Abstract
Background
Parathyroid carcinoma is a rare endocrine malignancy, rarer when synchronous with a non medullary well differentiated thyroid carcinoma. Parathyroid carcinoma accounts of 0.005% of all malignant tumors and it is responsible for less than 1% of primary hyperparathyroidism. The intrathyroidal localization of a parathyroid gland is not frequent with a reported prevalence of 0.2%. Carcinoma of parathyroids with intrathyroidal localization represents an even rarer finding, reported in only 16 cases described in literature. The rare constellation of synchronous parathyroid and thyroid carcinomas has prompted us to report our experience and perform literature review.
Case presentation
We herein report a case of a 63-years-old man with multinodular goiter and biochemical diagnosis of hyperparathyroidism. Total thyroidectomy with radio-guide technique using gamma probe after intraoperative sesta-MIBI administration and intraoperative PTH level was performed. The high radiation levels in the posterior thyroid lobe discovered an intrathyroidal parathyroid. Microscopic examination revealed a parathyroid main cell carcinoma at the posterior thyroidal left basal lobe, a classic papillary carcinoma at the same lobe and follicular variant of papillary carcinoma at the thyroidal right lobe. To the best of our knowledge, this is the first case documenting a synchronous multicentric non medullary thyroid carcinomas and intrathyroidal parathyroid carcinoma.
Conclusions
Our experience was reported and literature review underlining challenging difficulties in diagnostic workup and surgical management was carried out.
Keywords: Thyroid cancer, Parathyroid cancer, Hyperparathyroidism, Intrathyroidal parathyroid, Surgical approach
Background
The intrathyroidal localization of a parathyroid gland is not frequent, with a reported prevalence of 0.2% [1]. Intrathyroidal parathyroids (IP) could also become pathological and primary hyperparathyroidism supported by an IP has an incidence of 1% [2].
Parathyroid carcinoma (PC) accounts of 0.005% of all malignant tumors and it is responsible for less than 1% of primary hyperparathyroidism [3, 4]. Carcinoma of parathyroids with intrathyroidal localization represents an even rarer finding, reported in only 16 cases described in literature [5–20].
The association between hyperparathyroidism and non-medullary well differentiated thyroid carcinoma is found in 2.4–3.7% of hyperparathyroidism cases [21], but hyperparathyroidism is mainly observed in benign parathyroid diseases [21–23].
At the best of our knowledge, only 11 cases of synchronous extrathyroidal parathyroid carcinomas and non-medullary well differentiated thyroid carcinoma are reported up to date in literature [24–34]. Indeed, our patient is the first documented case of synchronous non-medullary well differentiated thyroid carcinoma and parathyroid carcinoma with an intrathyroidal localization.
The rare constellation of synchronous thyroid carcinoma and intrathyroidal parathyroid carcinoma has prompted us to report this case and perform literature review to stress the challenging difficulties in diagnostic workup and surgical management.
Case presentation
A 63 years-old Caucasian male patient, with a negative family history of thyroid diseases and without a personal anamnesis of neck irradiation, was referred to general surgery unit for multinodular goiter, bigger on the right side, complicated by a tracheal deviation and symptoms of compression.
Routine preoperative tests, unexpectedly, showed a mild serum hypercalcemia (13.3 mg / dL) [normal range (n.r.): 8.6–10.2)], hypophosphatemia [2.2 mg / dL (n.r. 2.7–4.5)], with no symptomatology of urolithiasis, myasthenia, osteoarthralgia, dyspepsia, constipation or psychotic depression. Serum parathyroid hormone (PTH) value was increased 159 pg / mL (n.r. 4.6–58.1), the urine calcium was 896 mg / 24 h (n.r. 100–400) and urine phosphorus was 1000 mg / 24 h (n.r. 400–1300). No kidney disorders were found thus a diagnosis of primary hyperparathyroidism was made.
A neck ultrasound (US) exam confirmed a multinodular goiter without extra-thyroid lump: in particular, a 14 mm-diameter nodule with intravascular spot signal at Doppler exam was observed at the left posterior base of the thyroid. The parathyroid subtraction scintigraphy with 99mTechnetium and Sesta-Meta-iodo-Benzylguanidine showed a strong posterior signal at the left thyroid base in correspondence of the nodule (Fig. 1). No pathological signal was observed in other extrathyroidal sites.
Fig. 1.
