Abstract
Background: After the beginning and during the worldwide pandemic caused by the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), patients with allergic and atopic diseases have felt and still feel insecure. Currently, four vaccines against SARS-CoV-2 have been approved by the Paul Ehrlich Institute in Germany, and vaccination campaigns have been started nationwide. In this respect, it is of utmost importance to give recommendations on possible immunological interactions and potential risks of immunomodulatory substances (monoclonal antibodies, biologicals) during concurrent vaccination with the approved vaccines. Materials and methods: This position paper provides specific recommendations on the use of immunomodulatory drugs in the context of concurrent SARS-CoV-2 vaccinations based on current literature. Results: The recommendations are covering the following conditions in which biologicals are indicated and approved: 1) chronic inflammatory skin diseases (atopic dermatitis, chronic spontaneous urticaria), 2) bronchial asthma, and 3) chronic rhinosinusitis with nasal polyps (CRSwNP). Patients with atopic dermatitis or chronic spontaneous urticaria are not at increased risk for allergic reactions after COVID-19 vaccination. Nevertheless, vaccination may result in transient eczema exacerbation due to general immune stimulation. Vaccination in patients receiving systemic therapy with biologicals can be performed. Patients with severe asthma and concomitant treatment with biologicals also do not have an increased risk of allergic reaction following COVID-19 vaccination which is recommended in these patients. Patients with CRSwNP are also not known to be at increased risk for allergic vaccine reactions, and continuation or initiation of a treatment with biologicals is also recommended with concurrent COVID-19 vaccination. In general, COVID-19 vaccination should be given within the interval between two applications of the respective biological, that is, with a time-lag of at least 1 week after the previous or at least 1 week before the next biological treatment planned. Conclusion: Biologicals for the treatment of atopic dermatitis, chronic spontaneous urticaria, bronchial asthma, and CRSwNP should be continued during the current COVID-19 vaccination campaigns. However, the intervals of biological treatment may need to be slightly adjusted (DGAKI/AeDA recommendations as of March 22, 2021).
Keywords: allergy, antibodies, atopy, biologicals, COVID-19, vaccination
Introduction
During March 2020, the World Health Organization (WHO) declared a pandemic for severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2)-transmitted corona virus infectious disease 19 (COVID-19) [1]. Since then, numerous position papers and practical guidance for optimal care of patients have been published by international and national allergy societies [2, 3, 4, 5, 6]. In addition, recommendations for the use of immunomodulatory antibodies (biologicals) and allergen immunotherapy (AIT) products were formulated early during the pandemic and adapted to the German healthcare situation [7, 8, 9].
Abbreviations. Abbreviations.
| AeDA | German Society for Applied Allergology (Aerzteverband deutscher Allergologen) |
| COVID-19 | Corona virus infectious disease 19 |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| DGAKI | German Society of Allergology and Clinical Immunology (Deutsche Gesellschaft für Allergologie und Klinische Immunologie) |
| GPA | Society for Pediatric Allergology and Environmental Medicine (Gesellschaft für Pädiatrische Allergologie und Umweltmedizin) |
| EADV | European Academy of Dermatology and Venereology |
| EMA | European Medicines Agency |
| IgE | Immunoglobulin |
| IL | Interleukin |
| LABA | Long-acting β-agonists |
| LABA | Long-acting muscarinic antagonists |
| SARS-CoV2 | Severe acute respiratory syndrome coronavirus type 2 |
| SmpC | Summary of Product Characteristics |
| STIKO | (German) Standing Committee on Vaccination (Ständige Impfkommission) |
| WHO | World Health Organization |
At present, two mRNA-based vaccines (Comirnaty from BioNTech [10] and COVID-19 Vaccine from Moderna [11]) and two vector-based vaccines (Vaxzevria from AstraZeneca [12] and COVID-19 Vaccine from Johnson & Johnson [13]) have been approved by the European Medicines Agency (EMA) in Europe. Vaccination campaigns were initiated at the end of December 2020 in Germany. An interdisciplinary expert group “Management of Anaphylaxis” formed by the German Society of Allergology and Clinical Immunology (DGAKI), the German Society for Applied Allergology (AeDA), and the Society for Pediatric Allergology and Environmental Medicine (GPA) have published recommendations for the risk assessment of allergic reactions during COVID-19 vaccinations [14, 15]. Moreover, practical guidance has been provided for the management of patients at risk of anaphylaxis [16].
