Abstract
Cortical grey matter (GM) demyelination is present from the earliest stages of multiple sclerosis (MS) and is associated with physical deficits and cognitive impairment. In particular, the rate of disability progression in MS, both in the relapsing and progressive phases, appears to be strictly associated with degenerative GM demyelination and diffuse cortical atrophy. In the last decade, several histopathological studies and advanced radiological methodologies have contributed to better identify the exact involvement/load of cortical pathology in MS, even if the specific inflammatory features and the precise cell and molecular mechanisms of GM demyelination and neurodegeneration in MS remain still not fully understood. It has been proposed that a combined neuropathology, imaging and molecular approach may help to define a more detailed characterization and precise assessment of the heterogeneous features of GM injury and inflammation in MS. This, in turn, will possibly identify specific imaging and biohumoral (cerebrospinal fluid/serum) correlates of cortical pathology that may have an important role in predicting and monitor the disease evolution.
Keywords: Grey matter lesions, Cortical pathology, Atrophy, Intrathecal inflammation, Meningeal infiltrates
CORTICAL LESIONS IN MULTIPLE SCLEROSIS
Multiple sclerosis (MS) has long been considered a predominantly white matter (WM) disease, based on the obvious demyelination detectable in the WM by histological staining and conventional magnetic resonance imaging (MRI). WM lesions and perivascular inflammation are undoubtedly of major importance in contributing to focal clinical deficits in MS during the relapsing‐remitting (RR) disease stage, but neurodegeneration is thought to play a key role in the progressive stage of the disease and to drive clinical disease progression 50.
Although already highlighted in earlier studies that investigated cortical grey matter (GM) pathology in MS 8, 18, it is only during the last 15 years that immunohistochemical and advanced imaging techniques have allowed establishing the true extent and clinical impact of GM involvement in MS. The pathology of progressive MS is characterized by the combined accumulation of chronic demyelinated lesions and axonal loss in the WM, diffuse changes to the normal appearing WM and diffuse and focal cortical GM demyelination and neurodegeneration 16, 26, 29, 33, 37, 43.
Even though demyelinated cortical lesions and brain atrophy are considered as one of the substrates of MS disease progression 12, 23, 25, 51, 65, 66, they have also been shown to be present from the earliest stages of the disease 16, 20, 40. Furthermore, significant neurodegeneration, including axonal abnormalities and neuronal and synaptic loss, is thought to represent the main pathological substrate of cognitive deterioration at all disease stages 11, 14, 30, 64.
The high prevalence of demyelination in GM areas in MS was detected by a study combining MRI and conventional histology data on 12 postmortem brains 34. It was hypothesized that the distribution of cortical lesions might be causally related to vascular anatomy, which resulted in the definition of seven distinct types of cortical lesions. Later, another classification system was developed that distinguished between three major cortical lesion types, based on immunohistochemical detection of myelin 47. This is the scoring system that is currently widely used for classification of cortical lesions in MS tissue: type 1 lesions extend across both WM and gray matter and are often called leucocortical lesions; type 2 lesions are contained within the cerebral cortex GM and often occur around a blood vessel; type 3 lesions are subpial and affect the largest cortical area.
In one study, type 3 leucocortical lesions represented 18% of lesions and accounted for 14.5% of the demyelinated area, type 2 intracortical lesions represented 17% of GM lesions and accounted for 1.2% of the cortical lesions area and type 3 subpial lesions represented 60% of the lesions and accounted for 67% of the total cortical demyelinated area 5. A common appearance of type 3 lesions was that of long ribbons of subpial demyelination, often affecting several adjacent gyri. Other type 3 lesions were wedge‐shaped, with the base at the surface of the brain. Additionally, a combination of these patterns, with wedge‐like lesion areas within bands of more superficial subpial demyelination, was often present.
When this classification system was applied to biopsy samples from patients with early stages of MS, 37% revealed clear evidence of cortical demyelination and general cortical subpial demyelination has been identified as a distinct pattern occurring in a significant subpopulation of MS patients, particularly those with a long and progressive disease course 5. A more recent study of GM and WM demyelination in different regions of the CNS (motor cortex, cingulate, cerebellum, spinal cord and thalamus) found that the area covered by demyelination was greater in the cortex than in the WM at each anatomical site, but in particular GM demyelination was most extensive in the spinal cord and cerebellum 29.
