Abstract
This research involved the production of polycaprolactone fiber layers via the alternating current electrospinning method. To construct the micro/nanofiber scaffold, mixtures of two molecular weight solutions, Mn 45 000 and Mn 80 000, were spun in differing proportions in a solvent system containing acetic acid, formic acid, and acetone in a ratio of 1:1:1. The composite fiber materials with hydroxyapatite particles were prepared from a solution that combined the different molecular weight solutions at a ratio of 1:3. The study resulted in the preparation of fiber layers containing 0, 5, 10, and 15% (wt) hydroxyapatite particles from the dry mass of the polycaprolactone. The strength, wettability, and surface energy of the composite materials were examined, and the results demonstrated that hydroxyapatite affects the fiber diameters, strength, and surface energy and, thus, the wettability of the fiber layers. The fibrous layers produced were further tested for cytotoxicity and cell viability and proliferation. The results obtained thus strongly indicate that the resulting bulky micro/nanofiber layers are suitable for further testing with a view to their eventual application in the field of bone tissue engineering.
1. Introduction
Polycaprolactone (PCL) constitutes one of the most frequently employed polymers in the field of bone tissue engineering.1 It comprises a semicrystalline hydrophobic polyester obtained via ring-opening polymerization that is both biocompatible and biodegradable. It has a low melting point of around 60 °C and a glass transition temperature of −60 °C.2,3
Polycaprolactone is widely used in the field of tissue engineering as a scaffold not only for bone regeneration but also for skin tendons, wound dressing, vascular grafts, abdominal adhesion prevention, or blood vessels.4−6 Scaffolds from polycaprolactone may also be combined with particles that promote cell adhesion and proliferation including hydroxyapatite, a material that is found in bones.7,8 Scaffolds containing hydroxyapatite enhance both osteoblast activity and bone formation.9 Nanofibrous layers from PCL are produced at the industrial scale principally by means of the direct current (DC) electrospinning technique.10−12 DC spinning technology leads to the production of very fine fibers from a polymer solution with diameters ranging from 50 to 1000 nm.13,14 However, DC spinning technology is unsuitable for the production of bulky materials since the thickness of the layers ranges from 20 to a maximum of 600 μm.15 This is due to the effort of the fibers to lose their charge, which they gained from the charged electrode, by the oppositely charged collector. This results in a tight arrangement of fibers and the impossibility of obtaining bulky materials.
Thus, due to the impossibility of producing voluminous samples via DC spinning technology, alternating current (AC) electrospinning method was selected for the research. In contrast to DC spinning, the AC technique does not require an opposite electrode since the fibers are charged both positively and negatively due to changes in polarity.16 The emission of nanofibers carrying one polarity is diverted from the electrode by an electric wind, and the newly created fibers of opposite polarity are then pulled toward existing fibers via the influence of the counter charge. The continuously produced nanofiber siding can be deposited on a static collector, rotating drum, or even a yarn.17 This study involved the use of a rotating drum as a collector for obtaining a voluminous fiber layer.
The technology of AC electrospinning is currently still relatively little used. Currently, there are only a few publications that deal with this technology. In recent publications, several polymers have been spun using AC electrospinning, such as gelatin,18 polyamide 6,19 poly(vinyl alcohol),20 poly(ethylene oxide),21 polyacrylonitrile,22 or polycaprolactone,23 which we have also addressed in this study.
2. Materials and Methods
2.1. Materials
Two molecular weights of PCL were used in the production of a micro/nanofiber system: Mn 45 000 and Mn 80 000 (Sigma-Aldrich, Germany). The solvent system was selected from a set of acetic acid, formic acid, and acetone (Penta, Czech Republic). Hydroxyapatite particles (particle size 1.838 ± 0.708 μm, Sigma-Aldrich, Germany) were added to the basic solution, thus resulting in a suspension.
2.2. Preparation of Solutions
The first stage of the research involved the determination of a solution that would produce a micro/nanofiber system without the occurrence of defects. The polycaprolactone solutions were prepared in a solvent system consisting of acetic acid, formic acid, and acetone at a ratio of 1:1:1. The polycaprolactone with Mn 45 000 was prepared at a concentration of 16 wt % and the polycaprolactone with Mn 80 000 at a concentration of 10 wt %. The PCL solutions were dissolved at room temperature for 24 h. Mixtures were prepared from these solutions at the ratios of 1:0, 3:1, 2:1, 1:1, 1:2, 1:3, and 0:1.
2.3. Viscosity
The viscosity of the prepared solutions was measured on a rotary rheometer HAAKE RotoVisco 1 (Thermo Fisher Scientific, Czech Republic). Two hundred microliters of each solution was dosed, and a total of 10 measurements were performed at a shear rate of 1000 s–1. The values from the measurements were averaged and evaluated.
2.4. Conductivity
The electrical conductivity of the solutions was measured with a CyberScan CON 510 (Thermo Fisher Scientific, Czech Republic) with an ATC electrode (ECCONSEN91W/35608-50). Ten measurements were performed, and the mean value was evaluated.
2.5. AC Spinning
The prepared solutions were spun using a custom-built AC spinning device that consisted of an ABB KGUG 36 high-voltage transformer and a Thalheimer-Trafowerke ESS 104 variable autotransformer. The spinning of each of the solutions was performed at an effective voltage of 34 kV and a frequency of 50 Hz at a temperature of 22 °C and a relative humidity of 40%. The spinning was performed using an electrically neutral rotary drum covered with a polypropylene spunbond fabric as the collector. Alternating spinning resulted in the fibers being pushed toward the collector by an electric wind. The distance of the rotating drum from the electrode was set at 250 mm, and the peripheral speed of the drum was set at 40 m/min. The spinning of the solutions was provided by an overflow electrode, and the polymer solution was dosed by a screw pump as described in ref (16).
