whereas B/Yamagata infections have a bimodal age distribution affecting children and adults aged ~30–64 years [7, 8]. In the United States, since 2000, the B lineage included in trivalent inactivated influenza vaccines (IIV3) was mismatched with the predominant B lineage in circulation during almost half of the seasons, confirming the difficulty of predicting which lineage might predominate [9, 10]. Models estimating the public health impact of quadrivalent inactivated influenza vaccines (IIV4) containing both B lineages suggested the potential for modest reductions in influenza-associated outcomes, assuming no cross-lineage protection for IIV3 [9]. However, limited data were available on cross-lineage immunogenicity and no relative efficacy studies were conducted. Formulations of IIV4 were first distributed in the United States in 2013–2014, and gradually replaced IIV3 in most age groups, leaving a unique, narrow window to fill the evidence gap comparing the vaccine effectiveness (VE) of IIV3 and IIV4 against the 2 B lineages [11, 12]. We were testing the hypothesis that the VE of IIV4 would be better than that of IIV3 against any influenza B illness, because of the additional B-lineage virus included in IIV4. We did not include the B lineage mismatched season of 2017–2018 in this analysis, because 97% of the network participants aged <65 years received IIV4 [13].
Because many countries have continued using IIV3, data comparing the effectiveness of IIV3 and IIV4 are important for informing the choice of the B lineage virus included in IIV3. A recent review found that >40% of 194 World Health Organization member states, most of which are low- and middle-income countries, have no national influenza immunization policy [14]. Despite global evidence for B lineage mismatches with IIV3 [15], national health authorities have diverse recommendations regarding the permissive or preferential use of IIV4 [16]. Herein, we report the effectiveness of IIV3 and IIV4 against medically attended illness due to influenza B and each B lineage separately, including cross-lineage protection for IIV3, using data from the US Influenza VE (Flu VE) Network during 6 seasons, 2011–2012 to 2016–2017, including 4 seasons when both trivalent and quadrivalent vaccines were widely used.
METHODS
Study Population
The US Flu VE Network methods have been reported previously [17–22]. We enrolled eligible participants during the 2011–2012 to 2016–2017 seasons, based on local influenza surveillance at sites in Wisconsin, Washington, Texas, Pennsylvania, and Michigan. Patients aged ≥6 months as of 1 September of each year who presented to ambulatory care with an acute respiratory illness with a cough of ≤7 days duration and who had not received influenza antivirals were eligible (see Supplementary Methods for details) [21].
Influenza Vaccines
B/Brisbane/60/2008 (Victoria) was included in IIV3 in 2010–2011 and 2011–2012, B/Wisconsin/1/2010 (Yamagata) in 2012–2013, B/Massachusetts/2/2012 (Yamagata) from 2013–2014 to 2014–2015, B/Phuket/3073/2013 (Yamagata) in 2015–2016, and B/Brisbane/60/2008 (Victoria) in 2016–2017 (Supplementary Table S1) [23–30]. In addition, IIV4 included B/Brisbane/60/2008 (Victoria) from 2013–2014 to 2015–2016 and B/Phuket/3073/2013 (Yamagata) in 2016–2017.
Influenza Vaccination
To verify receipt of IIV3 or IIV4 ≥14 days before illness onset, we used the vaccine lot number and/or trade name, manufacturer, and route of administration documented in electronic medical records, employee health records, and immunization registries. We excluded participants with an undocumented or indeterminate vaccination status (Supplementary Table S2).
Laboratory Methods
Combined nasal and oropharyngeal swab specimens (nasal swabs only in children aged <2 years) were tested for influenza type A and B virus infections and for B lineage, using real-time, reverse-transcriptase polymerase chain reaction with primers, probes, and standard methods provided by the Centers for Disease Control and Prevention. Antigenic and genetic characterization methods are provided in the Supplementary Methods [30, 31].
Statistical Analyses
Inclusion and exclusion criteria for analyses are provided in the Supplementary Methods and Supplementary Table S2. Unadjusted VE estimates were calculated for influenza B and for each B lineage as [1- OR]*100, where OR is the odds ratio for influenza among vaccinated versus unvaccinated persons from logistic regression models. A priori, estimates were adjusted for age, season, study site, calendar time, and the presence of any high-risk, chronic medical condition, according to the Advisory Committee on Immunization Practices [21, 32]. The addition of other variables did not change the estimates by ≥5%. Age in years was modeled as linear tail-restricted natural cubic spline functions with 5 percentile knots [18]. During each season, the calendar time in weeks was divided into tertiles (pre-peak, peak, and post-peak), based on the numbers of patients enrolled at each site. To account for small sample sizes, adjusted estimates were obtained from Firth’s penalized logistic regression.
We estimated IIV3 effectiveness against any influenza B and each B lineage separately during 6 influenza seasons from 2011–2012 to 2016–2017, and for IIV3 and IIV4 during 4 seasons from 2013–2014 to 2016–2017. Similarly, we used logistic regression to estimate the adjusted relative odds of influenza B and each B-lineage infection, comparing IIV3 to IIV4 during 2013–2014 to 2016–2017. Analyses were also performed after classifying the B lineage included in IIV3 each season as matched or mismatched to the predominant B lineage among circulating viruses (Supplementary Table S1). Sensitivity analyses were performed, and included partially vaccinated children aged <9 years.
RESULTS
Participant Characteristics
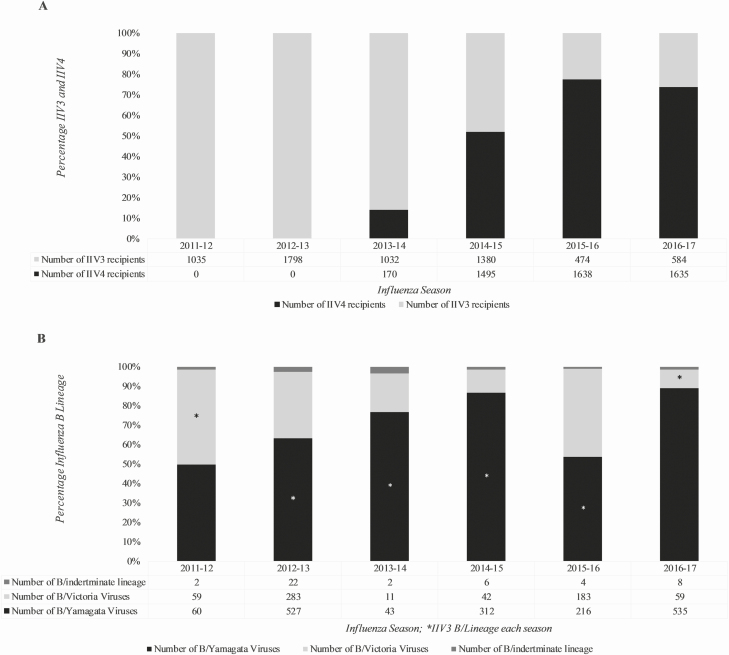
During 6 influenza seasons from 2011–2012 to 2016–2017, we enrolled 42 600 patients seeking ambulatory care for an acute respiratory illness. For estimating VE against any influenza B illness, we excluded 17 581 participants (Supplementary Results and Supplementary Table S2). Of the remaining 25 019 participants (Table 1), 2386 (10%) were reverse-transcriptase polymerase chain reaction–positive for influenza B. After excluding 12 (1%) patients with an influenza A and B virus coinfection, analyses included 2374 cases of influenza B infection, 1693 (71%) of which were B/Yamagata, 637 (27%) B/Victoria, and 44 (2%) infections of undetermined B lineage. There were significant differences between influenza B cases and influenza-negative patients for all reported variables except for race/ethnicity. Almost half of the B cases were children younger than 18 years. Unvaccinated and IIV3/IIV4 recipients also differed significantly for all reported variables. Among those vaccinated, IIV4 use increased from 7% in 2013–2014 to 52% in 2014–2015, and increased to ~75% during the last 2 seasons analyzed (Figure 1A).
