Dear editor,
The Kii Peninsula in Japan has a high incidence of endemic amyotrophic lateral sclerosis (ALS) and parkinsonism–dementia complex (PDC) (Kii ALS/PDC) 8. This disorder is a tauopathy that causes numerous neurofibrillary tangles (NFTs), especially in the medial temporal lobe and brainstem 9. A similar syndrome has been found in Guam, with dislocated and multinucleated Purkinje cells reported in the cerebellums of patients with Guam ALS/PDC 11. However, there are no detailed neuropathologic reports of the cerebellum in Kii ALS/PDC, with the exception of reports of tau pathology in the dentate nucleus 5, 9. Therefore, we examined the cerebellums of 10 patients with Kii ALS/PDC neuropathologically. We found dislocated and multinucleated Purkinje cells with tau deposition and various tau‐positive structures.
The cerebellums of 10 consecutive patients with clinicopathologically verified definite Kii ALS/PDC 6 and 10 controls (five female; mean age 69 ± 6.0 years, five male; mean age 73 ± 9.1 years, ± SD), recruited from the Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, were studied. There are some common cases between this study and previous studies 5, 9, and they were reanalyzed in a different higher sensitive method using a Ventana Discovery NX. We have added the patient numbers from the previous two studies to Table 1 to identify the common cases. To be eligible for a diagnosis of Kii ALS/PDC, patients had to be current or previous inhabitants of the Muro district or the surrounding areas of the Kii peninsula in the Wakayama and Mie prefectures. We defined the clinical forms as: (1) a, ALS type: classic ALS; b, PDC type: progressive parkinsonism (poor or non‐responsive to L‐dopa therapy) and/or dementia (abulia observed even in the early stage) or c, ALS/PDC type: combination of a and b. (2) Family history of ALS/PDC was taken into account also (ALS only, PDC only, or ALS/PDC). We also assessed neuropathological findings (3): a, Classic ALS neuropathology, with neurofibrillary tangles in the cerebral cortex and brainstem exceeding those that would normally be present because of the aging process; and b, substantia nigra and basal ganglionic degeneration, with abundant neurofibrillary tangles in the cerebral cortex and brainstem, exceeding that which would be usually present because of the aging process. “Exceeding those that would normally be present because of the aging process” means tau pathology was not limited to the limbic system and extended beyond the collateral sulcus or to the brain stem; or c, a + b. Based on these criteria, we diagnosed Possible Kii ALS/PDC, (1a) or (1b); Probable Kii ALS/PDC, (1c), (1a + 2) or (1b + 2), or Definite Kii ALS/PDC, possible or probable criteria met, along with 3a, 3b or 3c. Mean age was 70 years (range, 60–77 years). Previous repeat‐primed PCR analyses excluded repeat expansion mutations in C9orf72 3. The clinical and neuropathological profiles of the patients are shown in Table 1. Their cerebrum, brain stem and spinal cord pathology, including immunohistochemical results such as amyloid β, phosphorylated tau (p‐tau), phosphorylated alpha‐synuclein (p‐alpha‐synuclein) and phosphorylated TDP‐43 (p‐TDP‐43), were reminiscent of the previous reports 5, 7, 8, 9.
Table 1.
Clinical profiles, neuropathological abnormalities, and distribution of tau pathology in the cerebellums of patients with Kii ALS/PDC.