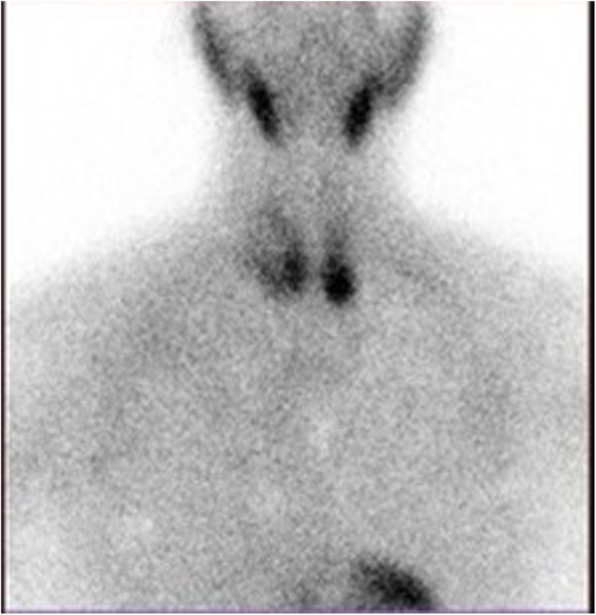
Scintigraphy with 99mtechnetium: Late hypercaptation at lower left pole of the thyroid, no mediastinal uptake
Therefore, these observations suggested the suspect of an IP adenoma: the decision was made not to proceed with a needle aspiration, due to the risk of severe fibrosis that could complicate the final histological diagnosis [35].
Otherwise fine-needle cytology (FNC) on the major nodule of the thyroidal right lobe diagnosed Thy 2 according to Bethesda System.
The patient received an intravenous injection of 12.3 mCi of Tc99m-sestamibi approximately 1 hour prior to surgery. A total thyroidectomy with radio-guide technique using gamma probe after intraoperative sesta-MIBI administration and intraoperative PTH level evaluation was performed.
The intraoperative PTH level evaluation, performed with a chemiluminescence immunoassay, allows the determination of the serum bio-intact PTH value. The basal serum PTH level before thyroidectomy was 164 pg/mL. After 20 min from the surgery, the value decreased at 38 pg/mL and 30 min later at 19 pg/mL.
The use of the gamma probe after surgery was essential to identify high radiation levels in the posterior left thyroid lobe, where a yellow-brown nodule was found (Fig. 2).
Fig. 2.

Posterior view of the operative specimen, node of the lower left pole of the thyroid subcapsular seat, reddish-yellow in color
Conversely, the postoperative thyroid lodge did not show any radiation activity.
The patient was discharged on the third post-operative day without complications and with normal range serum values of PTH, calcemia and phosphoremia.
Histopathological examination revealed a classic papillary carcinoma of 0.8 cm of diameter at the thyroidal left lobe (Fig. 3a), a follicular variant of a papillary carcinoma of 0.6 cm of diameter at the thyroidal right lobe (Fig. 3b) and a parathyroidal main cell carcinoma of 1.2 × 1.0 cm of diameter at the posterior thyroidal left basal lobe (Fig. 3c, d, Fig. 4). Photographs of histological slides were obtained using a digital acquisition system (Olympus DP2-SAL4).
Fig. 3.

Histopathological finding. a Conventional papillary carcinoma. Histological evaluation showing a neoplasm with a papillary architectural pattern (H&E, original magnification 200x). b Follicular variant of a papillary carcinoma. Histological evaluation showing a neoplastic proliferation with a follicular architectural pattern and cytological atypia including ground-glass nuclei and nuclear grooves (H&E, original magnification 200x). c-d Parathyroid carcinoma. Histological evaluation showing neoplastic islets separated by thick bands of connective tissue (yellow star). The neoplasm shows an infiltrative growth in the adjacent soft tissue (blue arrows). Thyroid tissue is present in the lower right (C, H&E, original magnification 40x). The neoplasm is constituted by main cells (D, H&E, original magnification 200x). Abbreviation: H&E: hematoxylin and eosin
Fig. 4.