Table 1.
Characteristics of United States Influenza Vaccine Effectiveness Network Study Participants, 2011–2012 to 2016–2017 Seasons
| Characteristic | Influenza B Cases, n = 2386 (10%), n (Column%) | Non-cases, n = 22 633 (90%), n (Column%) | IIV3 Vaccinated, n = 6303 (56%), n (Column %) | IIV4 Vaccinated, n = 4938 (44%), n (Column %) | Any IIV Vaccinated, n = 11 241 (45%), n (Column %) | Not Vaccinated, n = 13 778 (55%), n (Column %) |
|---|---|---|---|---|---|---|
| Sex, female | 1234 (52)a | 13 032 (58)a | 3716 (59) | 2975 (60) | 6691 (60)a | 7575 (55)a |
| Age group, years | ||||||
| 6 months to 8 years | 599 (25)a | 5733 (25)a | 1411 (22) | 1414 (29) | 2825 (25)a | 3507 (25)a |
| 9–17 years | 551 (23)a | 2917 (13)a | 557 (9) | 527 (11) | 1084 (10)a | 2384 (17)a |
| 18–49 years | 648 (27)a | 7858 (35)a | 1714 (27) | 1362 (28) | 3076 (27)a | 5430 (39)a |
| 50–64 years | 419 (18)a | 3806 (17)a | 1408 (22) | 959 (19) | 2367 (21)a | 1858 (14)a |
| ≥65 years | 169 (7)a | 2319 (10)a | 1213 (20) | 676 (14) | 1889 (17)a | 599 (4)a |
| Race/ethnicityb | ||||||
| White, non-Hispanic | 1755 (74) | 16 534 (73) | 5024 (80) | 3532 (72) | 8556 (76)a | 9733 (71)a |
| Black, non-Hispanic | 208 (9) | 1885 (8) | 328 (5) | 394 (8) | 726 (6)a | 1371 (10)a |
| Other, non-Hispanic | 210 (9) | 2102 (9) | 537 (9) | 522 (11) | 1059 (9)a | 1253 (9)a |
| Hispanic, race | 207 (9) | 2052 (9) | 401 (6) | 480 (10) | 881 (8)a | 1378 (10)a |
| High-risk health conditionc | 720 (30)a | 8593 (38)a | 2947 (47) | 2515 (51) | 5462 (49)a | 3851 (28)a |
| Self-rated general health statusd | ||||||
| Excellent | 971 (41)a | 7311 (32)a | 1803 (29) | 1541 (31) | 3344 (30)a | 4938 (36)a |
| Very good | 861 (36)a | 8228 (36)a | 2246 (36) | 1798 (36) | 4044 (36)a | 5045 (37)a |
| Good | 436 (18)a | 5445 (24)a | 1695 (27) | 1203 (24) | 2898 (26)a | 2983 (22)a |
| Fair | 104 (4)a | 1444 (6)a | 485 (8) | 337 (7) | 822 (7)a | 726 (5)a |
| Poor | 13 (1)a | 186 (1)a | 70 (1) | 55 (1) | 125 (1)a | 74 (1)a |
| Illness onset to enrollment | ||||||
| ≤2 days | 800 (34)a | 6771 (30)a | 1784 (28) | 1462 (30) | 3246 (29)a | 4325 (31)a |
| 3–4 days | 1058 (44)a | 8871 (39)a | 2484 (39) | 1920 (39) | 4404 (39)a | 5525 (40)a |
| 5–7 days | 528 (22)a | 6691 (31)a | 2035 (32) | 1556 (32) | 3591 (32)a | 3928 (29)a |
| Enrollment season | ||||||
| 2011–2012 | 121 (5)a | 2230 (10)a | 1035 (16) | 0 (0) | 1035 (9)a | 1316 (10)a |
| 2012–2013 | 832 (35)a | 3308 (15)a | 1798 (28) | 0 (0) | 1798 (16)a | 2342 (17)a |
| 2013–2014 | 56 (2)a | 2543 (11)a | 1032 (16) | 170 (3) | 1202 (11)a | 1397 (10)a |
| 2014–2015 | 362 (15)a | 5765 (26)a | 1380 (22) | 1495 (30) | 2875 (26)a | 3252 (24)a |
| 2015–2016 | 408 (17)a | 4445 (20)a | 474 (8) | 1638 (33) | 2112 (19)a | 2741 (20)a |
| 2016–2017 | 607 (25)a | 4342 (19)a | 584 (9) | 1635 (33) | 2219 (20)a | 2730 (20)a |
| Study site | ||||||
| Washington | 397 (17)a | 6394 (28)a | 2179 (35) | 1372 (28) | 3551 (32)a | 3240 (24)a |
| Wisconsin | 750 (31)a | 4054 (18)a | 2088 (33) | 201 (4) | 2289 (20)a | 2515 (18)a |
| Texas | 451 (19)a | 4359 (19)a | 552 (9) | 1222 (25) | 1774 (16)a | 3036 (22)a |
| Michigan | 431 (18)a | 3816 (17)a | 761 (12) | 1181 (24) | 1942 (17)a | 2305 (17)a |
| Pennsylvania | 357 (15)a | 4010 (18)a | 723 (12) | 962 (20) | 1685 (15)a | 2682 (20)a |
| Calendar-week tertilese | ||||||
| First tertile | 444 (19)a | 8018 (35)a | 2137 (34) | 1626 (33) | 3763 (34)a | 4699 (34)a |
| Second tertile | 857 (36)a | 7671 (34)a | 2084 (33) | 1683 (34) | 3767 (34)a | 4746 (35)a |
| Third tertile | 1085 (46)a | 6959 (31)a | 2082 (33) | 1629 (33) | 3711 (33)a | 4333 (32)a |
| Influenza B or B lineage cases | ||||||
| Influenza B cases | 2386 (100%) | … | 407 (7) | 244 (5) | 651 (6) | 1735 (13) |
| B/Yamagata | 1693 (71) | … | … | … | … | … |
| B/Victoria | 637 (27) | … | … | … | … | … |
| Undetermined | 44 (2) | … | … | … | … | … |
| Coinfectionsf | 12 (1) | … | … | … | … | … |
| Vaccination status | ||||||
| Any IIV | 651 (27)a | 10 590 (47)a | … | … | … | … |
| Unvaccinated | 1735 (73)a | 12 043 (53)a | … | … | … | … |
| Trivalent IIV3 | 407 (17)a | 5896 (26)a | … | … | … | … |
| Quadrivalent IIV4 | 244 (10)a | 4694 (21)a | … | … | … | … |
| Unvaccinated | 1735 (73)a | 12 043 (53)a | … | … | … | … |
n = 25 019.