Cortical GM lesions are characterized by the relative absence of lymphocyte infiltration, complement deposition and blood brain barrier (BBB) disruption compared to WM lesions 5, 7, 60, although this distinction is not absolute and significant intracortical lymphocytic infiltrates have been noted in some MS cases 44. Moreover, cortical lesions are thought to be characterized by a dominant effector cell population of ramified microglia 47.
A high frequency of vessels with tight junction abnormalities, which are a sign of BBB compromise, have been shown to be present in the GM, particularly in SPMS cases with long disease duration 39. These changes appeared less severe compared to the WM and appeared not associated with increased cortical inflammation, astrogliosis and complement deposition 60. A striking association between microglia and neurons has been reported in cortical MS lesions. Double‐labeling confocal microscopy detected elongated microglia oriented perpendicularly to the pial surface, closely apposed and often ensheathing apical dendrites and axons in active and chronic active cortical lesions. In addition, other more ramified stellate microglia often extended processes to neuronal perikarya and ensheathed dendrites or axons 21, 59. Unlike microglia/macrophages in WM lesions, which often apposed the terminal ends of transacted axons, microglia in cortical lesions did not consistently associate with the terminal ends of transected neurites.
INTRATHECAL INFLAMMATION AND SUBPIAL CORTICAL DAMAGE
The lack of substantial inflammatory infiltrates, complement deposition and BBB damage in MS cortical lesions led to the initial suggestion that the mechanisms underlying GM and WM pathology may substantially differ 5, 7, 60 and that activated microglia may represent one of the dominant effector cell populations mediating GM damage 47, 61. Only during the last 10 years has it become increasingly clear that immune activation in the meningeal compartment may have a key role in causing damage in the adjacent cerebral cortex. Besides the fact that most cortical lesions are subpial, numerous studies in experimental models and in postmortem MS brain tissue support this idea 32, 33, 37, 38, 40, 41, 42, 49, 55, 58. In particular, it has been shown that secondary progressive MS cases characterized by a high levels of inflammatory infiltrates in the meninges, diffuse or organized in B‐cell follicles‐like structures, (Figure 1) have more and larger subpial GM lesions, earlier age of disease and disability onset and earlier age of death 33, 42. The strong association between meningeal inflammation and severity of pathology has been recently substantiated in the spinal cord 2, 19, 32. In addition, increased meningeal inflammation has been shown to be strictly associated with a “surface‐in” gradient of neuronal, astrocyte and oligodendrocyte loss accompanied by microglia activation greatest in the most external cortical layers (I‐III) close to the CSF surface and decreasing in the most inner ones close to the WM 43. The fact that similar alterations have been found also in the normal appearing GM suggest that the pathology is not only restricted to demyelinated areas but is possibly a diffuse event occurring all over the brain and spinal cord.
Figure 1.