2.6. Scanning Electron Microscopy (SEM) and Analysis
The resulting layers were coated with a 10 nm layer of gold using Quorum Q150R ES (Quorum Technologies, U.K.), and images of the layers were obtained by means of a scanning electron microscope (SEM, TESCAN VEGA3, Czech Republic) at an accelerated voltage of 20 kV. The images were assessed using ImageJ software (NIH, Bethesda, MD).
2.7. Strength of the Nanofibrous Layers
The strength of the fiber layers was measured by LabTest 6.031 (LaborTech Instrument, Czech Republic) using a head with a range of 150 N, loading speed of 100 mm·s–1, and clamped length of 50 mm. Five 100 × 50 mm2 samples were tested from each set.
2.8. Contact Angle and Surface Energy of the Fiber Layers
The See System E (Advex Instruments, Czech Republic) was used to determine the contact angle. Distilled water (10 μL) was used for each droplet, and 30 measurements were made on each sample. The surface energy was then calculated via the Kwok–Neumann model.
2.9. Sterilization
Ethylene oxide sterilization was chosen as a suitable method for sterilizing low-melting-point polymers as described by Horakova et al.6 The materials were sterilized in an Anprolene AN-74i (Andersen Products) sterilizer using ethylene oxide at 37 °C for 12 h. The materials were then vented at room temperature for 2 weeks.
2.10. Cell Cultivation
MG-63 human osteoblasts (ATCC) were cultured in complete minimum essential medium (MEM, Biosera, Czech Republic) supplemented with 10% fetal bovine serum (Biosera, Czech Republic), 1% glutamine (Biosera, Czech Republic), 1% antibiotic—Pen/Strep amphotericin B (Lonza Biotec, Czech Republic), and 1% nonessential amino acids (NEAA, Lonza Biotec, Czech Republic). The cells were incubated in an incubator in 5% CO2 at 37 °C.
2.11. Cytotoxicity
According to the standard ISO 10993-5:2009, the material is considered to be cytotoxic if the viability of the cells incubated with an extract from the material evinces values of lower than 70% of the cell viability of the negative control.24 Cells from passage 19 at a concentration of 1 × 104 per well were seeded at the bottom of a 96-well microtiter plate and cultured for 24 h. For the positive control (PC) and the negative control (NC) and, each material were seeded 10 wells of cells. Six samples were prepared from each material: three of them were rinsed twice with phosphate buffer saline (PBS, Lonza Biotec, Czech Republic) solution and the remaining three were not rinsed with PBS. The materials were then incubated with the complete medium (MEM) for 24 h.
The culture medium was then changed to extracts (200 μL per well) of the test materials followed by 0.1% Triton in the MEM for the PC and pure complete medium (MEM) for the NC. The cells were further incubated for 24 h, following which their viability was determined by spectrophotometric analysis using the Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Japan) metabolic assay.
2.12. In Vitro Test
The MG-63 cell line was also used for the testing of the cell viability and proliferation on the test materials, which were prepared in the form of circular samples with diameters of 10 mm. The samples were placed in 24-well culture plates in a flow box, weighted with sterile glass rings, and washed twice with PBS. Cells from passage 27 were seeded onto the materials at a concentration of 1 × 104 per well. CCK-8 assay and fluorescent microscopy evaluations were conducted after the 1st, 3rd, 7th, and 14th days of culture.
2.13. CCK-8 Assay
A sufficient amount of a 10% solution of CCK-8 in MEM (200 μL per well) was prepared for testing purposes. Incubation lasted for 3 h at 37 °C, following which the 10% solution of CCK-8 in MEM was transferred to a clean 96-well microtiter plate and measured at 450 nm using a spectrophotometer (Synergy HTX, BioTek).
2.14. Fluorescence Microscopy
The morphological analysis of the cells was performed using a fluorescence microscope (Nikon Eclipse Ti-e, Nikon Imaging, Czech Republic). The cells on the materials were stained with phalloidin-fluorescein isothiocyanate (FITC) (Sigma-Aldrich, Germany) solution and, subsequently with 4′,6-diamidine-2-phenylindole (DAPI, Sigma-Aldrich, Germany) solution. Then, the samples were analyzed with a fluorescence microscope. The number of cells per unit area (1 mm2) was determined for each material from 10 images using ImageJ software for the comparison of the various materials.
2.15. Statistical Analysis
The statistical evaluation was performed using GraphPad Prism v7 software (GraphPad Software). The normality of the data was evaluated using the D’Agostino and Pearson normality test. The data were not subject to a normal distribution; therefore, the Kruskal–Wallis test was used to compare the data. There was a statistically significant difference at the level of significance p ≤ 0.05.
3. Results and Discussion
3.1. Viscosity and Conductivity of the PCL Solutions
The viscosity and conductivity of the polymer solutions affect the electrospinning process itself, and as a result of these quantities, there is also a change in the fibrous structure. Therefore, we investigated the viscosity and conductivity of the PCL solutions.