According to the Summary of Product Characteristics (SmPC) of the four authorized COVID-19 vaccines in Europe, immunosuppressive or immunomodulatory therapies including biologicals are not contraindicated, but it is noted in the SMPC’s leaflet that “efficacy may be lower in immunosuppressed patients” [10, 11, 12, 13]. On this basis, learned societies have issued preliminary recommendations for the use of biologicals during concurrent COVID-19 vaccinations. In a first ad hoc statement, the DGAKI advocates the concurrent use of monoclonal antibodies (benralizumab, dupilumab, mepolizumab, omalizumab, and reslizumab) and COVID-19 vaccines [17]. In line with a statement of the German Standing Committee on Vaccination (Ständige Impfkommission (STIKO)) on inactivated vaccines and concurrent immunomodulatory therapy [18], the German allergological societies published a joint declaration to schedule the COVID-19 vaccination in the middle of a therapy interval of biological treatment [17, 19, 20].
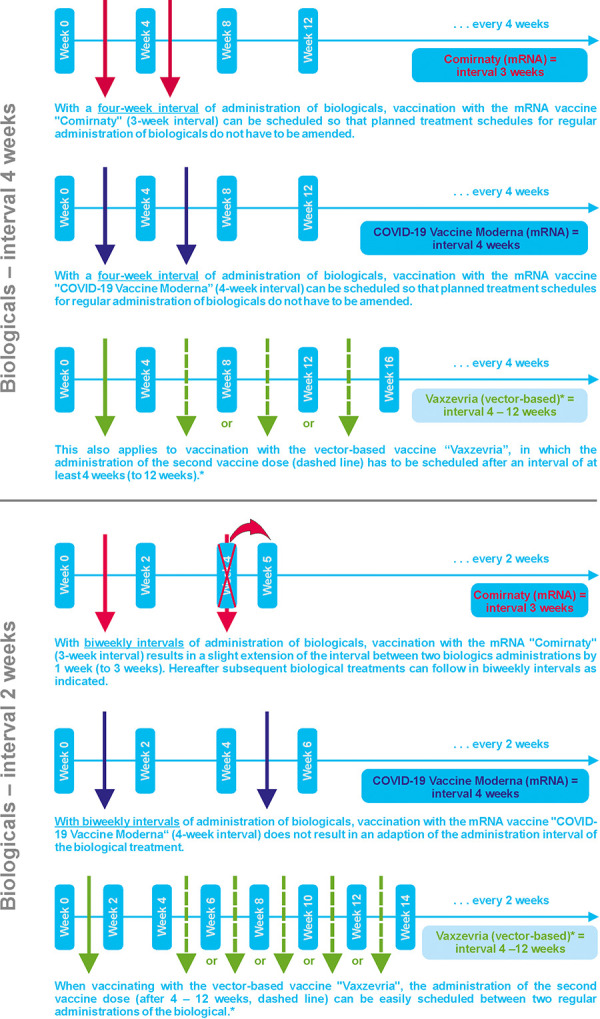
The aim of this position paper is to outline practical implications for the concurrent use of biologicals in different indications such as atopic dermatitis, chronic spontaneous urticaria, bronchial asthma, and chronic rhinosinusitis with nasal polyps (CRSwNP) with COVID-19 vaccination and to provide recommendations for best practice management (Table 1, Figure 1).
Table 1. DGAKI/AeDA recommendations (as of March 22, 2021).
| Diseases | Recommendations on COVID-19 vaccines | Recommendations on COVID-19 vaccines and biologicals |
|---|---|---|
| Atopic dermatitis | No increased risk of allergic reactions to COVID-19 vaccination. Vaccination possible at any time. Short-term eczema aggravation possible due to vaccination. | Vaccination can be applied at any time under dupilumab. Vaccination is recommended between two dupilumab injections with 1 week interval between vaccination and this biological treatment. |
| Chronic spontaneous urticaria | No increased risk of allergic reactions to COVID-19 vaccination. Vaccination possible at any time. Short-term eczema aggravation possible due to vaccination. | Vaccination can be applied at any time under omalizumab. Intervals between vaccinations and biological therapies as outlined above should be followed. |
| Bronchial asthma | No increased risk of allergic reactions to COVID-19 vaccination. Vaccination possible at any time. | Vaccination is recommended in patients with severe asthma and concurrent biological treatment. Intervals between vaccinations and biological therapies as outlined above should be followed. |
| Chronic rhinosinusitis with polyps (CRSwNP) | No increased risk of allergic reactions to COVID-19 vaccination. Vaccination possible at any time. | Continuation or initiation of biological therapy in CRSwNP is recommended (if indicated) with concurrent vaccination. Intervals between vaccinations and biological therapies as outlined above should be followed. |
Figure 1. Recommendations for time intervals between COVID-19 vaccines and biologicals. *The vector-based vaccine “COVID-19 Vaccine Janssen” is administered as a single-dose only.