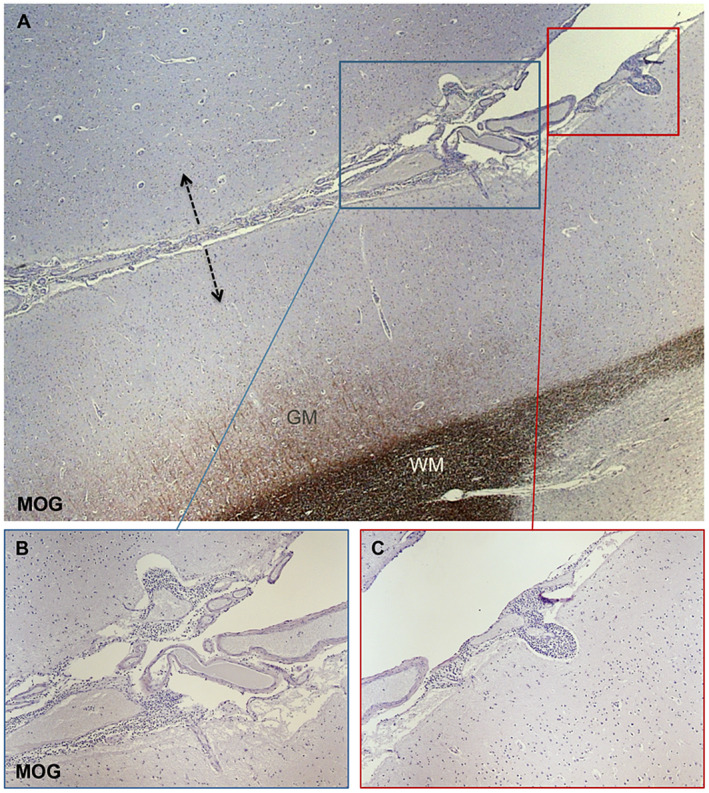
Neuropathology assessment of subpial cortical demyelination associated with meningeal inflammatory infiltrates. MOG immunostaining (A‐C) allows the detection of subpial cortical lesions that are often expanding from both sides (arrows in A) of cerebral sulci as specular images. Within the meninges lining on the pial surface numerous extravasating inflammatory infiltrates are evident (B and C). Some of them appeared invading the adjacent, superficial cortical layers (B and C).
At the same time, in type II intracortical lesions focal lymphocytic cuffing has been described in the more superficial GM layers of SPMS cases with prominent meningeal inflammation and extensive cortical pathology either in postmortem MS cases 44 or in brain biopsies from patients with a recent diagnosis of MS 40.
It has therefore been suggested that while WM demyelination and type II cortical lesions may be preferentially influenced by perivenular inflammation, CSF inflammation may mediate subpial and periventricular demyelination, possibly through a gradient “surface‐in” 14, 45, 49. In order to verify this hypothesis, a combined neuropathology, molecular and imaging study on postmortem MS cases and in MS patients at the time of diagnosis has been performed demonstrating that a common pattern of intrathecal (meninges and CSF) inflammatory profile strongly correlates with increased cortical pathology, both at time of the diagnosis and of death. In particular, increased expression of pro‐inflammatory cytokines (IFNγ, TNF, IL2 and IL22) and molecules related to sustained B‐cell activity and lymphoid‐neogenesis (CXCL13, CXCL10, LTα, IL6, IL10) was detected in paired meninges and CSF of rapidly progressive postmortem MS cases with high levels of meningeal inflammation and GM demyelination 45. Significant (P < 0.0001) positive correlation was, in fact, detected non only between degree of meningeal inflammation and percentage of cortical demyelination (r = 0.968), but also specifically between degree of meningeal inflammation and CSF levels of CXCL13 (r = 0.943), IFNγ (r = 0.718), IL10 (r = 0.627), CCL22 (r = 0.603), IL16 (r = 0.568) and TNF (r = 0.553) in postmortem progressive MS patients (Figure 2). .
Figure 2.

Graphs reporting Pearson correlations between degree of meningeal inflammation and percentage of cortical demyelination (r = 0.968) and CSF levels of CXCL13 (r = 0.943), IFNγ (r = 0.718), IL10 (r = 0.627), CCL22 (r = 0.603), IL16 (r = 0.568) and TNF (r = 0.553) in postmortem MS patients. All the correlations were significant (P < 0.001).
This finding corroborates the hypothesis that meningeal infiltrates, diffuse or aggregated in lymphoid‐structures, are strongly associated with CSF inflammatory milieu. Similar pro‐inflammatory patterns, including increased levels of CXCL13, TNF, IFNγ, CXCL12, IL6, IL8 and IL10, together with high levels of BAFF, APRIL, LIGHT, TWEAK, sTNFR1, sCD163, MMP2 and pentraxin III, were detected in the CSF of MS patients with higher levels of GM lesions detected by 3T double inversion recovery (DIR) imaging at diagnosis 46. Furthermore, cortical lesion load on MRI was found correlated not only with highly inflamed CSF and presence of oligoclonal bands (OCB), but also with elevated CSF levels of neurofilament protein (NF‐L), suggesting that intrathecal inflammatory milieu may be one of the key determinants of the rate of cortical demyelination and neurodegeneration in MS 22, 45. In particular, it has been proposed that a specific panel of molecules commonly identified in both neuropathological and clinical parts of the study, including CXCL13, IL10, TNF and IFNγ, might represent a useful prognostic signature of cortical damage and meningeal inflammation, possibly in a subgroup of patients with more rapid and severe disease progression 45. This strongly supports the existence of a “cortical variant” of MS in which the cerebral cortex is predominantly involved.