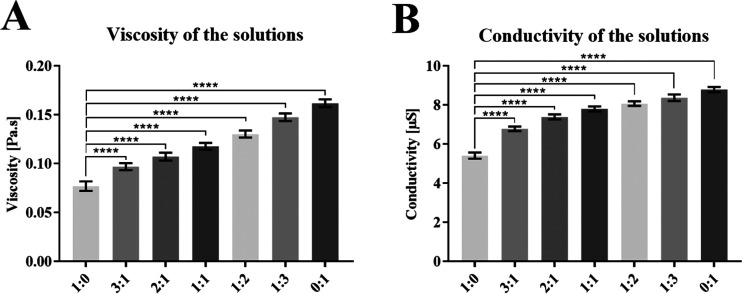
The mixtures with combinations of 16 wt %, Mn 45 000 PCL and 10 wt %, Mn 80 000 PCL were prepared at ratios of 1:0, 3:1, 2:1, 1:1, 1:2, 1:3, and 0:1. From the obtained results, it is evident that the viscosity and conductivity of the polymer solutions depend on the amount of 10 wt %, Mn 80 000 PCL. A higher molecular weight of this component results in a significant increase in the viscosity, and also the higher amount of solvent in this solution results in a significant increase in the conductivity, as shown in the graphs of Figure 1.
Figure 1.
Parameters of the prepared PCL solutions: (A) viscosity and (B) conductivity. 95% CI; ****p < 0.0001.
3.2. Fiber Morphology and Diameter Analysis of the PCL Fibers
The prepared PCL solutions were spun to ensure the production of a micro/nanofiber system that would not feature any defects in the fiber structure.
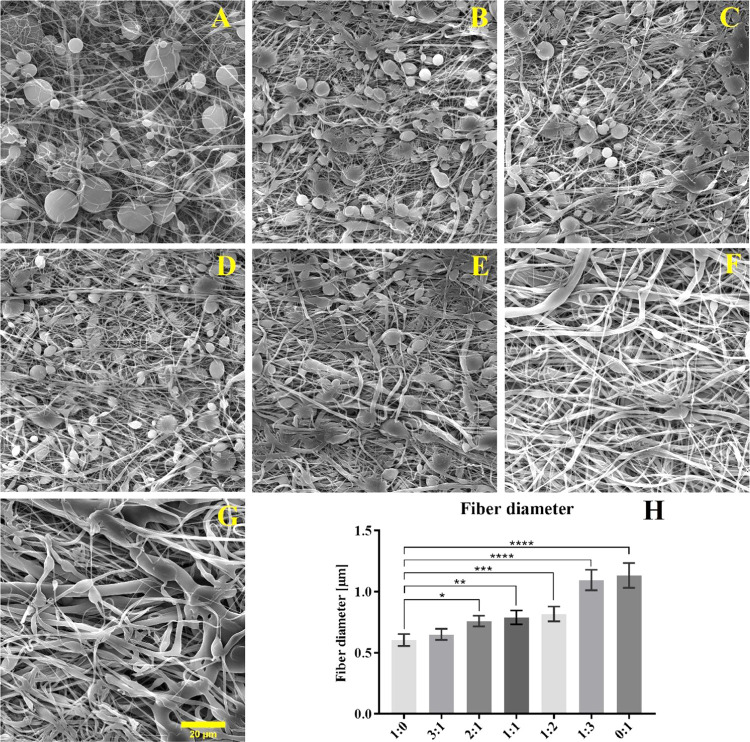
The fiber diameters of the various spun mixtures and the morphology of the fibrous layers are listed in Figure 2. The results clearly indicate that the molecular weight affected the morphology of the fabricated layers. While the molecular weight of 45 000 led to the production of smaller-diameter fibers, an increase in the occurrence of droplet defects was observed. The molecular weight of 80 000 resulted in significantly larger fiber diameters and a more expansive diameter distribution in the fiber layer. A homogeneous, defect-free micro/nanofiber layer was finally achieved via the spinning of a mixture of Mn 45 000 and Mn 80 000 solutions at a ratio of 1:3.
Figure 2.
SEM images of the fibrous layers produced from mixtures of Mn 45 000 and Mn 80 000 at the following ratios: (A) 1:0, (B) 3:1, (C) 2:1, (D) 1:1, (E) 1:2, (F) 1:3, and (G) 0:1. (20 μm scale). (H) Fiber diameter of spun mixtures. 95% CI; *p < 0.0368, **p < 0.0032, ***p < 0.0003, and ****p < 0.0001.
3.3. Viscosity and Conductivity of the PCL/HA Solutions
A mixture of Mn 45 000 and Mn 80 000 solutions at a ratio of 1:3 was selected for the combination of PCL with hydroxyapatite (HA) particles. Hydroxyapatite particles of 5, 10, and 15% (wt) from the dry mass of the PCL were then added to the prepared solutions.
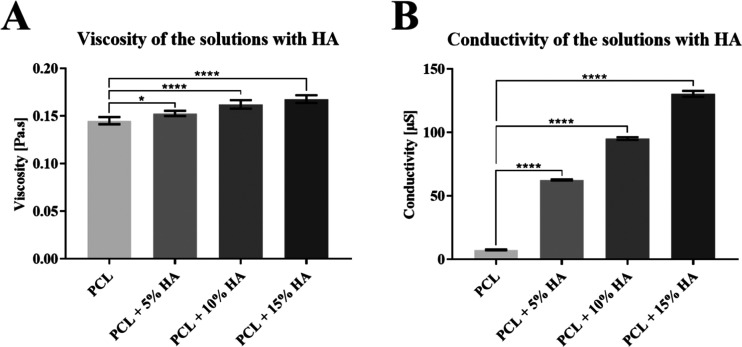
Solutions containing hydroxyapatite particles were also examined for their viscosity and conductivity; the results are shown in the graph in Figure 3. The values show that the hydroxyapatite particles affect the resulting viscosity and conductivity significantly. Higher amounts of HA in the solution lead to increased viscosity and conductivity.
Figure 3.
Parameters of the prepared PCL solutions with HA: (A) viscosity and (B) conductivity of the solutions. 95% CI; *p < 0.0177 and ****p < 0.0001.