| Case | Age | Gender | DOI (year) | Phenotype | Types of degeneration | Tau pathology | Amyloid β, p‐syn, p‐TDP‐43 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | P | D | Dislocation of PCs | Bi‐ or Tri‐nucleated PCs | Torpedo | Grumose degeneration | Cortex | WM | |||||||||
| ML | PCs | GL | I | D* | DN | p62, ubiquilin2 | |||||||||||
| Case 1 (Case 2: Ref 7, Case 1: Ref 11) | 63 | F | 5 | + | − | − | + | − | + | + | + | − | ± | + | + | + | − |
| Case 2 (Case 1: Ref 7) | 70 | F | 13 | + | − | − | + | − | + | + | − | − | ± | − | ± | ± | − |
| Case 3 | 70 | F | 10 | + | − | − | − | − | + | + | ± | − | − | + | ± | + | − |
| Case 4 (Case 5: Ref 7, Case 7: Ref 11) | 77 | M | 7 | + | − | + | + | + | + | + | + | + | + | + | + | + | − |
| Case 5 (Case 4: Ref 7) | 65 | M | 3 | + | − | + | + | + | + | + | + | + | + | + | + | + | − |
| Case 6 (Case 8: Ref 7, Case 5: Ref 11) | 76 | F | 6 | + | + | + | + | + | + | + | + | − | + | + | + | + | − |
| Case 7 (Case 7: Ref 7, Case 6: Ref 11) | 60 | F | 7 | + | + | + | + | + | + | + | + | + | + | + | + | + | − |
| Case 8 (Case 9: Ref 7) | 70 | F | 12 | − | + | + | − | − | + | + | + | + | + | + * | + * | + | − |
| Case 9 | 75 | M | 7 | − | + | + | + | + | + | + | + | − | − | + | ± | + | − |
| Case 10 | 70 | F | 11 | − | + | + | + | + | + | + | + | + | + | + | + | + | − |
DOI, duration of illness; A, amyotrophy; P, parkinsonism; D, dementia; p‐syn, phosphorylated α‐synuclein; p‐TDP‐43, phosphorylated TDP‐43; Ub, ubiquitin; ML, molecular layer; PCs, Purkinje cells; GL, granular layer; WM, white matter; I, interlobar white matter; D*, deep white matter; DN, dentate nucleus.
±Several neuropil threads without inclusions.
*Severe white matter pathology.
Formalin‐fixed, paraffin‐embedded specimens of the cerebellums were cut into 9‐µm‐thick sections for hematoxylin and eosin, Klüver–Barrera, and Gallyas–Braak staining. Sections 6‐µm‐thick were cut for immunohistochemical studies using the avidin–biotin–peroxidase complex (ABC) method with a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA). We used a Ventana Discovery NX autoimmunostainer for immunostaining (Ventana Medical Systems Inc., Tucson, AZ, USA). The antibodies used were as follows: anti‐p‐tau antibody (AT‐8; monoclonal, Innogenetics, Gent, Belgium; 1:100), anti‐human Aβ 11–28 (12B2, monoclonal, 1:50 dilution, IBL, Maebashi, Japan), p‐alpha‐synuclein (pSyn#64, monoclonal, 1:20000 dilution, Wako, Osaka, Japan), anti‐phosphorylated TDP‐43 (p‐TDP‐43) (pSer409/410, monoclonal, 1:10000 dilution, Cosmo Bio, Tokyo, Japan), anti‐p62 antibody (p62 lck ligand; monoclonal, BD Biosciences, USA; 1:100), anti‐ubiquilin 2 antibody (5F5; monoclonal, Novus Biologicals, USA; 1:20000), anti‐3‐repeat tau isoforms (RD3, monoclonal, 1:500 dilution, Merck Millipore, Darmstadt, Germany) and anti‐4‐repeat tau isoforms (RD4, monoclonal, 1:500 dilution, Merck Millipore, Darmstadt, Germany and anti‐4R, polyclonal, 1:1000 dilution, Cosmo Bio, Koto‐ku, Japan) 1, 2. For the double‐labeling immunofluorescence study, deparaffinized sections were simultaneously incubated with polyclonal anti‐phosphorylated tau antibody (pSer262; polyclonal, Calbiochem, Germany; 1:200) and anti‐calbindin antibody for Purkinje cells (calbindin; monoclonal, Swant, Switzerland; 1:5000). The sections were examined using immunofluorescent substrates attached directly to the primary antibodies (Alexa Fluor 488 and 546; Life Technologies, Carlsbad, CA, USA). This study was approved by the ethics committees of Mie University Graduate School of Medicine and Tokyo Metropolitan Institute of Gerontology. Informed consent was obtained from patients or their families. Statistical analyses were conducted using JMP 11 software (SAS Institute Inc., Cary, NC, USA), performing Fisher's exact test and χ 2 test for comparisons of categorical data (the relationship between sex and the presence of AT‐8‐positive Purkinje cells). Following the Shapiro–Wilk normality test, Student's t‐tests were used for parametric analyses (the relationship between age or duration of illness and the presence of AT‐8‐positive Purkinje cells). The criterion for statistical significance was set to P < 0.05.