Abbreviations: IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine.
a P value <.05 for comparisons; IIV3 and IIV4 recipients are not compared.
bSelf-reported race/ethnicity was missing in 66 participants, including 13 IIV3 and 10 IIV4 recipients.
cThe presence of a high-risk health condition is defined as ≥ 1 medical record–documented International Classification of Disease code from October 1 in the prior season to enrollment, as defined by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
dSelf-rated general health status was missing in 20 participants, including 4 each of IIV3 and IIV4 recipients.
eCalendar-week pre-peak and peak influenza activity and post-peak tertiles, per season per site, based on the enrollment week for B cases and non-cases.
fCoinfections of influenza types A and B or B/lineage coinfections.
Figure 1.
Influenza vaccine distribution and B lineage virus circulation in the US Influenza Vaccine Effectiveness (Flu VE) Network. A, Distribution of IIV3 and IIV4 during the 2011–2012 to 2016–17 seasons. B, Influenza B lineage circulation in the US Flu VE network during the 2011–2012 to 2016–2017 seasons. Abbreviations: IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine.
Influenza Surveillance in the United States During 2011–2012 to 2016–2017
Influenza B viruses comprised ~30% of all influenza cases during 2012–2013, 2015–2016, and 2016–2017, and ~15% during the remaining seasons (Supplementary Table S1). Although both B lineages cocirculated during the period, B/Yamagata viruses predominated in all 6 seasons. For IIV3 effectiveness analyses, 4 seasons (2012–2013 to 2015–2016) when IIV3 included B/Yamagata were considered lineage-matched, but there was an intra-lineage B/Yamagata clade mismatch during the 2014–15 season (see “Antigenic and Genetic Characterization of B Viruses” in Supplementary Results and Supplementary Tables S3 and S4). Each of the 2 seasons when IIV3 included B/Victoria viruses 2011–2012 and 2016–2017 were lineage-mismatched.
Influenza B and B Lineage Virus Activity in the United States Influenza Vaccine Effectiveness Network
Overall, during the 6 seasons from 2011–2012 to 2016–2017, influenza B viruses accounted for ~25% of all influenza cases. The US Flu VE Network enrolled more than 500 cases of influenza B in 2 seasons (2012–2013 and 2016–2017), between 100 and 400 cases in 3 seasons, and <60 cases during 2013–2014 (Figure 1B). Both B lineages circulated each season. During the 6 seasons, 1114 (48%) of the 2330 B cases with a known B lineage were due to the B lineage not contained in IIV3, including a majority (89%) of all B cases in 2016–17 and one-third to one-half of the cases during 3 seasons (2011–2012, 2012–2013, and 2015–2016; Figure 1B). Of 1693 B/Yamagata cases enrolled during the period, 595 (35%) occurred when B/Victoria was the IIV3 B lineage. Of 637 B/Victoria cases, 519 (82%) occurred when B/Yamagata was the IIV3 B lineage.
Vaccine Effectiveness Against Any Influenza B and Each B Lineage Infection
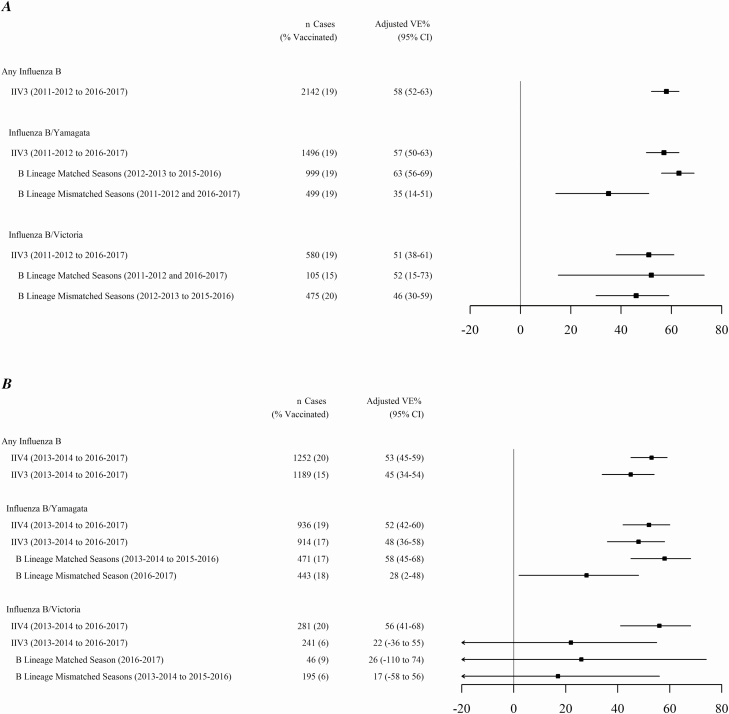
During 6 influenza seasons, the overall IIV3 effectiveness against any influenza B illness was 58% (95% confidence interval [CI], 52–63; Figure 2A). During 4 seasons with widespread use of both IIV3 and IIV4, the overall IIV3 effectiveness against influenza B was 45% (95% CI, 34–54), versus 53% (95% CI, 45–59) for IIV4 (Table 2; Figure 2B). The effectiveness against B/Yamagata was similar for IIV3 (48%; 95% CI, 37–58) and for IIV4 (52%; 95% CI, 43–60). However, during 3 seasons (2013–2014 to 2015–2016) when IIV3 included a B/Yamagata virus, IIV3 effectiveness against B/Yamagata was 58% (95% CI, 45–68), versus 28% (95% CI, 2–48) when IIV3 included B/Victoria (P < .001; Figure 2B). Against B/Victoria, during 4 seasons the IIV3 effectiveness was 22% (95% CI, −36 to 55) and the IIV4 effectiveness was 56% (95% CI, 41–68; Figure 2B). The IIV3 effectiveness against B/Victoria was not significantly different whether IIV3 included a B/Victoria or B/Yamagata virus (Figure 2B), although analyses were limited by the small numbers of B/Victoria cases. Age-specific VE estimates against any influenza B and each B lineage were similar for IIV3 and IIV4 for persons aged 6 months to 17 years, 18 to 49 years, and 50 years and older (Table 2).