3.4. Spinning of the PCL/HA Solutions
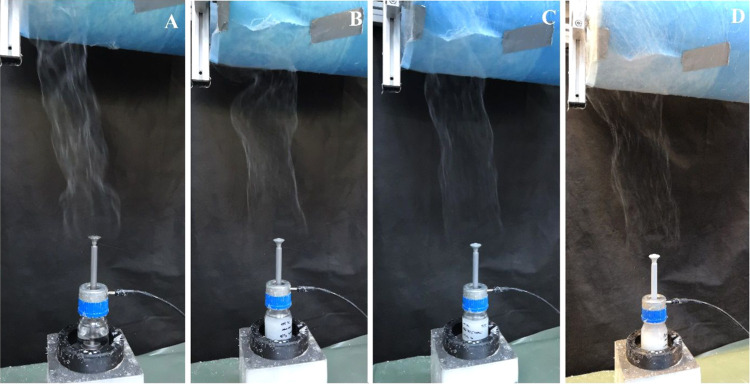
The solution was first spun without adding hydroxyapatite particles, followed by solutions containing hydroxyapatite particles. Figure 4 illustrates the spinning process for the various solutions. In all four cases, the spinning process was optimal with the attainment of continuous stable spinning and high productivity. Thus, the high conductivity of the solutions does not affect the spinning process itself. The resulting nanofibrous layers were found to be homogeneous and without defects.
Figure 4.
AC spinning of the prepared solutions: (A) pure PCL solution, (B) PCL solution with 5% HA, (C) PCL solution with 10% HA, and (D) PCL solution with 15% HA.
3.5. Fiber Morphology and Diameter Analysis of the PCL/HA Fibers
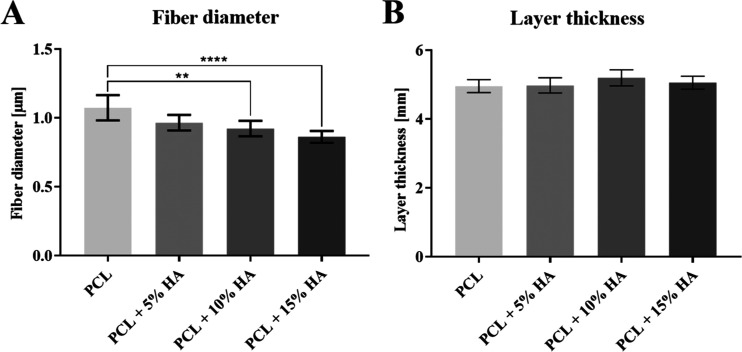
The various fiber diameters and layer thicknesses are shown in the graphs in Figure 5. The results reveal that the spinning of solutions with hydroxyapatite particles serves to significantly reduce the diameters of the fibers.
Figure 5.
Parameters of fiber layers. (A) Fiber diameters of PCL/HA layers. 95% CI; **p < 0.0065 and ****p < 0.0001. (B) Thicknesses of PCL/HA layers. 95% CI.
The aim of the experiment was to produce a bulkier fibrous layer than in the case of DC spinning. The layer thicknesses were measured using an Elcometer 456 (Gamin, Czech Republic) digital thickness gauge. During the spinning process, layers with almost the same thickness of 5 mm were produced. In all cases, significantly bulkier micro/nanofiber layers were produced than would be possible via DC spinning process.
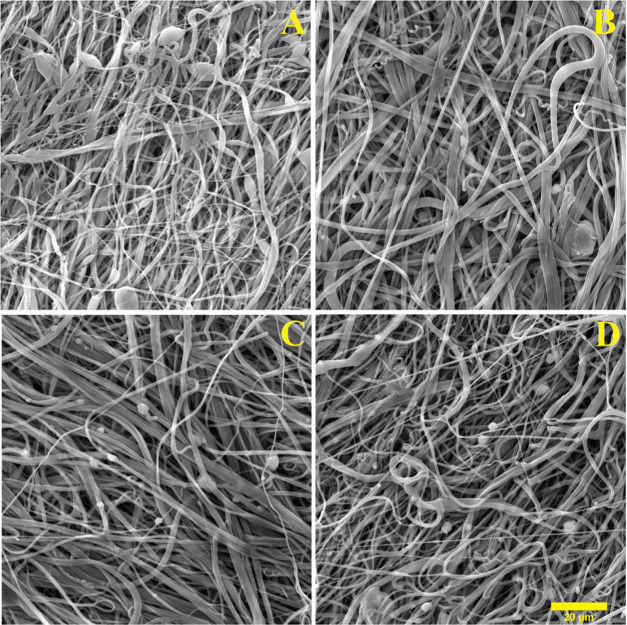
The four resulting nanofibrous PCL/HA layers are presented in Figure 6, which illustrates the effects of increasing the dose of hydroxyapatite particles. The solutions with the lowest dose of hydroxyapatite (Figure 6B) can be seen to retain particles within the fibrous structure, whereas the solutions with higher concentrations (Figure 6C,D) can be seen to feature particles outside the fiber masses.
Figure 6.
SEM images of the fibrous layers with differing concentrations of HA: (A) PCL, (B) PCL + 5% HA, (C) PCL + 10% HA, and (D) PCL + 15% HA (20 μm scale).