In all 10 patients with Kii ALS/PDC, there were some Purkinje cells that had torpedoes (Figure 1a), and many dentate nucleus neurons that were affected by grumose degeneration (Figure 1b). Six of the ten patients had dislocated and multinucleated Purkinje cells in the molecular layer (Figure 1c), and some of the dislocated Purkinje cells had AT‐8‐positive structures (Figure 1d). None of the controls had dislocated or multinucleated Purkinje cells. All patients showed p‐tau pathology of various types in the cerebellum. Five of the ten patients showed AT‐8‐positive structures, which had argyrophilic outlines of differing intensity, as shown by Gallyas–Braak silver staining or granular inclusions in the cytoplasm of Purkinje cells (Figure 1e–i). Double staining with pSer242 and calbindin confirmed that Purkinje cells had p‐tau protein (Figure 1s). The number of AT‐8‐positive structures in Purkinje cells of the posterior lobe was greater than that in the anterior lobe. They existed not only in both hemispheres (Figure 1t), but also in the vermis. There were no more than two AT‐8‐positive structures or granular inclusions in the Purkinje cells per slice. In the molecular layer, there was a stream of dendrites from the Purkinje layer to the surface of the molecular layer (Figure 1j), and argyrophilic structures that appeared as empty baskets beside the Purkinje cells. In the granular layer, the specific glial tau pathology included Bergmann glia‐like structures (Figure 1k), Golgi cells (Figure 1l), and sparkler‐shaped astrocytes (Figure 1m). Scattered coiled bodies (Figure 1n), neuronal loss, gliosis, NFTs (Figure 1o), patchy astrocytes (Figure 1p, q) and long neurites (Figure 1r) were present in the dentate nucleus. Many AT‐8‐positive argyrophilic threads appeared in the white matter, which had no tuft‐shaped astrocytes. The number of neuronal cells decreased in the dentate nucleus, and remained constant in Purkinje cell layer and granular layer. We found a mix of 3‐repeat and 4‐repeat tau splice variants deposited in the cerebellums of these cases. Anti‐amyloid β, p‐α‐synuclein, p‐TDP‐43, p62, and ubiquilin 2 immunostaining were negative in the cerebellums of all cases. None of the patients with tau‐positive inclusions had cerebellar symptoms. No relation was observed between NFTs in the Purkinje cells and the clinical profiles of age, sex or duration of illness statistically.
Figure 1.