Figure 2.
Adjusted VE estimates of trivalent and quadrivalent formulations against any influenza B and each B lineage in the US Influenza Vaccine Effectiveness Network. A, Adjusted VE % against influenza B and each B lineage, including the B lineage virus included in IIV3 during 6 seasons from 2011–2012 to 2016–2017. B, Adjusted VE % against any influenza B and each B lineage, including the B lineage virus included in IIV3 and IIV4 during 4 seasons when both IIV3 and IIV4 were used (2013–2014 to 2016–2017). A priori, VE estimates were simple adjusted for age, season, study site, calendar week (pre-peak and peak influenza activity and post-peak tertiles, per site per season), and the presence of any high-risk, chronic medical condition. Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; VE, vaccine effectiveness.
Table 2.
Trivalent and Quadrivalent Inactivated Influenza Vaccine Effectiveness Against Any Influenza B
| IIV3 or Unvaccinated | IIV4 or Unvaccinated | |||||
|---|---|---|---|---|---|---|
| Non-cases, n Vaccinated/Total (%) | Influenza B Cases, n Vaccinated/Total (%) | Adjusteda VE % (95% CI) | Non-cases, n Vaccinated/Total (%) | Influenza B Cases, n Vaccinated / Total (%) | Adjusted VE % (95% CI) | |
| Any Influenza B, ages | ||||||
| ≧6 months | 3289/12 401 (27) | 183/1189 (15) | 45 (34–54) | 4694/13 806 (34) | 244/1252 (20) | 53 (45–59) |
| 6 months to 17 years | 902/4620 (20) | 39/523 (7) | 58 (40–71) | 1844/5562 (33) | 97/581 (17) | 55 (43–65) |
| 18–49 years | 885/4620 (19) | 39/348 (11) | 45 (20–62) | 1307/5042 (26) | 55/364 (15) | 54 (37–66) |
| ≥50 years | 1502/3161 (48) | 103/318 (32) | 43 (24–57) | 1543/3202 (48) | 92/307 (30) | 42 (24–56) |
| B/Yamagata, ages | ||||||
| ≧6 months | 2706/10 959 (25) | 159/914 (17) | 48 (36–58) | 4605/12 858 (36) | 181/936 (19) | 52 (42–60) |
| 6 months to 17 years | 685/4069 (17) | 32/356 (9) | 63 (44–75) | 1802/5186 (35) | 61/385 (16) | 54 (37–66) |
| 18–49 years | 748/4106 (18) | 35/269 (13) | 41 (12–61) | 1292/4650 (28) | 39/273 (14) | 56 (38–70) |
| ≥50 years | 1273/2784 (46) | 92/289 (32) | 44 (24–59) | 1511/3022 (50) | 81/278 (29) | 45 (26–59) |
| B/Victoria, ages | ||||||
| ≧6 months | 2706/10 959 (25) | 15/241 (6) | 22 (−36 to 55) | 4605/12 858 (36) | 55/281 (20) | 56 (41–68) |
| 6 months to 17 years | 685/4069 (17) | 6/156 (4) | 17 (−94 to 64) | 1802/5186 (35) | 34/184 (19) | 57 (36–71) |
| 18–49 years | 748/4106 (18) | 4/68 (6) | 36 (−66 to 76) | 1292/4650 (28) | 13/77 (17) | 54 (18–75) |
| ≥50 years | 1273/2784 (46) | 5/17 (29) | 5 (−167 to 66) | 1511/3022 (50) | 8/20 (40) | 41 (−34 to 74) |
| B Lineage included in IIV3, ages | ||||||
| ≧6 months | 2706/10 959 (25) | 84/517 (16) | 56 (43–67) | 4605/12 858 (36) | 100/533 (19) | 53 (40–63) |
| 6 months to 17 years | 685/4069 (17) | 11/191 (6) | 73 (50–86) | 1802/5186 (35) | 34/214 (16) | 51 (27–67) |
| 18–49 years | 748/4106 (18) | 18/171 (11) | 61 (35–77) | 1292/4650 (28) | 18/171 (11) | 69 (49–81) |
| ≥50 years | 1273/2784 (46) | 55/155 (35) | 46 (20–63) | 1511/3022 (50) | 48/148 (32) | 41 (13–60) |
| B Lineage not included in IIV3, ages | ||||||
| ≧6 months | 2706/10 959 (25) | 90/638 (14) | 25 (1–43) | 4605/12 858 (36) | 136/684 (20) | 56 (46–64) |
| 6 months to 17 years | 685/4069 (17) | 27/321 (8) | 38 (2–61) | 1802/5186 (35) | 61/355 (17) | 58 (43–69) |
| 18–49 years | 748/4106 (18) | 21/166 (13) | −2 (−71 to 39) | 1292/4650 (28) | 34/179 (19) | 46 (21–63) |
| ≥50 years | 1273/2784 (46) | 42/151 (28) | 47 (14–67) | 1511/3022 (50) | 41/150 (27) | 45 (17–63) |
| Sensitivity analyses including partially vaccinated children from 2013–2014 to 2016–2017 | ||||||
| B Lineage included in IIV3, ages | ||||||
| ≧6 months | 2803/11 056 (25) | 84/517 (16) | 57 (44–67) | 4920/13 173 (37) | 103/536 (19) | 53 (40–63) |
| 6 months to 17 years | 782/4166 (19) | 11/191 (6) | 74 (52–86) | 2117/5501 (39) | 37/217 (17) | 51 (29–67) |
| B Lineage not included in IIV3, ages | ||||||
| ≧6 months | 2853/11 056 (25) | 90/638 (14) | 26 (2–43) | 4920/13 173 (37) | 145/693 (21) | 56 (46–64) |
| 6 months to 17 years | 782/4166 (19) | 27/321 (8) | 40 (5–62) | 2117/5501 (38) | 70/364 (19) | 57 (43–68) |
Data are shown by each B virus lineage and by B lineage included or not included in IIV3 during 4 influenza seasons from 2013–2014 to 2016–2017.
Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; VE, vaccine effectiveness.
a A priori VE estimates were simple adjusted for age, season, study site, calendar week (pre-peak and peak influenza activity and post-peak tertiles, per site per season), and the presence of any high-risk, chronic medical condition.