3.6. Contact Angle and Surface Energy of the Fiber Layers
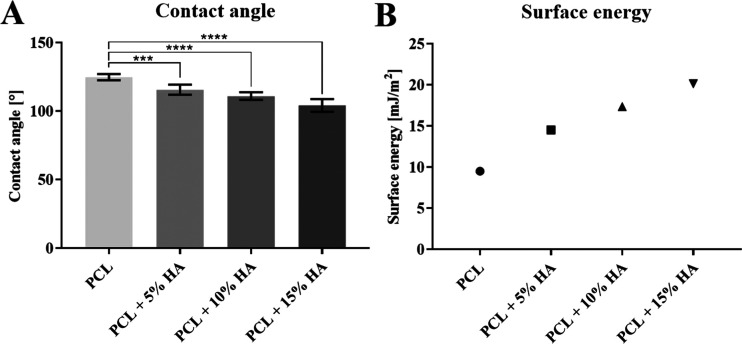
The contact angle is directly related to the surface energy of the fiber layers and the surface tension of the liquid. Distilled water with a surface tension of 73 mN was used for the measurement of the contact angle, the values of which are presented in Figure 7 along with the values of fiber layer surface energy. The values show that the contact angle significantly decreases with higher amounts of hydroxyapatite particles. Therefore, the surface energy of the fiber layers increases with higher amounts of hydroxyapatite particles, i.e., the fibrous layers are more hydrophilic and are wetted to a higher degree upon contact with liquids, such as cell suspensions.
Figure 7.
(A) Contact angle of the fiber layers. 95% CI; ***p < 0.0008 and ****p < 0.0001. (B) Surface energy of the fiber layers.
3.7. Strength of the Nanofibrous Layers
During the study, the effect of HA particles on the strength of the fibrous layers was investigated. The data in the graph in Figure 8 reveals that the hydroxyapatite particles dramatically influenced the strength and extension of the fibrous layers. A higher amount of hydroxyapatite particles led to a decrease in the strength and extension of the layers, which was most likely the result of a reduction in the fiber diameter and, in particular, to the encapsulation of the hydroxyapatite particles in the fiber structure. The encapsulation of the particles disrupted the uniform fiber structure, and less polymeric material was present at the encapsulation site, which led to a decrease in the strength and extension in the tensile test.
Figure 8.
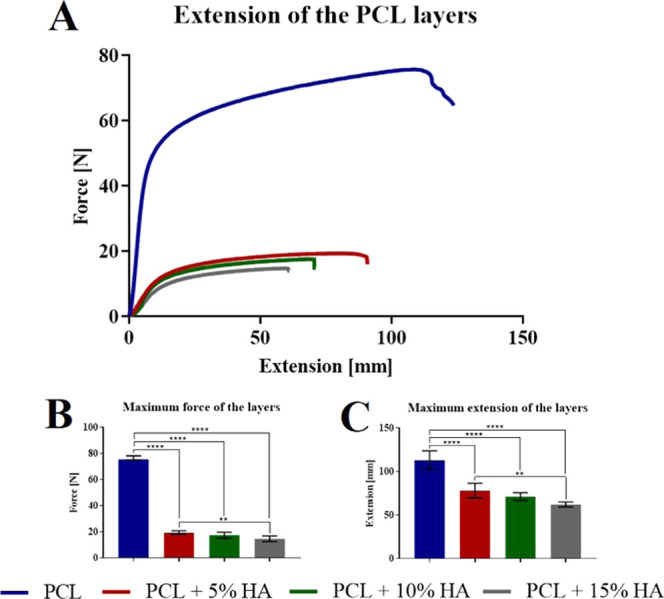
(A) Tensile tests of the fiber layers with differing concentrations of hydroxyapatite. (B) Maximum force of the layers. 95% CI; **p < 0.0016 and ****p < 0.0001. (C) Maximum extension of the layers. 95% CI; **p < 0.0027 and ****p < 0.0001.
3.8. Cytotoxicity
A material is considered to be cytotoxic if the viability of the cells incubated with an extract from the material evinces values of lower than 70% of the cell viability of the negative control. The metabolic assay graph in Figure 9 shows that materials were cytotoxic to human osteoblast MG-63 cells if they were not rinsed with PBS. Following the rinsing of the materials with PBS, the cytotoxic effect of the materials could no longer be observed. The cytotoxic behavior of the materials was most likely caused by the presence of residual amounts of acetic acid and formic acid that made up the spinning solution.
Figure 9.
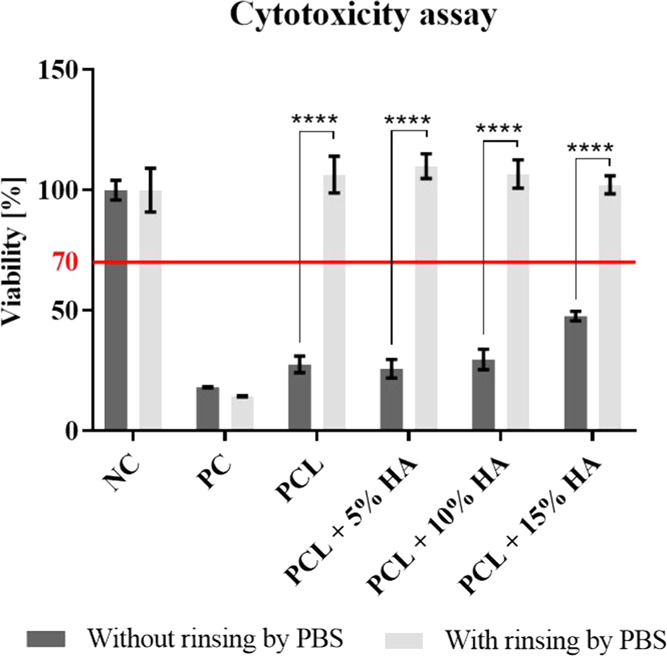
Cytotoxicity of the materials via the metabolic assay (CCK-8) following the culturing of human osteoblast MG-63 cells with extracts of the tested materials. 95% CI; ****p < 0.0001.