The finding that cortical pathology can be detected early on in vivo is fundamental in order to be able to effectively target the underlying mechanisms of progressive disease before significant disability develops 27, 53. Despite several improvements, including the introduction of GM specific pulse sequences at high field 3T 9 and ultra‐high field 7T MRI 35, detailed CSF profiling may give more potential for the development of surrogate biomarkers of cortical GM demyelination/neurodegeneration and compartmentalized meningeal inflammation. This in turn could help to identify those individuals at risk of a more rapid and severe disease course early in the disease course.
CORTICAL LESION DETECTION BY MAGNETIC RESONANCE
Despite this knowledge and the detailed histological studies, in vivo detection of cortical MS lesions using MRI is challenging. A large proportion of cortical lesions go undetected on conventional MRI using standard field strength 17, 27 for several reasons including their small size 53, the anatomical paucity of myelin in the cortex generating little MRI contrast upon demyelination and the partial volume effects from adjacent CSF and WM 34, 47. Furthermore, most of the GM lesions affect the outermost layers of the cortex, in close contact with the sub‐arachnoid space, resulting in susceptibility artifact at the interface between cortex and CSF 52. In addition, in contrast to WM lesions, cortical GM lesions show a lack of substantial focal infiltration of blood‐derived leukocytes into the cortex 5, 40, 44, 47, complement deposition and BBB damage 5, 7, 60.
Several improvements have been achieved in the last decades, through the introduction of inversion recovery 6 and by developing GM specific pulse sequences such as DIR, DIR (Figure 3) or phase sensitive inversion recovery 9, 28, 54, 56, 57, 62, and by moving to high field 3 T and ultra‐high field 7 T MRI systems 12, 24, 35, 63. High field strength analysis of postmortem MS brain slices has allowed detection of a larger number and a more precise localization of the cortical lesions 3, 29, 36, 52. MP2RAGE and T2*‐weighted imaging at 7T have recently been shown to improve detection of leukocortical and juxtacortical lesions in the early disease stages, but not intracortical or subpial lesions 4, 31. However, it still remains unclear whether it could be possible to translate these methods into routine clinical practice.
Figure 3.
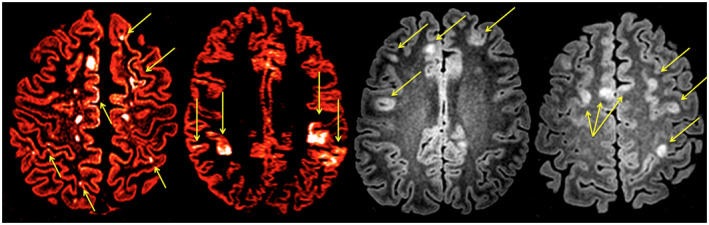
Axial double inversion recovery (DIR) sequences from 4 different subject suffering from relapsing remitting multiple sclerosis (MS). Several cortical lesions are highlighted; intracortical lesions (Yellow arrows), leucocortical lesions (Blu arrows), subpial cortical lesions (White arrow). DIR (A and B) are red colored to better highlight some cortical lesions.
During the last 10 years, increased focus on the neuroimmunological mechanisms underlying GM pathology has demonstrated that meningeal immune cell accumulation, compartmentalized within the subarachnoid space, as well as altered microglial activity, may have a key role in the pathogenesis of the damage in the cerebral cortex. Similarly, advanced 7 Tesla MRI methodology has been able to show the presence of a gradient of subpial cortical alterations 46, strictly resembling the increased severity of demyelination, neuronal loss and microglia activation in the most external cortical layers respect to the inner ones 43. Furthermore, the use of 3D DIR and 3D‐ Echo Planar Imaging (EPI) SWI has recently shown that CLs susceptibility maps highly reproduce the heterogeneous activity features of GM damage, even at individual level and that QSM hyperintensity edge found in proximity to the pial surface might be caused by the subpial gradient of microglial activation 15.