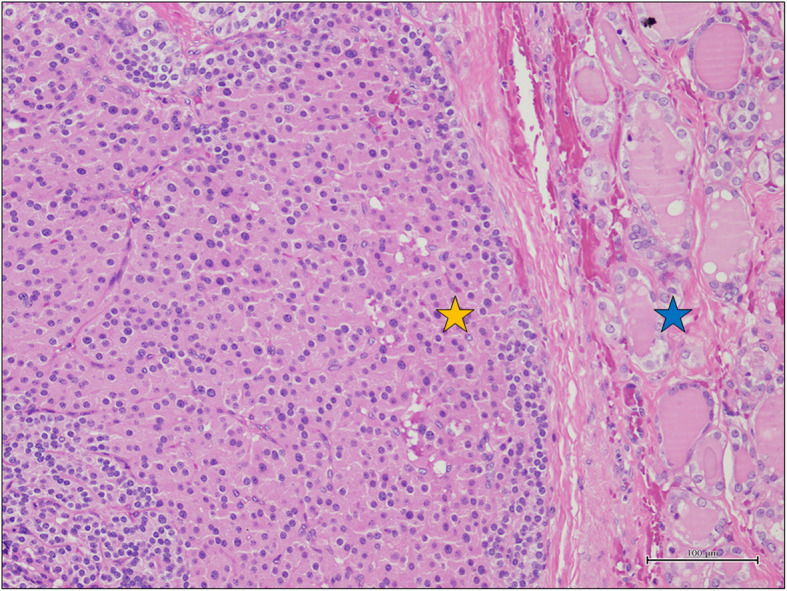
Nests of main cells (yellow star) associated with thyroid follicles (blue star) (H&E, original magnification 200x). Abbreviation: H&E: hematoxylin and eosin
Postoperatively, 131I ablation therapy was administered at a dose of 50 mCi.
The patient was followed up closely for both malignancies. US examination was performed every 6 months in the first year and every year later. TSH, FT3, FT4, Thyreoglobulin (Tg), Thyreoglobulin Antibodies, PTH, calcemia and phosphoremia was scheduled every 3 months.
Tg value was 6 ng/ml before I-131 therapy and remained < 0.2 ng/ml after treatment and for the 7 years follow-up; serum calcium, phosphorus and PTH levels remained in the respective normal ranges, with multiple negative neck US.
Discussion and conclusions
In literature only 11 cases of simultaneous occurrence of extrathyroidal parathyroid carcinoma and thyroid tumor has been reported. To the best of our knowledge, the association of intrathyroidal parathyroid carcinoma and thyroid tumor has never been described.
We have illustrated this unique association and performed a review of the last 40 years’ literature on the association of synchronous parathyroid carcinomas and non-medullary well differentiated thyroid carcinomas.
Parathyroid carcinoma is usually sporadic or related to other diseases [(e.g., multiple Endocrine Neoplasia (MEN) 1 and MEN 2, single familial hyperparathyroidism (FIHP), hyperparathyroidism “jaw tumor” (HPTJT)].
Studies of hereditary and syndromic forms of PC have revealed some genetic mechanisms underlying PC. Somatic mutations of CDC73 gene, involved in the Jaw tumor syndrome, would be the cause of hypercalcemic disorders and can be identified in up to 70% of patients with PC. In one-third of cases the mutations are germline [4, 36–38].
Nearly 90% of parathyroid carcinomas are hyperfunctioning versus 7–10% of non-functioning forms [4]; this percentage is confirmed in our literature review of synchronous parathyroid carcinoma and well differentiated thyroid tumor with a value of 9.09%. In non-functioning forms the patient typically presents late because of “mass effect” of a palpable tumor or lymphadenopathies (15–30%), whereas the lung, liver or bone metastases are found in one third of the cases [3].
Instead, hyperfunctioning forms usually present with nephrolithiasis, myasthenia, psychotic depression, osteoarthralgia; 7–12% of the cases have a parathyroid crisis, usually with serum PTH value > 16 mg/dl, characterized by cardiac arrhythmias and altered consciousness that can lead to coma [4, 39].
Currently, clinical, biochemical or imaging criteria are not able to distinguish benignant from malignant diseases. A malignant diagnosis should be suspected in the following conditions: a) fast onset of acute symptoms; b) serum calcemia > 14 mg/dL, usually associated to parathyroid carcinomas in 65–75% of the cases; c) serum PTH level beyond 10 times the normal value (81% predictive positive value); d) metastasis detection on the radiological investigations [4, 39, 40].
The routine use of FNC is unnecessary and should be avoided because of fibrosis and related reactive changes that can make histologic interpretation of benign versus malignant disease difficult [35]. Moreover, the risk of lump rupture with malignant cell spread should be considered. Therefore, FNC is indicated only in case of recurrence suspicion.
In order to obtain a definitive diagnosis, histopathological examination is necessary using Shantz and Castleman criteria, such as trabecular pattern, mitotic figures detection, thick fibrous band and capsular and blood vessel invasion [41]. The immunohistochemical exam (PTH, GATA3, TTF-1, PAX8 and thyroglobulin) is useful to identify the uncertain forms [20].
According to our literature review summarized in Table 1, in 12 reports with synchronous parathyroid malignancy and thyroid disease, 11 patients were hypercalcemic, whereas the one reported by Savli et al. [28] was normocalcemic; in all of the 11 extrathyroidal cases reported there were no preoperative suspect of malignancy.