3.9. In Vitro Test
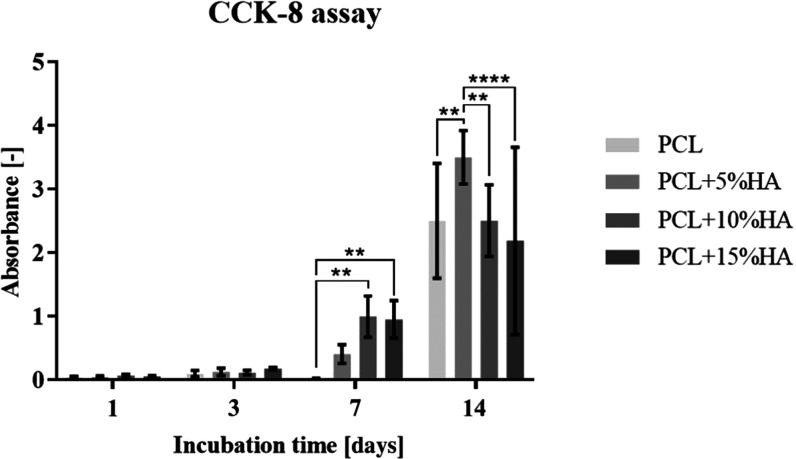
The cell viability of MG-63 human osteoblasts cultured on PCL and PCL with hydroxyapatite was monitored after 1, 3, 7, and 14 days of incubation. The average measured absorbance values (after deducting the negative control) are shown in the graph in Figure 10.
Figure 10.
Graph showing the metabolic activity of the MG-63 cell line cultured on PCL and on PCL with hydroxyapatite after 1, 3, 7, and 14 days of incubation. 95% CI; **p < 0.0023 and ****p < 0.0001.
The metabolic activity results revealed that the initial cell adhesion on the tested materials was with no statistical differences for all of the materials. The same trend was also observed on the 3rd day of cell proliferation on the materials. Significant difference was observed on the 7th day of cell cultivation; the PCL + 10% HA and PCL + 15% HA had higher cell viability than the other two tested materials. However, on day 14, the viability of the PCL + 5% HA sample was observed to be higher than that of the other materials.
3.10. Fluorescence Microscopy
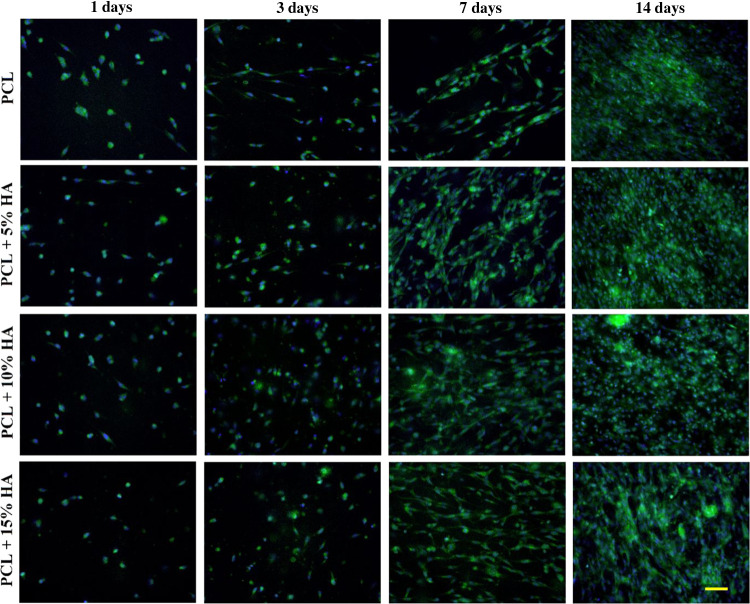
The morphology of the cells on the tested materials was monitored by fluorescence microscopy. Images were taken after 1, 3, 7, and 14 days of culture; see Figure 11.
Figure 11.
Morphology of the cells that adhered to the surface of the tested materials stained with phalloidin-FITC (green) and DAPI (blue) after days 1, 3, 7, and 14 of incubation at 200× magnification (50 μm scale).
The differences in the cell viability determined via the CCK-8 assay results correspond with the images from fluorescence microscopy. Cell adhesion on the 1st and 3rd days was visibly the same for all of the tested materials. A clear difference between the PCL and the other materials was observed on the 7th day, with a visibly lower number of cells on the PCL material. After 14 days, an almost confluent layer of cells was observed on all of the materials. However, it can be seen that the PCL + 5% HA material evinced a higher cell count than the other materials after 14 days of MG-63 cell line culturing.
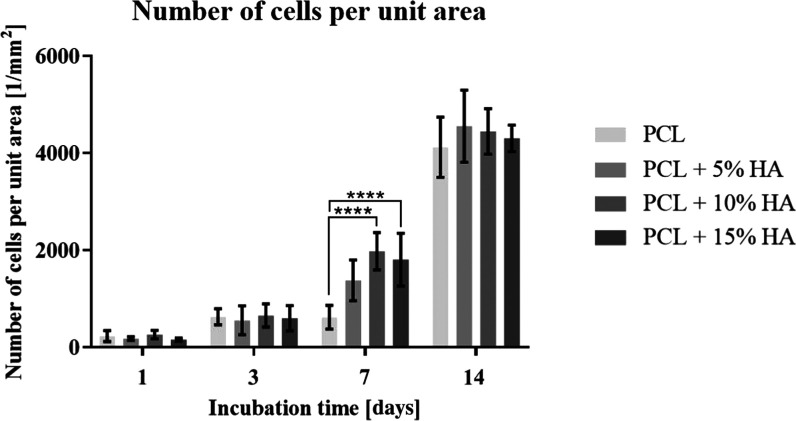
Furthermore, a series of images of the cell nuclei (DAPI) that adhered to the monitored materials on the various sampling days (1st, 3rd, 7th, and 14th days of incubation) were taken by means of fluorescence microscopy. Ten images of samples of each of the materials were taken on all of the sampling days. The number of cells per unit area (1 mm2) was then calculated from the images of the cell nuclei using ImageJ for each image. The various values of the number of cells per unit area were then statistically assessed via the use of software; see the graph in Figure 12.