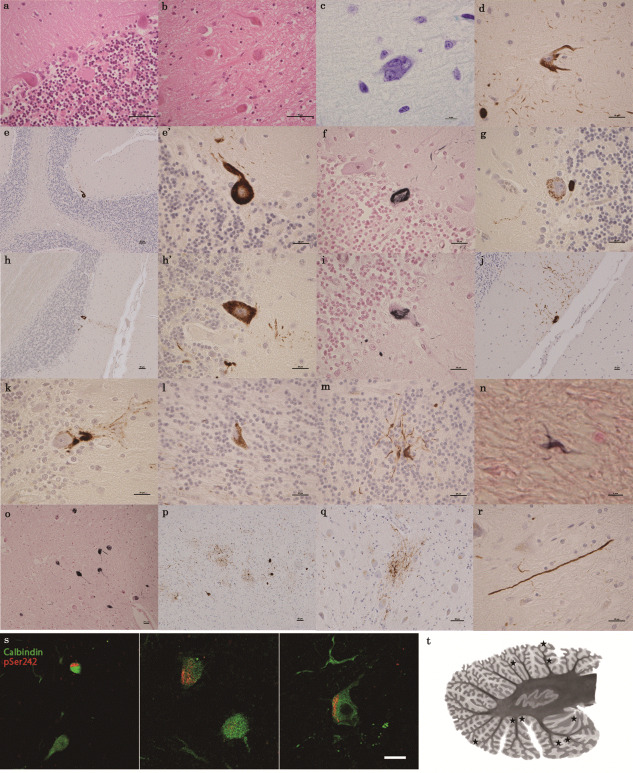
(a–r) Histopathological features of the cerebellar dentate nucleus and cerebellar cortex of patients with amyotrophic lateral sclerosis (ALS) and parkinsonism–dementia complex (PDC) endemic to the Kii Peninsula in Japan (Kii ALS/PDC). a: Purkinje cell torpedoes (hematoxylin and eosin [H&E]) (Case 7); b: grumose degeneration (H&E) (Case 7); c: dislocated multinuclear Purkinje cell (Klüver–Barrera stain) (Case 10); d: AT‐8‐positive dislocated Purkinje cell immunostained for phosphorylated tau (p‐tau) (AT‐8) (Case 7); e, e′ and h, h′: low‐power and high‐power images of tau‐immunostained Purkinje cells (AT‐8) (Case 7, 4); f, i: argyrophilic cytoplasmic inclusion of a Purkinje cell (Gallyas–Braak [GB] stain) (Case 7); g: tau‐positive granular cytoplasmic inclusion (AT‐8) (Case 5); j: AT‐8‐positive, waterfall‐like dendrites coexisting with Purkinje cell in the molecular layer (AT‐8) (Case 7); k: Bergmann glia–like structure in the Purkinje cell layer (AT‐8) (Case 4); l: Golgi cell in the granular cell layer (AT‐8) (Case 8); m: sparkler‐shaped astrocyte in the granular cell layer (AT‐8) (Case 6). In the dentate nucleus, n: coiled bodie (GB) (Case 7); o, p: neurofibrillary tangles (GB, AT‐8) (Case 7, 10); q, r: patchy astrocyte and long neurite (AT‐8) (Case 9, 6). (s) Double immunofluorescence staining showing localization of p‐tau (pSer242) and calbindin. Confocal images of Purkinje cells show colocalization. Alexa 546 (red) for p‐tau and Alexa 488 (green) for calbindin. Scale bar = 20 µm. (t) Distribution schema of all AT‐8‐positive Purkinje cells (stars). We plotted the location of a total of nine AT‐8 positive Purkinje cells in the five cases.
We detected unusual tau pathology in Purkinje cells as well as unique tau pathology in the dentate nucleus and glial cells in the cerebellums of patients with Kii ALS/PDC. Tau pathology in Purkinje cells has been reported only in the cerebellar ataxia form of progressive supranuclear palsy (PSP), in which tau‐positive inclusion bodies in Purkinje cells, neuronal loss with gliosis and higher densities of coiled bodies in the cerebellar dentate nucleus were observed 4. No tau pathology was observed in the Purkinje cells of patients with Guamanian ALS/PDC in previous studies (personal communication with Professor Asao Hirano at Montefiore Medical Center). However, the cerebellums of Guamanian ALS/PDC patients have not been analyzed using modern and the same methodologies such as the kind of antibodies for immunostaining and use of an auto‐immunostainer. Unlike patients with PSP, our patients with Kii ALS/PDC did not show cerebellar symptoms, although the reason is unclear. It may be that the cerebellar lesions of Kii ALS/PDC are milder than those of PSP, or that cerebellar ataxia may be masked by motor neuron symptoms or parkinsonism in these Kii ALS/PDC patients, unlike in patients with the cerebellar type of PSP. Ubiquitin‐related proteins such as p62 and ubiquilin 2 are not responsible for cerebellar lesions in patients with Kii ALS/PDC, although ubiquitin, p62 and ubiquilin 2 were reported in the granular layer in patients with the C9orf72 mutation.