Chronic inflammatory skin diseases
Chronic inflammatory skin diseases such as atopic dermatitis and chronic spontaneous urticaria are commonly treated with systemic immunomodulatory therapy in severe courses, which arises concern about potential risks of COVID-19 infection and vaccination under this systemic therapy [21].
Patients with atopic dermatitis are not at increased risk for allergic reactions to COVID-19 vaccination. Nevertheless, vaccination may result in transient eczema exacerbation due to general immune stimulation.
Topic anti-inflammatory local therapy with both steroids and calcineurin inhibitors does not influence the efficacy of vaccination. Besides, patients receiving systemic therapy with ciclosporin, methotrexate, azathioprine, or baricitinib can be vaccinated at any time. However, the effect of vaccination may be reduced by the systemic immunosuppression [22, 23]. Therefore, the European Task Force on Atopic Dermatitis of the European Academy of Dermatology and Venereology (EADV) recommends either a temporary interruption of treatment or a reduced dosage of the aforementioned substances (details in [24]).
Patients receiving systemic therapy with dupilumab can also be vaccinated at any time. Data from a previous study of patients treated with dupilumab receiving vaccination with tetanus toxoid demonstrated that the efficacy of the vaccination was not adversely affected by dupilumab treatment [25]. Currently, for practical reasons, it is recommended that vaccination be administered between dupilumab injections. If specific time intervals need to be observed regarding vaccination, dupilumab injections should be adjusted accordingly [24]. In general, COVID-19 vaccination should be applied in the treatment interval between two injections of the biological, that is, with a time-lag of at least 1 week after the previous or at least 1 week before the next biological treatment planned. This recommendation also applies to the biologicals listed below.
Patients with chronic spontaneous urticaria also have no increased risk of allergic reactions to COVID-19 vaccinations. Nevertheless, vaccination may result in transient exacerbation due to general immune stimulation. Systemic antihistamines do not impact the effect of the vaccination. Patients treated with systemic steroids and/or ciclosporin can be vaccinated at any time. However, the effect of vaccination may be reduced by this systemic immunosuppression as well, so immunological response to COVID-19 vaccination should be verified by serum antibody levels, if applicable. Patients treated with omalizumab can be vaccinated at any time. As with other biologicals, it is recommended that vaccination be administered between omalizumab injections (see above) and slight amendments of the intervals for biological treatment may be followed as stated above.
Bronchial asthma
For the treatment of severe uncontrolled bronchial asthma, biologicals are approved as an add-on treatment option to standard anti-inflammatory treatment with inhaled corticosteroids + long-acting β-agonists (LABA) and long-acting muscarinic antagonists (LAMA). They are targeting key mechanisms of the inflammatory cascade in bronchial asthma with high efficacy and a good safety profile in this indication. In this respect, it is of utmost importance to further focus on possible immunological interactions of biologicals during concurrent vaccination with the approved vaccines.
The asthmatic inflammatory response is based on the T2 immune response, in which immunoglobulin E (IgE) and the cytokines interleukin (IL-)4, IL-5, and IL-13 in particular are key elements targeted by biologicals. It is important to note that neither IgE nor the aforementioned cytokines play a role in the antiviral immune response. Study data on vaccine responses to other antiviral vaccines and concurrent treatment with biologicals are also available with no hint for a blunting of the immune response by these vaccinations [25, 26]. Moreover, previous studies have demonstrated that anti-IgE treatment indeed increases the type-1 interferon production, thereby enhancing antiviral response [27, 28]. Preliminary data in asthmatic patients with a COVID-19 infection indicate no difference in antibody production against SARS-CoV2 in patients with concurrent biological treatment in comparison to patients without this therapy [29].