Figure 12.
Graph showing the number of cells per unit area (1 mm2) on PCL and on PCL with hydroxyapatite after 1, 3, 7, and 14 days of incubation. 95% CI; ****p < 0.0001.
The cell count results were found to be almost identical to the cell viability results determined via the CCK-8 metabolic assay. The mild discrepancies may have been due to the temporary attenuation of the cells in terms of metabolic activity during their culturing with the CCK-8 solution.
4. Conclusions
The experiment involved the successful AC spinning of a PCL solution in a solvent system comprising acetic acid, formic acid, and acetone at a ratio of 1:1:1. The process was fully optimized, and fibrous layers with differing molecular weight ratios were produced.
A planar bulky layer without the presence of defects was obtained by combining PCL with Mn 45 000 and Mn 80 000 at a ratio of 1:3. The layer exhibited no droplet defects and provided a micro/nanofibrous system that allowed for cell adhesion and proliferation.
The study focused on the spinnability of a PCL solution with the addition of hydroxyapatite particles, which were found to significantly influence the parameters of the fibrous layers. Hydroxyapatite affects the diameters of the fibers, which evince a decreasing tendency with the increasing proportions of hydroxyapatite. Furthermore, the effect of hydroxyapatite on the contact angle and surface energy was to render the fiber layer more hydrophilic. Finally, it was also determined that hydroxyapatite dramatically affects the strength and extension of the fiber layers.
The results of the in vitro testing revealed that cells adhered better and proliferated on materials modified via the addition of hydroxyapatite, in particular, on the PCL + 5% HA and PCL + 10% HA materials. Cells were found to proliferate least on the pure PCL material, which evinced the lowest cell viability results over the first 3 days of testing. Moreover, cell adhesion to the PCL material was observed to be negligible over the first 7 days of testing compared to the other tested materials as determined by both the fluorescent and SEM images of the cells on the tested materials. After 14 days of culturing, the cell viability and proliferation were found to be almost identical for all of the materials, with confluent layers of cells being observed over the whole area of the samples.
The results obtained thus strongly indicate that the resulting bulky micro/nanofiber layers are suitable for further testing with a view to their eventual application in the field of bone tissue engineering.
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic and the European Union, European Structural and Investment Funds in the frames of Operational Programme Research, Development and Education, project Hybrid Materials for Hierarchical Structures (HyHi, Reg. No. CZ.02.1.01/0.0/0.0/16_019/0000843), and by the project of the Specific University Research Grant (Reg. No. 21403) provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2020.
The authors declare no competing financial interest.
References
- Dwivedi R.; Kumar S.; Pandey R.; Mahajan A.; Nandana D.; Katti D. S.; Mehrotra D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. 10.1016/j.jobcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair N. R.; Sekhar V. C.; Nampoothiri K. M.; Pandey A.. 32—Biodegradation of Biopolymers. In Current Developments in Biotechnology and Bioengineering; Pandey A.; Negi S.; Soccol C. R., Eds.; Elsevier, 2017; pp 739–755. [Google Scholar]
- McKeen L.12—Renewable Resource and Biodegradable Polymers. In The Effect of Sterilization on Plastics and Elastomers; 3rd ed.; McKeen L., Ed.; William Andrew Publishing: Boston, 2012; pp 305–317. [Google Scholar]
- Erben J.; Jencova V.; Chvojka J.; Blazkova L.; Strnadova K.; Modrak M.; Kostakova E. K. The Combination of Meltblown Technology and Electrospinning—The Influence of the Ratio of Micro and Nanofibers on Cell Viability. Mater. Lett. 2016, 173, 153–157. 10.1016/j.matlet.2016.02.147. [DOI] [Google Scholar]
- Klicova M.; Klapstova A.; Chvojka J.; Koprivova B.; Jencova V.; Horakova J. Novel Double-Layered Planar Scaffold Combining Electrospun PCL Fibers and PVA Hydrogels with High Shape Integrity and Water Stability. Mater. Lett. 2020, 263, 127281 10.1016/j.matlet.2019.127281. [DOI] [Google Scholar]
- Horakova J.; Mikes P.; Saman A.; Jencova V.; Klapstova A.; Svarcova T.; Ackermann M.; Novotny V.; Suchy T.; Lukas D. The Effect of Ethylene Oxide Sterilization on Electrospun Vascular Grafts Made from Biodegradable Polyesters. Mater. Sci. Eng. C 2018, 92, 132–142. 10.1016/j.msec.2018.06.041. [DOI] [PubMed] [Google Scholar]