Table 1.
Clinical features of 12 patients with synchronous parathyroid and thyroid carcinoma
| Author | Sex | Age | Calcium (mg/dl) | PTh (pg/ml) | Parathyroid Size (cm) | Carcinoma location | Thyroid Carcinoma- | Surgical Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Kurita et al., 1979 [27] | F | 68 | 12.2 | 6.3 | 4.2 × 3.2 × 2.4 | Left | Papillary | En-bloc resection | Normocalcemia |
| Christmas et al., 1988 [29] | F | 62 | Hypercalcemia | Unknown | Unknown | Lower Unknown | Follicular | Unknown | Died from metastatic parathyroid carcinoma |
| Savli et al., 2001 [28] | F | 47 | Normal | ND | Unknown | Unknown | Papillary | Total thyroidectomy, parathyroidectomy (Excision of 2 hyperplastic glands) |
Normocalcemia (1 year) |
| Schoretsanitis et al., 2002 [30] | F | 55 | 14.2 | > 1000 | 3 × 3 | Left Lower | Papillary (Follicular variant) | En-bloc resection |
Normocalcemia (2 years) |
| Lin et al., 2005 [24] | M | 38 | 16.5 | 351 | 4x3x3 | Left Lower | Papillary |
Total thyroidectomy, left parathyroidectomy |
Normocalcemia (6 years) |
| Goldfarb et al., 2009 [25] | M | 58 | 14.4 | 2023 | 3.4 × 3.3 × 2.2 | Left lower | Papillary | En-bloc resection | Normocalcemia after excision of contralateral parathyroid adenoma (1 year) |
| Marcy et al., 2009 [31] | F | 42 | 12 | 180 | Unknown | Right | Papillary | Total thyroidectomy, parathyroidectyomy | Normocalcemia |
| Chaychi et al., 2010 [26] | F | 79 | 10.4 | 89 | 5 | Left Lower | Papillary Multifocal | En-bloc Resection | Normocalcemia |
| Zakerkish et al., 2015 [32] | M | 21 | 11.5 | 1311 | Unknown | Unknown | Hürthle | Left thyroid lobo-istmectmy and cervical lymph node dissection | Persistent hypercalcemia after left lobectomy of thyroid and finally expire |
| Dikmen et al., 2017 [33] | M | 57 | 11.4 | 184 | 21 × 11 and 30 × 20 retrosternal | Left inferior and anterior mediastinum | Micropapillary | Excission en-bloc with thoracoscopy, parathytoidectomy, left inferior and lobectomy ipsilateral | Normocalcemia |
| Baek et al., 2017 [34] | F | 68 | 12.8 | 1247 | 40 × 30 | Left inferior | Papillary (follicular variant) | Parathyroidectomy, left inferior and lobectomy left thyroid | Normocalcemia (6 months) |
| Present case | M | 63 | 13.3 | 159 | 1.2 × 1 | Left-intra-thyroidal | Bilateral: Papillary classic and Papillary variant Follicular | Total thyroidectomy and left inferior parathyroidectomy | Normocalcemia (7 years) |
Preoperative diagnostic management is paramount to lead to the correct surgical approach of thyroidal and parathyroidal synchronous carcinomas.
In five out of 11 reported cases, a preoperative thyroidal FNC has been performed and was suggestive of malignancy; in the other six cases the diagnosis of malignancy was rendered at the histological examination.
In patients in whom the preoperative findings supported the diagnosis of thyroidal carcinoma, the planned surgical technique was to perform an en-bloc resection of parathyroids with total thyroidectomy, while ipsilateral and/or contralateral hemithyroidectomy was reserved to patients with the suspect of malignancy or with follicular thyroid neoplasm (Table 1).
Our experience and literature review assess that nowadays the treatment of this synchronous oncologic disease is incredibly challenging and specific guidelines are still missing.
In our case the preoperative clinical findings supported the diagnosis of a multinodular goiter (FNC on the major nodule Thy 2 according to Bethesda System), complicated by a tracheal deviation and symptoms of compression with a suspect of a parathyroidal adenoma.
Therefore, our patient underwent a total thyroidectomy because of his diagnosis and according to the gold standard surgical procedure. Moreover, this approach allowed the incidental discovery of the multicentric thyroid malignancy and the accidental excision of intracapsular parathyroid carcinoma.
In our experience, radioguided technique (gamma-probe) and the intraoperative PTH dosage turned out indispensable, in order to assess the completeness of the excision.