As a general rule, a risk/benefit assessment must be followed for all vaccines and concurrent treatment with biologicals. In patients with severe asthma and concurrent biological therapy, vaccination against SARS-CoV-2 is recommended, and the intervals for biological treatment may be slightly amended as stated above.
Chronic rhinosinusitis with nasal polyps
To date, there is no evidence that patients with chronic rhinosinusitis (with or without nasal polyps) may have an increased likelihood of allergic reactions following any of the various COVID-19 vaccines. This holds also true for susceptibility to SARS-CoV-2 infection or severity of COVID-19 disease [30].
In the majority of patients with CRSwNP, a type-2 directed inflammation is the underlying pathomechanism involved with multiple inflammatory pathways [31, 32]. As those are also included in the inflammatory pathways of bronchial asthma, most of the antibodies approved in this indication have also been shown to be effective in CRSwNP [33].
As an add-on treatment for severe, uncontrolled forms of CRSwNP, two biologicals have been recently approved in Germany (dupilumab and omalizumab). Based on preliminary trial data, two additional antibodies may gain market authorization in due time (mepolizumab and benralizumab). The application and dosage of these four preparations are identical to those for asthma therapy. Therefore, the same considerations regarding their compatibility with COVID-19 vaccinations specified above for bronchial asthma also apply in principle to biological treatment of CRSwNP.
In line with the recommendations outlined above, continuation or initiation of a biological treatment for CRSwNP is recommended with a concurrent planning of a COVID-19 vaccination. For the biologicals indicated in CRSwNP, general recommendations regarding the interval between vaccinations and biological therapies as outlined above should be followed [17, 19, 20].
Conclusion
Currently, there is no evidence of an increased allergic risk from COVID-19 vaccinations in patients with chronic inflammatory skin diseases (atopic eczema, chronic spontaneous urticaria), bronchial asthma, and CRSwNP. Approved immunomodulatory biologicals with proven high efficacy and safety are indicated in uncontrolled courses of these diseases.
These therapies should not be temporarily interrupted due to a planned COVID-19 vaccination as they are not contraindicated for these vaccines. However, a COVID-19 vaccination should be given within the interval between two applications of the respective biological, that is, with a time-lag of at least 1 week after the previous or at least 1 week before the next biological treatment planned.
Funding
None.
Conflict of interest
O. Pfaar reports grants and/or honoraria from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B. V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Inmunotek S.L., Lofarma, Biomay, Circassia, ASIT Biotech Tools S. A., Laboratorios LETI/LETI Pharma, MEDA Pharma/MYLAN, Anergis S. A., Mobile Chamber Experts (a GA2LEN Partner), Indoor Biotechnologies, GlaxoSmithKline, Astellas Pharma Global, EUFOREA, ROXALL Medizin, Novartis, Sanofi Aventis, Sanofi Genzyme, Med Update Europe GmbH, streamedup! GmbH, Pohl-Boskamp GmbH, John Wiley and Sons AS, Paul Martini Foundation (PMS), during the last 36 months and all outside the present work.
L. Klimek reports grants and/or honoraria from Allergopharma, Bioprojet, Viatris, HAL Allergie, ALK Abelló, Aimmune, LETI Pharma, Stallergenes, Quintiles, Sanofi, ASIT Biotech, Lofarma, Thermofisher, Roxall, Allergy Therapeutics, AstraZeneca, GSK, Inmunotek, outside the submitted work; and membership in the following organizations: AeDA, DGHNO, German Academy of Allergology and Clinical Immunology, German Allergy League; ENT-BV, GPA, EAACI.
R. Brehler reports honoraria from ALK Abelló, Allergopharma, Allmiral, AstraZeneca, Bencard, Gesellschaft zur Förderung der Dermatologischen Forschung und Fortbildung e.V., GSK, HAL Allergie, LETI Pharma, MedUpdate, Merck, Novartis, Sanofi, Stallergenes, outside the submitted work; and membership in the following organizations: AeDA, DGAKI, EAACI, ABD.
M. Wagenmann reports honoraria from ALK-Abello, Allergopharma, AstraZeneca, Bencard, Genzyme, GlaxoSmithKline, HAL Allergy, LETI, Meda Pharma, Novartis, Sanofi Aventis, Stallergenes, all outside the present work.