Overall and age group–specific IIV3 and IIV4 effectiveness against any influenza B and B lineage included in IIV3 were similar (Table 2). For IIV3 B-lineage mismatched viruses, the IIV3 effectiveness (25%; 95% CI, 1–43) was lower than that of IIV4 (56%; 95% CI, 46–64; P < .001), with the greatest difference observed among adults 18–49 years old.
Relative Odds of Influenza B Among Trivalent and Quadrivalent Inactivated Vaccine Recipients
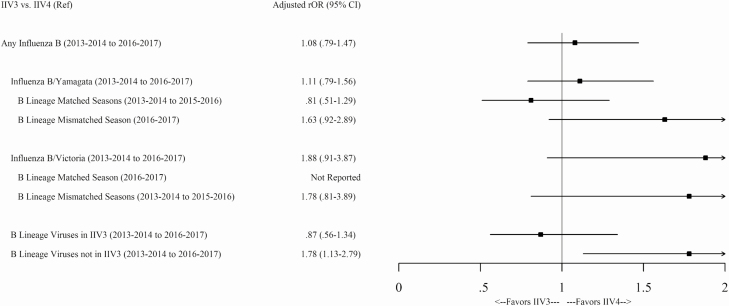
During 2013–2014 to 2016–2017, the odds of any influenza B infection were similar among IIV3 versus IIV4 recipients aged ≥6 months (adjusted relative odds ratio [rOR], 1.08; 95% CI, .79–1.47), including the odds of infection with B/Yamagata (rOR, 1.11; 95% CI, .79–1.56) or B/Victoria viruses (rOR, 1.88; 95% CI, .91–3.87; Figure 3). The relative odds of a B/Yamagata infection were similar in IIV3 versus IIV4 recipients, regardless of the IIV3 B-lineage match. Similarly, the relative odds of a B/Victoria infection were not significantly different in IIV3 versus IIV4 recipients during 3 seasons in which IIV3 contained a B/Yamagata virus. The relative odds of infection with the B lineage that was included in IIV3 during the four seasons indicated no difference between IIV3 versus IIV4 (rOR, 0.87; 95% CI, .56–1.34). However, IIV3 recipients had significantly higher odds of infection with mismatched B-lineage viruses compared to patients who received IIV4 (rOR, 1.78; 95% CI, 1.13–2.79; Figure 3).
Figure 3.
Adjusted rOR of IIV3 versus IIV4 (reference) during 4 seasons from 2013–2014 to 2016–2017 of any influenza B; of each B lineage virus infection, including B/Yamagata– or B/Victoria–containing IIV3 seasons; and of the B lineage virus included in IIV3 versus lineage-mismatched IIV3. A priori, rOR estimates were simple adjusted for age, season, site, calendar week (pre-peak and peak influenza activity and post-peak tertiles, per site per season) and the presence of any high-risk, chronic medical condition. Data were not reported when the number of vaccinated cases was <5. Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; rOR, relative odds ratios.
During 2016–2017, when IIV3 included a B/Victoria virus, the relative odds of a B/Yamagata infection showed no statistically significant difference among IIV3- and IIV4-vaccinated children aged 6 months to 17 years (rOR, 1.68; 95% CI, .63–4.49; Supplementary Table S5). However, the relative odds of a B/Yamagata infection were higher among adults aged 18 to 49 years who were vaccinated with IIV3, compared to those who received IIV4 (rOR, 3.43; 95% CI, 1.07–11.00; Supplementary Table S5). During the 4 seasons, the odds of infection with the mismatched B-lineage virus not included in IIV3 were higher in all patients aged ≥6 months (rOR, 1.78; 95% CI, 1.13–2.79); however, the odds were not significantly higher in age group–specific estimates, including among children aged 6 months to 17 years (rOR, 1.99; 95% CI, .91–4.36; Supplementary Table S5).
Sensitivity Analyses
Including partially vaccinated children did not change VE point estimates by ≥5%, either for any influenza B or for each B lineage, nor did it change IIV3 effectiveness against infection with mismatched lineage viruses (Table 2; Supplementary Table S6).
DISCUSSION
Over 6 influenza seasons during 2011–2012 to 2016–2017, including 4 seasons (beginning with 2013–2014) during which quadrivalent vaccines gradually replaced trivalent vaccines, data from this large, observational study of VE suggested that trivalent and quadrivalent inactivated vaccines provided comparable protection against influenza B and each B lineage. This is the first US Flu VE Network analysis to compare IIV4 to IIV3 VE against influenza B–related illness over multiple influenza seasons [17–22]. The results suggest that IIV3 containing a B/Yamagata virus provided some cross-protection against B/Victoria illness. During the 4 seasons from 2013–2014 to 2016–2017, the relative odds of infection with influenza B favored IIV4 when IIV3 was lineage mismatched (ie, when the predominant B lineage was not included in IIV3). However, we found the relative odds of any influenza B to be the same among persons vaccinated with IIV3 or IIV4 over the 4 seasons. A recent report from Canada from the 2010–2011 to 2017–2018 seasons showed greater than 50% effectiveness for predominantly used IIV3 during 3 of 5 lineage-mismatched seasons, including 2 seasons with greater than 65% effectiveness against lineage-mismatched influenza B illnesses [33].
During 4 seasons in which IIV3 included a B/Yamagata virus, the trivalent VE was 63% against B/Yamagata and 46% against B/Victoria. However, we observed lower protection (35%) against B/Yamagata illness during 2 lineage-mismatched seasons. The comparative effectiveness of IIV3 versus IIV4 over 4 seasons from the US Flu VE Network adds to reports of VE against influenza B illness from European and Canadian VE networks during the same period. However, all 3 networks were able to report effectiveness against influenza B only once during 2014–2015 [17–22, 34–45]. In those reports, the VE against influenza B ranged from 53% to 58% in the United States, except during 2013–2014, when B virus circulation was limited.
There is some evidence for effectiveness of trivalent vaccine against lineage-matched versus mismatched B infections. During 4 seasons during which IIV3 included a B/Yamagata virus (2012–2013 to 2015–2016), the effectiveness of any vaccination against B/Yamagata illness in the United States and Canada ranged from 42% to 73% [19–22, 34–38]. During 2011–2012, when IIV3 was lineage-mismatched to the predominant B/Yamagata virus, a trivalent VE of 66% (38–81%) against B/Yamagata was reported in the United States but not in Canada [17, 34]. Similarly, during the 2012–2013 season, trivalent VE estimates ranging from 51–75% against the lineage-mismatched B/Victoria virus were reported in the United States and Canada, and the effectiveness against the mismatched B/Victoria virus was 54% during 2015–2016 in Canada, where trivalent vaccines accounted for more than 85% of the doses distributed [18, 35, 38]. In the United States during 2015–2016, IIV3 containing a B/Yamagata virus accounted for approximately 20% of the doses distributed, versus 80% for IIV4. In the US Flu VE Network during this season, in which B/Victoria viruses accounted for 45% of influenza B cases, the trivalent VE against any influenza B was only 17%: 41% against B/Yamagata and −99% against B/Victoria [21]. In comparison, the quadrivalent VE was 63% against any influenza B: 66% against B/Yamagata and 57% against B/Victoria.