For the parathyroid carcinoma, indeed, bloc excision of the mass and any involved surrounding structures, preserving the integrity of the capsule, is the only curative approach for long term survival.
Ipsilateral lymphadenectomy of the central and/or laterocervical compartment is recommended in case of lymph node involvement evidence [3].
Recurrence of parathyroid cancer occurs in a range between 49 and 60% of the cases, with a mean time of 2.5–4.8 years after surgery [3]. Negative prognostic factors include young age and lymphadenopathies or metastases present at the onset. The most common recurrences are locoregional or distant metastasis involving lung, liver and bones [3, 39, 40, 42, 43].
Because of the exceptional rare condition for parathyroid cancer, there is no general consensus on treatment and follow-up [44]. According to the literature, patients should be followed up life-long measuring calcium and PTH and performing regular US surveillance [38, 44]. Moreover, our treatment strategy was based also on the evidence of thyroid malignancy; even if histopathological examinations revealed a multifocal thyroid microcarcinoma without signs of aggressive histology, the specific features of the patient, such as age > 60, sex, Tg value and patient preference have prompted us to recommend RAI remnant ablation, also according to nuclear counselling.
At 7 years-follow up, there is no evidence of both thyroidal and parathyroidal malignant recurrences or parathyroid hyperfunction.
According to our experience and recent studies, radical surgical approach is currently the goal standard of treatment for recurrences [3, 39, 40, 42, 45–47].
In fact, according to literature, only a few authors [48–54] attest the usefulness of radiotherapy and chemotherapy in improving rate of recurrence and survivor but the efficacy of adjuvant therapies has recently been disappointed in the management of recurrent parathyroid carcinoma, even if multicentric studies for improved long-term outcomes are still lacking [46, 47].
Nevertheless it is paramount to consider that the causes of death in patient with lymphatic recurrence or secondary malignance are more closely related to chronic and refractory hypercalcemia than the presence of metastatic mass [54].
In conclusion, our experience emphasizes that in case of intraoperative suspect of parathyroid carcinoma, an en-bloc resection of the parathyroid tumor and the ipsilateral thyroid lobe is mandatory, even if there is no evidence of thyroidal involvement.
This approach avoids a successive neck surgical procedure that could be justified by the high local and vascular aggressiveness of the carcinoma and could be related to serious and frequent complications as recurrent laryngeal nerve injury [3, 4, 45].
Acknowledgements
Not applicable.
Abbreviations
- IP
Intradthyroidal parathyroids
- FNC
Fine-needle cytology
- n.r
Normal range
- US
Ultrasound
- Tg
Thyreoglobulin
- PTH
Parathyroid hormone
- PC
Parathyroid carcinoma
- MEN
Multiple endocrine Neoplasia
- FIHP
Single familial hyperparathyroidism
- HPTJT
Hyperparathyroidism “jaw tumor”
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MDF, GS, MA, AR, GP, NDF and FC. AC, MGS, DP e SC participated substantially in the analysis and interpretation of the data. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The patient gave written informed consent to publish.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lappas D, Noussios G, Anagnostis P, Adamidou F, Chatzigeorgiou A, Skandalakis P. Location, number and morphology of parathyroid glands: results from a large anatomical series. Anat Sci Int. 2012;87(3):160–164. doi: 10.1007/s12565-012-0142-1. [DOI] [PubMed] [Google Scholar]