N. Novak reports honoraria from Alk Abello, Stallergens Geer, Hal Allergy, Leti Pharma, Sanofi Genzyme, Abbvie, Leo Pharma, Novartis, streamed up und Blueprint, all outside the present work.
M. Worm reports conflicts of interest by Regeneron Pharmaceuticals, DBV Technologies S.A, Stallergenes GmbH, HAL Allergy GmbH, Bencard Allergie GmbH, Allergopharma GmbH & Co. KG, ALK-Abelló Arzneimittel GmbH, Mylan Germany GmbH, Leo Pharma GmbH, Sanofi-Aventis Deutschland GmbH, Aimmune Therapeutics UK Limited, Actelion Pharmaceuticals Deutschland GmbH, Novartis AG, Biotest AG, AbbVie Deutschland GmbH & Co. KG, Lilly Deutschland GmbH – all outside the present work.
The other authors declare no conflicts of interest.
References
- 1. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Date of accessed: March 02, 2021).
- 2. Pfaar O Torres MJ Akdis CA COVID-19: A series of important recent clinical and laboratory reports in immunology and pathogenesis of SARS-CoV-2 infection and care of allergy patients. Allergy. 2021; 76: 622–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cardinale F Ciprandi G Barberi S Bernardini R Caffarelli C Calvani M Cavagni G Galli E Minasi D Del Giudice MM Moschese V Novembre E Paravati F Peroni DG Tosca MA Traina G Tripodi S Marseglia GL Consensus statement of the Italian society of pediatric allergy and immunology for the pragmatic management of children and adolescents with allergic or immunological diseases during the COVID-19 pandemic. Ital J Pediatr. 2020; 46: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaker MS Oppenheimer J Grayson M Stukus D Hartog N Hsieh EWY Rider N Dutmer CM Vander Leek TK Kim H Chan ES Mack D Ellis AK Lang D Lieberman J Fleischer D Golden DBK Wallace D Portnoy J Mosnaim G COVID-19: Pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020; 8: 1477–1488.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riggioni C Comberiati P Giovannini M Agache I Akdis M Alves-Correia M Antó JM Arcolaci A Azkur AK Azkur D Beken B Boccabella C Bousquet J Breiteneder H Carvalho D De Las Vecillas L Diamant Z Eguiluz-Gracia I Eiwegger T Eyerich S A compendium answering 150 questions on COVID-19 and SARS-CoV-2. Allergy. 2020; 75: 2503–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pfaar O Klimek L Jutel M Akdis CA Bousquet J Breiteneder H Chinthrajah S Diamant Z Eiwegger T Fokkens WJ Fritsch HW Nadeau KC O’Hehir RE O’Mahony L Rief W Sampath V Schedlowski M Torres MJ Traidl-Hoffmann C Wang Y COVID-19 pandemic: Practical considerations on the organization of an allergy clinic-An EAACI/ARIA Position Paper. Allergy. 2021; 76: 648–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klimek L Pfaar O Worm M Eiwegger T Hagemann J Ollert M Untersmayr E Hoffmann-Sommergruber K Vultaggio A Agache I Bavbek S Bossios A Casper I Chan S Chatzipetrou A Vogelberg C Firinu D Kauppi P Kolios A Kothari A Use of biologicals in allergic and type-2 inflammatory diseases during the current COVID-19 pandemic: Position paper of Ärzteverband Deutscher Allergologen (AeDA)A, Deutsche Gesellschaft für Allergologie und Klinische Immunologie (DGAKI)B, Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA)C, Österreichische Gesellschaft für Allergologie und Immunologie (ÖGAI)D, Luxemburgische Gesellschaft für Allergologie und Immunologie (LGAI)E, Österreichische Gesellschaft für Pneumologie (ÖGP)F in co-operation with the German, Austrian, and Swiss ARIA groupsG, and the European Academy of Allergy and Clinical Immunology (EAACI)H. Allergol Select. 2020; 4: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klimek L Pfaar O Worm M Bergmann KC Bieber T Buhl R Buters J Darsow U Keil T Kleine-Tebbe J Lau S Maurer M Merk H Mösges R Saloga J Staubach P Stute P Rabe K Rabe U Vogelmeier C Allergen immunotherapy in the current COVID-19 pandemic: A position paper of AeDA, ARIA, EAACI, DGAKI and GPA: Position paper of the German ARIA GroupA in cooperation with the Austrian ARIA GroupB, the Swiss ARIA GroupC, German Society for Applied Allergology (AEDA)D, German Society for Allergology and Clinical Immunology (DGAKI)E, Society for Pediatric Allergology (GPA)F in cooperation with AG Clinical Immunology, Allergology and Environmental Medicine of the DGHNO-KHCG and the European Academy of Allergy and Clinical Immunology (EAACI)H. Allergol Select. 2020; 4: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfaar O Klimek L Worm M Bergmann KC Bieber T Buhl R Buters J Darsow U Keil T Kleine-Tebbe J Lau S Maurer M Merk H Mösges R Saloga J Staubach P Stute P Rabe K Rabe U Vogelmeier C [Handling of allergen immunotherapy in the COVID-19 pandemic: An ARIA-EAACI-AeDA-GPA-DGAKI Position Paper (Pocket-Guide)]. Laryngorhinootologie. 2020; 99: 676–679. [DOI] [PubMed] [Google Scholar]
- 10. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (Date of accessed: March 12, 2021)
- 11. https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-moderna-epar-product-information_en.pdf (Date of accessed: March 12, 2021)
- 12. https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf (Date of accessed: March 28, 2021).