Even though both B lineages circulated during each season since 2011–2012, B/Yamagata evolved more rapidly, requiring newer strains to be incorporated frequently in seasonal vaccines. Quadrivalent vaccines did not outperform trivalent vaccines during seasons when B/Yamagata viruses predominated. The relative odds tended to favor IIV4 against B/Victoria during season with a IIV3 B-lineage mismatch. However, from the 2013–2014 through the 2016–2017 season, the B/Victoria viruses were not predominant and trivalent vaccine use declined substantially.
To our knowledge, this is the first direct comparison of the odds of an influenza B infection among persons vaccinated with trivalent or quadrivalent vaccines, including among children aged 6 months to 17 years. We found that since 2013–2014, the relative odds of any influenza B infection have been similar among persons vaccinated with IIV3 versus IIV4, although small numbers of B/Victoria cases limited our ability to estimate the relative odds of a B/Victoria infection during 2016–2017, when trivalent vaccines included a B/Victoria virus. Our ability to add additional years, including the trivalent lineage-mismatched 2017–2018 season, is limited by the declining use of IIV3.
The potential for cross-lineage protection needs to be considered during the selection of the B lineage for inclusion in trivalent vaccines each season. A possible mechanism for cross-lineage protection may be the neuraminidase (NA) of the B-lineage virus included in IIV3. The circulation of reassortants with B/Victoria hemagglutinin and B/Yamagata NA was reported during the early 2000s; this reassortment is a possible mechanism for cross-lineage protection against B/Yamagata infection from those trivalent vaccines including a B/Victoria virus [3, 46–48]. An experimental study showed that mice immunized with adjuvanted recombinant influenza B virus NA (from B/Yamagata/16/88) were completely protected from morbidity and mortality when challenged with a lethal dose of the prototype B/Victoria-lineage strain or recently circulating B/Victoria-lineage virus [49]. However, the amount of NA in IIV3 or IIV4 is not standardized, may vary with different vaccine products, and is absent in the recombinant hemagglutinin–containing influenza vaccine. Public health impact and cost-effectiveness modeling studies that assume no cross-lineage protection may overestimate the benefit of switching from trivalent to quadrivalent inactivated influenza vaccines [9, 50, 51].
Prior influenza B infection or vaccination may be another factor influencing VE against B lineage viruses. Immunogenicity studies in adults, children, and mice have suggested B/Yamagata dominance over B/Victoria for priming and recall [52–54]. Priming with a B/Yamagata-containing vaccine induces a good homologous response and a boost with a B/Victoria-containing vaccine induces strong recall against B/Yamagata but a low B/Victoria response. On the other hand, priming with a B/Victoria-containing vaccine has a low homologous response but a boost with a B/Yamagata-containing vaccine is associated with a response to both lineages.
This study has several limitations. First, we were unable to assess any bias in the use of trivalent versus quadrivalent vaccines during the study period. Second, the sample size was inadequate both to estimate VE in possibly naive children aged <9 years and for subgroup analyses, especially for estimating effectiveness against B/Victoria in those season where it had lower circulation than B/Yamagata viruses. Third, we did not examine the possible waning of VE against influenza B that may circulate later during influenza seasons, including during mismatched seasons. Lastly, residual confounding is possible despite adjustments for age and other factors. Other influenza VE networks transitioning from trivalent to quadrivalent vaccines could contribute data so that future meta-analyses may be possible.
CONCLUSIONS
Equivalent protection against any influenza B illness was provided by trivalent and quadrivalent influenza vaccines from 2013–2014 to 2016–2017 in the United States, even though quadrivalent vaccines afforded better relative protection against the trivalent vaccine’s mismatched B lineage viruses. Enhanced influenza surveillance to understand the epidemiology of B-lineage circulation, including the burden of illness with each B lineage, should help national health authorities to make informed decisions regarding recommendations of trivalent versus quadrivalent influenza vaccines, as should cost-effectiveness estimates for trivalent versus quadrivalent vaccine distributions in different age groups.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Study group. The US Influenza Vaccine Effectiveness investigators at Baylor Scott and White Health are Lydia Clipper, Michael Smith, Anne Robertson, Ashley Kossie, Vanessa Hoelscher, Kimberley Walker, Marcus Volz, Arundhati Rao, Robert Fader, and Yolanda Munoz-Maldonado; at the Baylor College of Medicine, Houston, are Pedro Piedra, W. Paul Glezen, and Vasanthi Avadhanula; at the Marshfield Clinic Research Institute are Lynn C. Ivacic, Jennifer P. King, Jennifer K. Meece, Madalyn M. Palmquist, and Sherri A. Guzinski; at the Kaiser Permanente Washington Health Research Institute are C. Hallie Phillips, Stacie Wellwood, Erika Kiniry, Lawrence Madziwa, and Matt Nguyen; at the University of Michigan School of Public Health are Joshua G. Petrie, Ryan E. Malosh, E. J. McSpadden, Hannah E. Segaloff, Caroline K. Cheng, Rachel Truscon, and Emileigh Johnson; at the Henry Ford Health System is Lois E. Lamerato; at the University of Pittsburgh and University of Pittsburgh Medical Center (UPMC) are G. K. Balasubramani, Todd M. Bear, Heather Eng, Robert Hickey, Donald B. Middleton, Krissy K. Moehling, Jonathan M. Raviotta, Evelyn C. Reis, Edmund M. Ricci, Charles Rinaldo Jr, Theresa Sax, Michael Susick, Joe Suyama, and John V. Williams; and the Centers for Disease Control and Prevention are LaShondra Berman, Angie Foust, Wendy Sessions, Juliana DaSilva, Thomas Stark, and John Barnes.