- 2.Mazeh H, Kouniavsky G, Schneider DF, Makris KI, Sippel RS, Dackiw AP, et al. Intrathyroidal parathyroid glands: small, but mighty (a Napoleon phenomenon) Surgery. 2012;152(6):1193–1200. doi: 10.1016/j.surg.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol. 2012;13(1):11–23. doi: 10.1007/s11864-011-0171-3. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kurd A, Mekel M, Mazeh H. Parathyroid carcinoma. Surg Oncol. 2014;23(2):107–114. doi: 10.1016/j.suronc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Vila Duckworth L, Winter WE, Vaysberg M, Moran CA, Al-Quran SZ. Intrathyroidal parathyroid carcinoma: report of an unusual case and review of the literature. Case Rep Pathol. 2013;2013:198643. doi: 10.1155/2013/198643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook MI, Qureshi YA, Todd CE, Cummins RS. An unusual ectopic location of a parathyroid carcinoma arising within the thyroid gland. J Clin Endocrinol Metab. 2012;97(6):1829–1833. doi: 10.1210/jc.2011-3115. [DOI] [PubMed] [Google Scholar]
- 7.Temmim L, Sinowatz F, Hussein WI, Al-Sanea O, El-Khodary H. Intrathyroidal parathyroid carcinoma: a case report with clinical and histological findings. Diagn Pathol. 2008;3(1):46. doi: 10.1186/1746-1596-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera-Hernández AA, Aranda-Valderrama P, Díaz-Pérez JA, Herrera LP. Intrathyroidal parathyroid carcinoma in a pediatric patient. Pediatr Surg Int. 2011;27(12):1361–1365. doi: 10.1007/s00383-011-2904-6. [DOI] [PubMed] [Google Scholar]
- 9.Tejera Hernández AA, Gutiérrez Giner MI, Vega Benítez V, Fernández San Millan D, Hernández JR. Intrathyroidal parathyroid carcinoma. A case report and review of literature. Endocrinol Nutr. 2016;63(1):46–48. doi: 10.1016/j.endonu.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Foppiani L, Del Monte P, Sartini G, Arlandini A, Quilici P, Bandelloni R, et al. Intrathyroidal parathyroid carcinoma as cause of hypercalcemia and pitfall of localization techniques: clinical and biologic features. Endocr Pract. 2007;13(2):176–181. doi: 10.4158/EP.13.2.176. [DOI] [PubMed] [Google Scholar]
- 11.Hussein WI, El-Maghraby TA, Al-Sanea O. Hyperfunctioning intrathyroidal parathyroid carcinoma. Saudi Med J. 2006;27(8):1226–1229. [PubMed] [Google Scholar]
- 12.Crescenzo DG, Shabahang M, Garvin D, Evans SR. Intrathyroidal parathyroid cancer presenting as a left neck mass. Thyroid. 1998;8(7):597–599. doi: 10.1089/thy.1998.8.597. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt JL, Perry RC, Philippsen LP, Wu HH. Intrathyroidal parathyroid carcinoma presenting with only hypercalcemia. Otolaryngol Head Neck Surg. 2002;127(4):352–353. doi: 10.1067/mhn.2002.128553. [DOI] [PubMed] [Google Scholar]
- 14.Ernst M, Lippmann M, Fleige B. Primary hyperparathyroidism in intrathyroid parathyroid cancer. Zentralbl Chir. 1993;118(11):682–685. [PubMed] [Google Scholar]
- 15.Kirstein LJ, Ghosh BC. Intrathyroid parathyroid carcinoma. J Surg Oncol. 2001;77(2):136–138. doi: 10.1002/jso.1084. [DOI] [PubMed] [Google Scholar]
- 16.Lee KM, Kim EJ, Choi WS, Park WS, Kim SW. Intrathyroidal parathyroid carcinoma mimicking a thyroid nodule in a MEN type 1 patient. J Clin Ultrasound. 2014;42(4):212–214. doi: 10.1002/jcu.22090. [DOI] [PubMed] [Google Scholar]
- 17.Balakrishnan M, George SA, Rajab SH, Francis IM, Kapila K. Cytological challenges in the diagnosis of intrathyroidal parathyroid carcinoma: a case report and review of literature. Diagn Cytopathol. 2018;46(1):47–52. doi: 10.1002/dc.23847. [DOI] [PubMed] [Google Scholar]
- 18.Alharbi N, Asa SL, Szybowska M, Kim RH, Ezzat S. Intrathyroidal parathyroid carcinoma: an atypical thyroid lesion. Front Endocrinol. 2018;9:641. doi: 10.3389/fendo.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quartey B, Shriver C, Russell D. Intrathyroidal parathyroid carcinoma presenting as asymptomatic high Normal serum calcium and slightly elevated intact parathyroid hormone: a case report and review of literature. World J Oncol. 2011;2(3):138–142. doi: 10.4021/wjon311w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong YP, Sharifah NA, Taan GC, Gill AJ, Ali SZ. Intrathyroidal oxyphilic parathyroid carcinoma: a potential diagnostic caveat in cytology? Diagn Cytopathol. 2016;44(8):688–692. doi: 10.1002/dc.23493. [DOI] [PubMed] [Google Scholar]