- 13. https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf (Date of accessed: March 28, 2021).
- 14. Worm M Ring J Klimek L Jakob T Lange L Treudler R Beyer K Werfel T Biedermann T Bircher A Fischer M Fuchs T Heller AR Hoffmann F Huttegger I Kopp MV Kugler C Lommatzsch M Pfaar O Rietschel E [Covid-19 vaccination and risk of anaphylaxis – Recommendations for practical management]. MMW Fortschr Med. 2021; 163: 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klimek L Novak N Hamelmann E Werfel T Wagenmann M Taube C Bauer A Merk H Rabe U Jung K Schlenter W Ring J Chaker A Wehrmann W Becker S Mülleneisen N Nemat K Czech W Wrede H Brehler R Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA: Position statement of the German Allergy Societies: Medical Association of German Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J Int. 2021; 24: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klimek L Worm M Lange L Beyer K Rietschel E Vogelberg C Schnadt S Stöcker B Brockow K Hagemann J Bieber T Wehrmann W Becker S Freudelsperger L Mülleneisen NK Nemat K Czech W Wrede H Brehler R Fuchs T Management von Anaphylaxie-gefährdeten Patienten während der Covid-19-Pandemie: Ein Positionspapier des Ärzteverbandes Deutscher Allergologen (AeDA)A, der Deutschen Gesellschaft für Allergologie und klinische Immunologie (DGAKI)B, der Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA)C und des Deutschen Allergie- und Asthmabundes (DAAB)D. Allergo J. 2020; 29: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleine-Tebbe J Klimek L Hamelmann E Pfaar O Taube C Wagenmann M Werfel T Worm M Severe allergic reactions to the COVID-19 vaccine – statement and practical consequences. Allergol Select. 2021; 5: 26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wagner N Assmus F Arendt G Baum E Baumann U Bogdan C Burchard G Föll D Garbe E Hecht J Müller-Ladner U Niehues T Überla K Vygen-Bonnet S Weinke T Wiese-Posselt M Wojcinski M Zepp F Impfen bei Immundefizienz : Anwendungshinweise zu den von der Ständigen Impfkommission empfohlenen Impfungen. (IV) Impfen bei Autoimmunkrankheiten, bei anderen chronisch-entzündlichen Erkrankungen und unter immunmodulatorischer Therapie. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019; 62: 494–515. [DOI] [PubMed] [Google Scholar]
- 19. https://dgaki.de/wp-content/uploads/2021/03/Impf_info_A4_web_1503.pdf (Date of accessed: March 12, 2021).
- 20. Klimek L Bergmann KC Brehler R Allergologische Diagnostik und Therapien bei COVID-19-Impfungen: Praktische Handlungsempfehlungen. Allergo J Int. 2021; in press.