Acknowledgements. The authors thank the team at Baylor Scott and White Health, including Juhee Song, Jessica Pruszynski, Archana Nangrani, Joann Nichols, Crystal Hodges, Teresa Ponder, Ineshia Jackson, Deborah Furze, Gabriela Gonzales, Martha Zayed, Melissa Zdroik, Kevin Dunlap, Iosefo Iosefo, Chooihoong Choo, Mary Kylberg, Lea Mallett, Hania Wehbe-Janek, Madhava Beeram, Natalie Settele, Jennifer Thomas, Jaime Walkowiak, Adelfa Alcozer, Evangeline Knight, Jeremy Ray, Renee Day, Deborah Price, Jennifer Fox, Robert Probe, and Alejandro Arroliga; the team at the Marshfield Clinic Research Institute, including Elizabeth Armagost, Deanna Cole, Terry J. Foss, Klevi Hoxha, Dyan Friemoth, Katherine Graebel-Khandakani, Linda Heeren, Tami Johnson, Tara Johnson, Nicole Kaiser, Diane Kohnhorst, Sarah Kopitzke, Ariel Marcoe, Karen McGreevey, Madalyn Minervini, Vicki Moon, Suellyn Murray, Jillette Peterson, Rebecca Pilsner, DeeAnn Polacek, Emily Redmond, Miriah Rotar, Carla Rottscheit, Samantha Smith, Jackie Salzwedel, Sandra Strey, Tammy Koepel, Nan Pan, Annie Steinmetz, Gregg Greenwald, Jane Wesely, Jennifer Anderson, and Laurel Verhagen; the Center for Clinical Epidemiology and Population Health Staff; the Integrated Research & Development Laboratory Staff; the team at the Kaiser Permanente Washington Health Research Institute, including C. Hallie Phillips, Stacie Wellwood, Lawrence Madziwa, Matthew Nguyen, Erika Kiniry, Suzie Park, and Julia Anderson; the team at the University of Pittsburgh and UPMC, including Rose Azrak, Arlene Bullotta, Jonathan Steele, Donald S. Burke, Samantha Ford, Edward Garafolo, Philip Iozzi, Monika Johnson, Sean Saul, Leonard Urbanski, Stephen Wisniewski, and Bret Rosenblum; the team at the University of Michigan School of Public Health, including Richard Evans, Anne Kaniclides, Joey Lundgren, Erika Chick, Lindsey Benisatto, Tosca Le, and Dexter Hobdy; the team at the Henry Ford Health System, including Heather R. Lipkovich, Nishat Islam, Michelle Groesbeck, Shirley Zhang, Andrea Lee, Kristyn Brundidge, Christina Rincon, Stephanie Haralson, Jennifer Hessen, and Ahn Trinh; and the team at the Centers for Disease Control and Prevention, including LaShondra Berman, Angie Foust, Steve Lindstrom, Wendy Sessions, Swathi Thaker, Xiyan Xu, Erin Burns, Jackie Katz, Daniel Jernigan, Dave Wentworth, Mark Thompson, and Jerome Tokars.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This project was supported by the Centers for Disease Control and Prevention (CDC) through cooperative agreements with Baylor Scott and White Health (grant numbers 1–5U01 IP000473 and 1U01 IP001039), the Marshfield Clinic Research Institute (grant numbers 1–5U01 IP000471 and 1U01 IP001038), the Kaiser Permanente Washington Research Institute (grant numbers 1–5U01 IP000466 and 1U01 IP001037), the University of Pittsburgh (grant numbers 1–5U01 IP000467 and 1U01 IP001035) and the University of Michigan (grant numbers 1–5U01 IP000474and 1U01 IP001034). CDC staff assisted in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; and the decision to submit for publication. In addition, infrastructure at the University of Pittsburgh was supported by the National Institutes of Health (grant number UL1 TR001857).
Potential conflicts of interest. M. G., E. A. B., H. Q. M., M. L. J., L. A. J., R. K. Z., M. P. N., E. T. M., and A. S. M. received grants from the CDC, during the conduct of the study. M. G. has received grants from the CDC, CDC subcontract through Abt Associates, and MedImmune/Astra Zeneca, outside the submitted work. H. Q. M. has received grants from Seqirus, outside the submitted work. M. L. J. has received grants from Sanofi Pasteur, outside the submitted work. L. A. J. has received grants from Pfizer, outside the submitted work. R. K. Z. has received grants from Sanofi Pasteur, Pfizer Inc, and Merck & Co, outside the submitted work. M. P. N. has received grants from Merck & Co., outside the submitted work. E. T. M. has received personal fees from Pfizer and grants from Merck, outside the submitted work. A. S. M. has received personal fees from Sanofi Pasteur and Seqirus, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health 2013; 103:e43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175:59–68. [DOI] [PubMed] [Google Scholar]
- 3. Shaw MW, Xu X, Li Y, et al. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000-2001 and 2001-2002 seasons. Virology 2002; 303:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Hampson A, Barr I, Cox N, et al. Improving the selection and development of influenza vaccine viruses - report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China, 18-20 November 2015. Vaccine 2017; 35:1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003 [published correction appears in J Virol 2005; 79:5886]. J Virol 2004; 78:12817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCullers JA, Wang GC, He S, Webster RG. Reassortment and insertion-deletion are strategies for the evolution of influenza B viruses in nature. J Virol 1999; 73:7343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vijaykrishna D, Holmes EC, Joseph U, et al. The contrasting phylodynamics of human influenza B viruses. Elife 2015; 4:e05055. doi: 10.7554/eLife.05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skowronski DM, Chambers C, De Serres G, et al. Age-related differences in influenza B infection by lineage in a community-based sentinel system, 2010-2011 to 2015-2016, Canada. J Infect Dis 2017; 216:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–8. [DOI] [PubMed] [Google Scholar]
- 10. Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999-2012. Clin Infect Dis 2014; 59:1519–24. [DOI] [PubMed] [Google Scholar]
- 11. Camilloni B, Neri M, Lepri E, Iorio AM. Cross-reactive antibodies in middle-aged and elderly volunteers after MF59-adjuvanted subunit trivalent influenza vaccine against B viruses of the B/Victoria or B/Yamagata lineages. Vaccine 2009; 27:4099–103. [DOI] [PubMed] [Google Scholar]
- 12. Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010; 28:2149–56. [DOI] [PubMed] [Google Scholar]
- 13. Rolfes MA, Flannery B, Chung JR, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. doi: 10.1093/cid/ciz075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ortiz JR, Perut M, Dumolard L, et al. A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine 2016; 34:5400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jennings L, Huang QS, Barr I, et al. Literature review of the epidemiology of influenza B disease in 15 countries in the Asia-Pacific region. Influenza Other Respir Viruses 2018; 12:383–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ray R, Dos Santos G, Buck PO, et al. A review of the value of quadrivalent influenza vaccines and their potential contribution to influenza control. Hum Vaccin Immunother 2017; 13:1640–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaglani M, Pruszynski J, Murthy K, et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013-2014 in the United States. J Infect Dis 2016; 213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zimmerman RK, Nowalk MP, Chung J, et al. 2014-2015 Influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016; 63:1564–73. doi: 10.1093/cid/ciw635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015-2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flannery B, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in the United States during the 2016-2017 season. Clin Infect Dis 2019; 68:1798–806. doi: 10.1093/cid/ciy775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep 2011; 60:705–12. [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Update: influenza activity—United States, 2011–12 season and composition of the 2012–13 influenza vaccine. MMWR Morb Mortal Wkly Rep 2012; 61:414–20. [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Influenza activity—United States, 2012–13 season and composition of the 2013–14 influenza vaccine. MMWR Morb Mortal Wkly Rep 2013; 62:473– 9. [PMC free article] [PubMed] [Google Scholar]