- 21.Leitha T, Staudenherz A. Concomitant hyperparathyroidism and nonmedullary thyroid cancer, with a review of the literature. Clin Nucl Med. 2003;28(2):113–117. doi: 10.1097/01.RLU.0000048680.30820.52. [DOI] [PubMed] [Google Scholar]
- 22.Ghorra C, Rizk H, Abi Hachem R, Tannoury J, Abboud B. Association of pathyroid pathology with well- differentiated thyroid carcinoma. Presse Med. 2012;41(6):e265–e271. doi: 10.1016/j.lpm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Campennì A, Giovinazo S, Pignata SA, Di Mauro F, Santoro D, Curtò L, et al. Association of parathyroid carcinoma and thyroid disorders: a clinical review. Endrocine. 2017;56:19–26. doi: 10.1007/s12020-016-1147-7. [DOI] [PubMed] [Google Scholar]
- 24.Lin SD, Tu ST, Hsu SR, Chang JH, Yang KT, Yang LH. Synchronous parathyroid and papillary thyroid carcinoma. J Chin Med Assoc. 2005;68(2):87–91. doi: 10.1016/S1726-4901(09)70141-8. [DOI] [PubMed] [Google Scholar]
- 25.Goldfarb M, O'Neal P, Shih JL, Hartzband P, Connolly J, Hasselgren PO. Synchronous parathyroid carcinoma, parathyroid adenoma, and papillary thyroid carcinoma in a patient with severe and long-standing hyperparathyroidism. Endocr Pract. 2009;15(5):463–468. doi: 10.4158/EP09075.CRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaychi L, Belbruno K, Golding A, Memoli V. Unusual manifestation of parathyroid carcinoma in the setting of papillary thyroid cancer. Endocr Pract. 2010;16(4):664–668. doi: 10.4158/EP10061.CR. [DOI] [PubMed] [Google Scholar]
- 27.Kurita S, Mihashi S, Hirano M, Nakashima T, Tanimura A. Hyperfunctioning parathyroid carcinoma combined with papillary carcinoma of the thyroid gland--report of a case (author's transl) Nihon Gan Chiryo Gakkai Shi. 1979;14(7):1127–1135. [PubMed] [Google Scholar]
- 28.Savli H, Sevinc A, Sari R, Ozen S, Buyukberber S, Ertas E. Occult parathyroid carcinoma in a patient with papillary thyroid carcinoma and Hashimoto's thyroiditis. J Endocrinol Investig. 2001;24(1):42–44. doi: 10.1007/BF03343807. [DOI] [PubMed] [Google Scholar]
- 29.Christmas TJ, Chapple CR, Noble JG, Milroy EJ, Cowie AG. Hyperparathyroidism after neck irradiation. Br J Surg. 1988;75:873–874. doi: 10.1002/bjs.1800750914. [DOI] [PubMed] [Google Scholar]
- 30.Schoretsanitis G, Melissas J, Kafousi M. Synchronous parathyroid and papillary thyroid carcinoma: a case report. Am J Otolaryngol. 2002;23(6):382–385. doi: 10.1053/ajot.2002.126317. [DOI] [PubMed] [Google Scholar]
- 31.Marcy PY, Thariat J, Sudaka A, Poissonnet G. Synchronous parathyroid and papillary thyroid carcinomas. Thyroid. 2009;19(10):1131–1133. doi: 10.1089/thy.2009.0016. [DOI] [PubMed] [Google Scholar]
- 32.Zakerkish M, Rajaei E, Dargahi M, Bahadoram M. A Rare Constellation of Hürthle cell Thyroid Carcinoma and Parathyroid Carcinoma. J Clin Diagn Res. 2015;9(12):OD08–OD10. doi: 10.7860/JCDR/2015/16540.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dikmen K, Bostanci H, Gobut H, Yildiz A, Ertunc O, Celik A, et al. Nonfunctional double parathyroid carcinoma with incidental thyroid micropapillary carcinoma : a rare case. Pan Afr Med J. 2017;27:241. doi: 10.11604/pamj.2017.27.241.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baek C-O, Kim KH, Song SK. Synchronous parathyroid carcinoma and papillary thyroid carcinoma in a patient with long-standing schizophrenia. Korean J Intern Med. 2017;32(6):1104–1107. doi: 10.3904/kjim.2015.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norman J, Politz D, Browarsky I. Diagnostic aspiration of parathyroid adenomas causes severe fibrosis complicating surgery and final histologic diagnosis. Thyroid. 2007;17(12):1251–1255. doi: 10.1089/thy.2007.0081. [DOI] [PubMed] [Google Scholar]
- 36.Sharretts JM, Simonds WF. Clinical and molecular genetics of parathyroid neoplasms. Best Pract Res Clin Endocrinol Metab. 2010;24(3):491–502. doi: 10.1016/j.beem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao L, Sun LH, Liu DM, He XY, Tao B, Ning G, Liu JM, Zhao HY. Copy number variation in CCND1 gene is implicated in the pathogenesis of sporadic parathyroid carcinoma. World J Surg. 2014;38(7):1730–1737. doi: 10.1007/s00268-014-2455-9. [DOI] [PubMed] [Google Scholar]