CLINICAL RELEVANCE OF CORTICAL LESIONS
1) Cortical lesions are an early and frequent phenomenon in MS and correlate with disability
In a study conducted in a large patient population, cortical lesions were detected by MRI in the majority (64%) of patients with relapsing remitting (RRMS) and secondary progressive (70%) MS (SPMS), as well as in more than one‐third (36.8%) of patients with clinically isolated syndromes (CIS) suggestive of MS 9. In some cases, cortical lesions have been detected even before the appearance of WM lesions, thus suggesting that the development of cortical inflammation is an early phenomenon in MS at least partly independent of WM lesions 10.
2) Cortical lesions correlate with physical disability and its progression
The number of cortical lesions convincingly correlate with the severity of the disease: patients with cortical lesions show a higher Expanded Disability Status Scale (EDSS) score, a higher WM T2 lesion volume, a lower brain parenchyma fraction and a higher frequency of CSF IgG oligoclonal bands (IgGOBs), when compared with patients without cortical lesions. A recent study based on 5‐year longitudinal observations of more than 300 MS patients with different clinical phenotypes showed that patients with a high cortical lesion load at baseline had the worst clinical evolution and the fastest progression of cortical atrophy after 5 years. Cortical lesion load associated not only with baseline EDSS, but also with the EDSS change and with the % change of GM fraction; such an association being observed in all clinical subsets. Based on the multivariate analysis the authors concluded that CL volume was an independent predictor of disability progression 12. Interestingly, RRMS and SPMS patients were found to accumulate new cortical lesions at a similar rate (0.8/year in RRMS vs. 1.0/year in SPMS), suggesting that, in relapse‐onset MS, the medium‐term dynamics of cortical lesion evolution may not be influenced by the disease stage. The higher number of cortical lesions observed in SPMS vs. RRMS patients is clearly the consequence of the longer disease duration of the former subgroup, as also indicated by postmortem studies 37, 42 showing that demyelination and axonal damage in the GM are already present in RRMS patients but become more prominent in the chronic stage of the disease. Finally, the cortical lesion load in the relapsing remitting phase together with the cerebellar volume and age have been suggested as independent markers of the evolution to the secondary progressive phase of the disease 13.
In line with these results are the data coming from patients with the Primary Progressive form of MS (PPMS) 10. In the PPMS population, cortical lesions were observed in up to 80% of the patients examined and were significantly correlated with disease duration, EDSS score, as well as with increasing GM atrophy and disability during the follow up. A multivariate analysis revealed that cortical lesion volume at baseline was an independent predictor of percentage GM volume change and disability accumulation during the subsequent two‐year period.
On the contrary, patients with a “benign” MS course (EDSS < 3.0 after 15 years from clinical onset and without any cognitive dysfunction) showed significantly lower cortical lesion number and volume compared to early RRMS patients with the same degree of disability, but much shorter disease duration. After one‐year follow‐up, “benign” MS patients did not show an accumulation of cortical lesions compared to early RRMS patients 10. Also in these subgroups the multivariate analysis indicated that cortical lesion number and volume at baseline and their volume change were independent predictors of the clinical status. These findings suggest that the relative sparing of the cortex in benign MS might contribute to the more favorable clinical status in these patients.