- 21. Buhl T Beissert S Gaffal E Goebeler M Hertl M Mauch C Reich K Schmidt E Schön MP Sticherling M Sunderkötter C Traidl-Hoffmann C Werfel T Wilsman-Theis D Worm M COVID-19 and implications for dermatological and allergological diseases. J Dtsch Dermatol Ges. 2020; 18: 815–824. [DOI] [PubMed] [Google Scholar]
- 22. Furer V Rondaan C Heijstek MW Agmon-Levin N van Assen S Bijl M Breedveld FC D’Amelio R Dougados M Kapetanovic MC van Laar JM de Thurah A Landewé RB Molto A Müller-Ladner U Schreiber K Smolar L Walker J Warnatz K Wulffraat NM 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020; 79: 39–52. [DOI] [PubMed] [Google Scholar]
- 23. Winthrop KL Bingham CO Komocsar WJ Bradley J Issa M Klar R Kartman CE Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther. 2019; 21: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thyssen JP Vestergaard C Barbarot S de Bruin-Weller MS Bieber T Taieb A Seneschal J Cork MJ Paul C Flohr C Weidinger S Trzeciak M Werfel T Heratizadeh A Darsow U Simon D Torrelo A Chernyshov PV Stalder JF Gelmetti C Szalai Å Svensson Å von Kobyletzki LB De Raeve L Fölster-Holst R Christen-Zaech S Hijnen DJ Gieler U Gutermuth J Bangert C Spuls PI Kunz B Ring J Wollenberg A Deleuran M European Task Force on Atopic Dermatitis: position on vaccination of adult patients with atopic dermatitis against COVID-19 (SARS-CoV-2) being treated with systemic medication and biologics. J Eur Acad Dermatol Venereol. 2021; epub ahead of print. [DOI] [PMC free article] [PubMed]
- 25. Blauvelt A Simpson EL Tyring SK Purcell LA Shumel B Petro CD Akinlade B Gadkari A Eckert L Graham NMH Pirozzi G Evans R Dupilumab does not affect correlates of vaccine-induced immunity: A randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J Am Acad Dermatol. 2019; 80: 158–167.e1. [DOI] [PubMed] [Google Scholar]
- 26. Zeitlin PL Leong M Cole J Mallory RM Shih VH Olsson RF Goldman M Benralizumab does not impair antibody response to seasonal influenza vaccination in adolescent and young adult patients with moderate to severe asthma: results from the Phase IIIb ALIZE trial. J Asthma Allergy. 2018; 11: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Durrani SR Montville DJ Pratt AS Sahu S DeVries MK Rajamanickam V Gangnon RE Gill MA Gern JE Lemanske RF Jackson DJ Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012; 130: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esquivel A Busse WW Calatroni A Togias AG Grindle KG Bochkov YA Gruchalla RS Kattan M Kercsmar CM Khurana Hershey G Kim H Lebeau P Liu AH Szefler SJ Teach SJ West JB Wildfire J Pongracic JA Gern JE Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am J Respir Crit Care Med. 2017; 196: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhalla A Mukherjee M Radford K Nazy I Kjarsgaard M Bowdish DME Nair P Dupilumab, severe asthma airway responses, and SARS‐CoV‐2 serology. Allergy. 2021; 76: 957–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang M Wang C Zhang L Inflammatory endotypes of CRSwNP and responses to COVID-19. Curr Opin Allergy Clin Immunol. 2021; 21: 8–15. [DOI] [PubMed] [Google Scholar]
- 31. Bachert C Han JK Wagenmann M Hosemann W Lee SE Backer V Mullol J Gevaert P Klimek L Prokopakis E Knill A Cavaliere C Hopkins C Hellings P EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J Allergy Clin Immunol. 2021; 147: 29–36. 33227318 [Google Scholar]
- 32. Agache I Song Y Alonso-Coello P Vogel Y Rocha C Solà I Santero M Akdis C Akdis M Canonica GW Chivato T Del Giacco S Eiwegger T Fokkens W Georgalas C Gevaert P Hopkins C Klimek L Lund V Naclerio R O’Mahony L Palkonen S Pfaar O Schwarze MB Soyka MB Wang Y Zhang L Canelo-Aybar C Palomares O Jutel M Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps A systematic review for the EAACI Guidelines. Allergy. 2021; epub ahead of print. [DOI] [PubMed]
- 33. Fokkens WJ Lund V Bachert C Mullol J Bjermer L Bousquet J Canonica GW Deneyer L Desrosiers M Diamant Z Han J Heffler E Hopkins C Jankowski R Joos G Knill A Lee J Lee SE Mariën G Pugin B EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019; 74: 2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]