- 38.Salcuni AS, Cetani F, Guarnieri V, Nicastro V, Romagnoli E, De Martino D, et al. Parathyroid carcinoma. Best Pract Res Clin Endocrinol Metab. 2018;32(6):877–889. doi: 10.1016/j.beem.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Harari A, Waring A, Fernandez-Ranvier G, Hwang J, Suh I, Mitmaker E, Shen W, Gosnell J, Duh QY, Clark O. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab. 2011;96(12):3679–3686. doi: 10.1210/jc.2011-1571. [DOI] [PubMed] [Google Scholar]
- 40.Busaidy NL, Jimenez C, Habra MA, Schultz PN, El-Naggar AK, Clayman GL, et al. Parathyroid carcinoma: a 22-year experience. Head Neck. 2004;26(8):716–726. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 41.Shantz A, Castleman B. Parathyroid carcinoma: a study of 70 cases. Cancer. 1973;31(3):600–605. doi: 10.1002/1097-0142(197303)31:3<600::AID-CNCR2820310316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine. 1992;71(4):197–205. doi: 10.1097/00005792-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Talat N, Schulte KM. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann Surg Oncol. 2010;17(8):2156–2174. doi: 10.1245/s10434-010-1003-6. [DOI] [PubMed] [Google Scholar]
- 44.Ferraro V, Sgaramella LI, Di Meo G, Prete FP, Logoluso F, Minerva F, et al. Current concepts in parathyroid carcinoma: a single Centre experience. BMC Endocr Disord. 2019;19(Suppl 1):46. doi: 10.1186/s12902-019-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kebebew E, Arici C, Duh QY, Clark OH. Localization and reoperation results for persistent and recurrent parathyroid carcinoma. Arch Surg. 2001;136(8):878–885. doi: 10.1001/archsurg.136.8.878. [DOI] [PubMed] [Google Scholar]
- 46.Goswamy J, Lei M, Simo R. Parathyroid carcinoma. Curro Opin Otolaryngol Head Neck Surg. 2016;24(2):155–162. doi: 10.1097/MOO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 47.Limberg J, Stefanova D, Ullman TM, Thiesmeyer JW, Bains S, Beninato T, et al. The use and benefit of adjuvant radiotherapy in parathyroid carcinoma: a National Cancer Database Analysis. Ann Surg Oncol. 2021;28(1):502–511. doi: 10.1245/s10434-020-08825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calandra DB, Chejfec G, Foy BK, Lawrence AM, Paloyan E. Parathyroid carcinoma: biochemical and pathologic response to DTIC. Surgery. 1984;96(6):1132–1137. [PubMed] [Google Scholar]
- 49.Munson ND, Foote RL, Northcutt RC, Tiegs RD, Fitzpatrick LA, Grant CS, van Heerden JA, Thompson GB, Lloyd RV. Parathyroid carcinoma: is there a role for adjuvant radiation therapy? Cancer. 2003;98(11):2378–2384. doi: 10.1002/cncr.11819. [DOI] [PubMed] [Google Scholar]
- 50.Tochio M, Takaki H, Yamakado K, Uraki J, Kashima M, Nakatsuka A, Takao M, Shimamoto A, Tarukawa T, Shimpo H, Takeda K. A case report of 20 lung radiofrequency ablation sessions for 50 lung metastases from parathyroid carcinoma causing hyperparathyroidism. Cardiovasc Intervent Radiol. 2010;33(3):657–659. doi: 10.1007/s00270-009-9730-4. [DOI] [PubMed] [Google Scholar]
- 51.Denney AM, Watts NB. The effect of octreotide on parathyroid carcinoma. J Clin Endocrinol Metab. 2004;89(2):1016. doi: 10.1210/jc.2003-031825. [DOI] [PubMed] [Google Scholar]
- 52.Bukowski RM, Sheeler L, Cunningham J, Esselstyn C. Successful combination chemotherapy for metastatic parathyroid carcinoma. Arch Intern Med. 1984;144(2):399–400. doi: 10.1001/archinte.1984.00350140229032. [DOI] [PubMed] [Google Scholar]
- 53.Warrell RP, Jr, Issacs M, Alcock NW, Bockman RS. Gallium nitrate for treatment of refractory hypercalcemia from parathyroid carcinoma. Ann Intern Med. 1987;107(5):683–686. doi: 10.7326/0003-4819-107-5-683. [DOI] [PubMed] [Google Scholar]
- 54.Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM, Smallridge RC, Schwanauer LE, Olson KA, Klassen P, Bilezikian JP. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab. 2007;92(10):3803–3808. doi: 10.1210/jc.2007-0585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