Shiraki and Yase reported dislocated multinucleated Purkinje cells that deviated into the molecular layer in both Guam ALS/PDC and Kii ALS/PDC and postulated that this indicated some precocious senility process 11. Our results confirm the presence of dislocated and multinucleated Purkinje cells in the molecular layer of recent, consecutive patients with Kii ALS/PDC. Ectopic Purkinje cells scattered through the molecular layer have been reported in the context of developmental migration defects 10, Examining precocious senility processes and developmental anomalies may give insight into the etiology of Kii ALS/PDC.
In summary, patients with Kii ALS/PDC had dislocated, multinucleated Purkinje cells and various tau pathologies in the cerebellum. These cerebellar abnormalities may provide new insights into the pathomechanism of Kii ALS/PDC and may provide a neuropathological marker for the condition.
References
- 1. Dan A, Takahashi M, Masuda‐Suzukake M, Kametani F, Nonaka T, Kondo H et al (2013) Extensive deamidation at asparagine residue 279 accounts for weak immunoreactivity of tau with RD4 antibody in Alzheimer's disease brain. Acta Neuropathol Commun 211:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hasegawa M, Watanabe S, Kondo H, Akiyama H, Mann DM, Saito Y, Murayama S (2014) 3R and 4R tau isoforms in paired helical filaments in Alzheimer's disease. Acta Neuropathol 127:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ishiura H, Takahashi Y, Mitsui J, Yoshida S, Kihira T, Kokubo Y et al (2012) C9ORF72 repeat expansion in amyotrophic lateral sclerosis in the Kii peninsula of Japan. Arch Neurol 69:1154–1158. [DOI] [PubMed] [Google Scholar]
- 4. Kanazawa M, Shimohata T, Toyoshima Y, Tada M, Kakita A, Morita T et al (2009) Cerebellar involvement in progressive supranuclear palsy: a clinicopathological study. Mov Disord 24:1312–1318. [DOI] [PubMed] [Google Scholar]
- 5. Kokubo Y, Taniguchi A, Hasegawa M, Hayakawa Y, Morimoto S, Yoneda M et al (2012) α‐Synuclein pathology in the amyotrophic lateral sclerosis/parkinsonism dementia complex in the Kii Peninsula, Japan. J Neuropathol Exp Neurol 71:625–630. [DOI] [PubMed] [Google Scholar]
- 6. Kokubo Y (2015) Diagnostic criteria for amyotrophic lateral sclerosis/parkinsonism‐dementia complex in the Kii Peninsula, Japan. Brain Nerve 67:961–966. [DOI] [PubMed] [Google Scholar]
- 7. Kuzuhara S (2007) Revisit to Kii ALS–the innovated concept of ALS‐Parkinsonism‐dementia complex, clinicopathological features, epidemiology and etiology. Brain Nerve 59:1065–1074. [PubMed] [Google Scholar]
- 8. Kuzuhara S, Kokubo Y, Sasaki R, Narita Y, Yabana T, Hasegawa M, Iwatsubo T (2001) Familial amyotrophic lateral sclerosis and parkinsonism‐dementia complex of the Kii Peninsula of Japan: clinical and neuropathological study and tau analysis. Ann Neurol 49:501–511. [PubMed] [Google Scholar]
- 9. Mimuro M, Kokubo Y, Kuzuhara S (2007) Similar topographical distribution of neurofibrillary tangles in amyotrophic lateral sclerosis and parkinsonism‐dementia complex in people living in the Kii peninsula of Japan suggests a single tauopathy. Acta Neuropathol 113:653–658. [DOI] [PubMed] [Google Scholar]
- 10. Yang H, Jensen P, Goldowitz D (2002) The community effect and Purkinje cell migration in the cerebellar cortex: analysis of scrambler chimeric mice. J Neurosci 22:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yase Y, Shiaraki H (1991) ALS and parkinsonism‐dementia in the Kii peninsula. In: Handbook of Clinical Neurology. Vinken PJ, Bruyn GW, Klaxons HL (eds), pp. 273–300. Amsterdam, the Netherlands: North Holland Publishing Co. [Google Scholar]