- 26. Epperson S, Blanton L, Kniss K, et al. ; Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention . Influenza activity - United States, 2013-14 season and composition of the 2014-15 influenza vaccines. MMWR Morb Mortal Wkly Rep 2014; 63:483–90. [PMC free article] [PubMed] [Google Scholar]
- 27. Appiah GD, Blanton L, D’Mello T, et al. ; Centers for Disease Control and Prevention . Influenza activity—United States, 2014–15 season and composition of the 2015–16 influenza vaccine. MMWR Morb Mortal Wkly Rep 2015; 64:583–90. [PMC free article] [PubMed] [Google Scholar]
- 28. Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–75. doi: 10.15585/mmwr.mm6522a3. [DOI] [PubMed] [Google Scholar]
- 29. Blanton L, Alabi N, Mustaquim D, et al. Update: influenza activity in the United States during the 2016–17 season and composition of the 2017–18 influenza vaccine. MMWR Morb Mortal Wkly Rep 2017; 66:668–76. doi: 10.15585/mmwr.mm6625a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garten R, Blanton L, Elal AIA, et al. Update: influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou B, Lin X, Wang W, et al. Universal influenza B virus genomic amplification facilitates sequencing, diagnostics, and reverse genetics. J Clin Microbiol 2014; 52:1330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 influenza season. MMWR Recomm Rep 2018; 67:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skowronski DM, Chambers C, De Serres G, et al. Vaccine effectiveness against lineage-matched and -mismatched influenza B viruses across 8 seasons in Canada, 2010-2011 to 2017-2018. Clin Infect Dis 2019; 68:1754–7. doi: 10.1093/cid/ciy876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011-2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 35. Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLOS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skowronski DM, Chambers C, Sabaiduc S, et al. Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013-2014 influenza season. J Infect Dis 2015; 212:726–39. [DOI] [PubMed] [Google Scholar]
- 37. Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014-2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skowronski DM, Chambers C, Sabaiduc S, et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015-2016 season in Canada. J Infect Dis 2017; 216:1487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skowronski DM, Chambers C, Sabaiduc S, et al. Interim estimates of 2016/17 vaccine effectiveness against influenza A(H3N2), Canada, January 2017. Euro Surveill 2017; 22:1–8. pii: 30460. doi: 10.2807/1560-7917.ES.2017.22.6.30460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kissling E, Valenciano M, Larrauri A, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro Surveill 2013; 18(5):1–10. pii.20390. doi: 10.2807/ese.18.05.20390-en [DOI] [PubMed] [Google Scholar]
- 41. Kissling E, Valenciano M, Buchholz U, et al. Influenza vaccine effectiveness estimates in Europe in a season with three influenza type/subtypes circulating: the I-MOVE multicentre case-control study, influenza season 2012/13. Euro Surveill 2014; 19:1–12. pii:20701. doi: 10.2807/1560-7917.es2014.19.6.20701 [DOI] [PubMed] [Google Scholar]
- 42. Valenciano M, Kissling E, Reuss A, et al. The European I-MOVE multicentre 2013-2014 case-control study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogenous results by country against A(H3N2). Vaccine 2015; 33:2813–22. doi: 10.1016/j.vaccine.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 43. Valenciano M, Kissling E, Reuss A, et al. Vaccine effectiveness in preventing laboratory-confirmed influenza in primary care patients in a season of co-circulation of influenza A(H1N1)pdm09, B and drifted A(H3N2), I-MOVE Multicentre Case-Control Study, Europe 2014/15. Euro Surveill 2016; 21:1–17. pii=30139. doi: 10.2807/1560-7917.ES.2016.21.7.30139 [DOI] [PubMed] [Google Scholar]
- 44. Kissling E, Valenciano M, Pozo F, et al. 2015/16 I-MOVE/I-MOVE+ multicentre case-control study in Europe: Moderate vaccine effectiveness estimates against influenza A(H1N1)pdm09 and low estimates against lineage-mismatched influenza B among children. Influenza Other Respir Viruses 2018; 12:423–37. doi: 10.1111/irv.12520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kissling E, Rondy M; I-MOVE/I-MOVE+ study team. Early 2016/17 vaccine effectiveness estimates against influenza A(H3N2): I-MOVE multicentre case control studies at primary care and hospital levels in Europe. Euro Surveill 2017; 22:1–9. pii: 30464. doi: 10.2807/1560-7917.ES.2017.22.7.30464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Skowronski DM, Masaro C, Kwindt TL, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005-2006 season of dual A and B vaccine mismatch in Canada. Vaccine 2007; 25:2842–51. [DOI] [PubMed] [Google Scholar]
- 47. Puzelli S, Frezza F, Fabiani C, et al. Changes in the hemagglutinins and neuraminidases of human influenza B viruses isolated in Italy during the 2001-02, 2002-03, and 2003-04 seasons. J Med Virol 2004; 74:629–40. [DOI] [PubMed] [Google Scholar]
- 48. Barr IG, Komadina N, Hurt A, et al. Reassortants in recent human influenza A and B isolates from South East Asia and Oceania. Virus Res 2003; 98:35–44. [DOI] [PubMed] [Google Scholar]
- 49. Wohlbold TJ, Nachbagauer R, Xu H, et al. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015; 6:e02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Boer PT, Kelso JK, Halder N, et al. The cost-effectiveness of trivalent and quadrivalent influenza vaccination in communities in South Africa, Vietnam and Australia. Vaccine 2018; 36:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hendriks J, Hutubessy RCW, Grohmann G, Torelli G, Friede M, Kieny MP. Quadrivalent influenza vaccines in low and middle income countries: cost-effectiveness, affordability and availability. Vaccine 2018; 36:3993–7. [DOI] [PubMed] [Google Scholar]
- 52. Levandowski RA, Gross PA, Weksler M, Staton E, Williams MS, Bonelli J. Cross-reactive antibodies induced by a monovalent influenza B virus vaccine. J Clin Microbiol 1991; 29:1530–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skowronski DM, Hottes TS, De Serres G, et al. Influenza Β/Victoria antigen induces strong recall of Β/Yamagata but lower Β/Victoria response in children primed with two doses of Β/Yamagata. Pediatr Infect Dis J 2011; 30:833–9. [DOI] [PubMed] [Google Scholar]
- 54. Skowronski DM, Hamelin ME, Janjua NZ, et al. Cross-lineage influenza B and heterologous influenza A antibody responses in vaccinated mice: immunologic interactions and B/Yamagata dominance. PLOS One 2012; 7:e38929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.