- Shkarina S.; Shkarin R.; Weinhardt V.; Melnik E.; Vacun G.; Kluger P. J.; Loza K.; Epple M.; Ivlev S. I.; Baumbach T.; Surmeneva M. A.; Surmenev R. A. 3D Biodegradable Scaffolds of Polycaprolactone with Silicate-Containing Hydroxyapatite Microparticles for Bone Tissue Engineering: High-Resolution Tomography and in Vitro Study. Sci. Rep. 2018, 8, 8907 10.1038/s41598-018-27097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. M.; Chu P. K.; Leung F. K. L.; Cheung K. M. C.; Luk K. D. K.; Yeung K. W. K. Engineered Polycaprolactone–Magnesium Hybrid Biodegradable Porous Scaffold for Bone Tissue Engineering. Prog. Nat. Sci.: Mater. Int. 2014, 24, 561–567. 10.1016/j.pnsc.2014.08.013. [DOI] [Google Scholar]
- Michel J.; Penna M.; Kochen J.; Cheung H. Recent Advances in Hydroxyapatite Scaffolds Containing Mesenchymal Stem Cells. Stem Cells Int. 2015, 2015, 305217 10.1155/2015/305217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrč T.; Baumgartner S.; Roškar R.; Planinšek O.; Lavrič Z.; Kristl J.; Kocbek P. Electrospun Polycaprolactone Nanofibers as a Potential Oromucosal Delivery System for Poorly Water-Soluble Drugs. Eur. J. Pharm. Sci. 2015, 75, 101–113. 10.1016/j.ejps.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Šrámková I. H.; Carbonell-Rozas L.; Horstkotte B.; Háková M.; Erben J.; Chvojka J.; Švec F.; Solich P.; García-Campaña A. M.; Šatínský D. Screening of Extraction Properties of Nanofibers in a Sequential Injection Analysis System Using a 3D Printed Device. Talanta 2019, 197, 517–521. 10.1016/j.talanta.2019.01.050. [DOI] [PubMed] [Google Scholar]
- Háková M.; Havlíková L. C.; Chvojka J.; Erben J.; Solich P.; Švec F.; Šatínský D. A Comparison Study of Nanofiber, Microfiber, and New Composite Nano/Microfiber Polymers Used as Sorbents for on-Line Solid Phase Extraction in Chromatography System. Anal. Chim. Acta 2018, 1023, 44–52. 10.1016/j.aca.2018.04.023. [DOI] [PubMed] [Google Scholar]
- RenChun F.; Jun D.; hui H.; Zhong-Cheng G. Fabrication and Evaluation of Polyaniline Nanofibers via Ethyl Cellulose Template. High Perform. Polym. 2014, 26, 27–33. 10.1177/0954008313495067. [DOI] [Google Scholar]
- Beachley V.; Wen X. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C 2009, 29, 663–668. 10.1016/j.msec.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. K.; Bang J. Y.; Xu G.; Lee J.-H.; Kim Y.-J.; Lee H.-J.; Kim H. S.; Kwon S.-M. Thickness-Controllable Electrospun Fibers Promote Tubular Structure Formation by Endothelial Progenitor Cells. Int. J. Nanomed. 2015, 10, 1189–1200. 10.2147/IJN.S73096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtera J.; Kalous T.; Pokorny P.; Batka O.; Bilek M.; Chvojka J.; Mikes P.; Kostakova E. K.; Zabka P.; Ornstova J.; Beran J.; Stanishevsky A.; Lukas D. Fabrication of Dual-Functional Composite Yarns with a Nanofibrous Envelope Using High Throughput AC Needleless and Collectorless Electrospinning. Sci. Rep. 2019, 9, 1801 10.1038/s41598-019-38557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny P.; Kostakova E.; Sanetrnik F.; Mikes P.; Chvojka J.; Kalous T.; Bilek M.; Pejchar K.; Valtera J.; Lukas D. Effective AC Needleless and Collectorless Electrospinning for Yarn Production. Phys. Chem. Chem. Phys. 2014, 16, 26816–26822. 10.1039/C4CP04346D. [DOI] [PubMed] [Google Scholar]
- Jirkovec R.; Kalous T.; Brayer W. A.; Stanishevky A. V.; Chvojka J. Production of Gelatin Nanofibrous Layers via Alternating Current Electrospinning. Mater. Lett. 2019, 252, 186–190. 10.1016/j.matlet.2019.05.132. [DOI] [Google Scholar]
- Kalous T.; Holec P.; Jirkovec R.; Lukas D.; Chvojka J. Improved Spinnability of PA 6 Solutions Using AC Electrospinning. Mater. Lett. 2021, 283, 128761 10.1016/j.matlet.2020.128761. [DOI] [Google Scholar]
- Erben J.; Kalous T.; Chvojka J. Ac Bubble Electrospinning Technology for Preparation of Nanofibrous Mats. ACS Omega 2020, 5, 8268–8271. 10.1021/acsomega.0c00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessick R.; Fenn J.; Tepper G. The Use of AC Potentials in Electrospraying and Electrospinning Processes. Polymer 2004, 45, 2981–2984. 10.1016/j.polymer.2004.02.056. [DOI] [Google Scholar]
- He H.; Wang Y.; Farkas B.; Nagy Z. K.; Molnar K. Analysis and Prediction of the Diameter and Orientation of AC Electrospun Nanofibers by Response Surface Methodology. Mater. Des. 2020, 194, 108902 10.1016/j.matdes.2020.108902. [DOI] [Google Scholar]
- Sivan M.; Madheswaran D.; Asadian M.; Cools P.; Thukkaram M.; Van Der Voort P.; Morent R.; De Geyter N.; Lukas D. Plasma Treatment Effects on Bulk Properties of Polycaprolactone Nanofibrous Mats Fabricated by Uncommon AC Electrospinning: A Comparative Study. Surf. Coat. Technol. 2020, 399, 126203 10.1016/j.surfcoat.2020.126203. [DOI] [Google Scholar]
- ISO 10993-5:2009. https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/64/36406.html (accessed October 23, 2020).