3) Cortical lesions as a major substrate of cognitive dysfunction
Cortical pathology may have a significant impact not only on clinical disability but also on cognitive dysfunction in MS. A recent study clearly demonstrated that cortical GM damage significantly contributes to the cognitive decline observed in MS patients. Indeed, patients suffering from RRMS with cognitive deficits had more cortical lesions and atrophy than cognitively normal MS patients. These results are in agreement with neuropathology 28 and imaging 1 studies, which demonstrated a significant cortical volume reduction in cognitive impaired RRMS patients, but neither investigated the role of focal demyelinating lesions in the cortex, nor elucidated how the burden of cortical lesions and the extent of cortical atrophy are interrelated. We observed that cortical lesions number and volume were significantly higher in cognitively impaired RRMS patients compared to those without cognitive deficits 10. Similar results were also obtained when using a conservative approach to the definition of cognitive impairment. Considering that previous studies assessing the relationship between the extent of WM damage and cognitive impairment in MS patients have given conflicting results 1, 51, our data strengthen the notion that the overall burden of WM MRI‐visible lesions does not fully account for the severity of cognitive impairment in MS. Such a notion was reinforced further by the results of the multivariate analysis, which revealed that only cortical lesion volume and, even if at a lesser extent, neocortical volume loss are independent predictors of the composite cognitive score 10. On the contrary the evidence is quite strong that early cortical lesions might be helpful in the identification of MS patients at high risk of disability progression 48.
CONCLUSIONS
Despite the exact underlying molecular mechanism are not completely understood, the above‐mentioned studies suggest that meningeal inflammation and the consequent cortical microglia activation lead to progressive cortical demyelination and neurodegeneration since the MS onset to end‐stage of the disease. Such GM pathology has been demonstrated to have a great impact on each clinical parameter, from disability progression to cognitive dysfunction, thus influencing the long‐term prognosis of the disease.
For such reason we should improve our ability to detect it since the earliest phases of the disease, we should identify the molecular mechanisms underlying it and finally we should detect specific biomarkers able to highlight those patients at high risk of early neurodegeneration.
Thus, a more detailed characterization and precise assessment of the features and the extent of GM demyelination and of the meningeal and cortical inflammation in MS is required in order to identify imaging and biohumoral (CSF/serum) correlates of cortical pathology that may have an important role in predicting and monitor the disease evolution.
By defining more precisely the substrates of the cerebral cortical damage in MS and their relationship with cortical changes detectable by combined imaging, neuropathological and molecular analysis, this, in turn, will open up an avenue for the development of an effective clinical and therapeutic approach for slowing or stopping the progressive course of MS.
References
- 1. Amato MP, Zipoli V, Portaccio E (2006) Multiple sclerosis‐related cognitive changes: a review of cross‐sectional and longitudinal studies. J Neurol Sci 245:41–46. [DOI] [PubMed] [Google Scholar]
- 2. Androdias G, Reynolds R, Chanal M, Ritleng C, Confavreux C, Nataf S (2010) Meningeal T cells associate with diffuse axonal loss in multiple sclerosis spinal cords. Ann Neurol 68:465–476. [DOI] [PubMed] [Google Scholar]
- 3. Bagnato F, Yao B, Cantor F, Merkle H, Condon E, Montequin M et al (2009) Multisequence‐imaging protocols to detect cortical lesions of patients with multiple sclerosis: observations from a post‐mortem 3 Tesla imaging study. J Neurol Sci 282:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beck ES, Sati P, Sethi V, Kober T, Dewey B, Bhargava P et al (2018) Improved visualization of cortical lesions in multiple sclerosis using 7T MP2RAGE. Am J Neuroradiol 39:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bø L, Vedeler C, Nyland H, Trapp B, Mørk S (2003) Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 9:323–331. [DOI] [PubMed] [Google Scholar]
- 6. Boggild MD, Williams R, Haq N, Hawkins CP (1996) Cortical plaques visualised by fluid‐attenuated inversion recovery imaging in relapsing multiple sclerosis. Neuroradiology 38(Suppl 1):S10–S13. [DOI] [PubMed] [Google Scholar]
- 7. Brink BP, Veerhuis R, Breij ECW, Van Der Valk P, Dijkstra CD, Bö L (2005) The pathology of multiple sclerosis is location‐dependent: no significant complement activation is detected in purely cortical lesions. J Neuropathol Exp Neurol 64:147–155. [DOI] [PubMed] [Google Scholar]
- 8. Brownell B, Hughes JT (1962) The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 25:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calabrese M, De Stefano N, Atzori M, Bernardi V, Mattisi I, Barachino L et al (2007) Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol 64:1416–1422. [DOI] [PubMed] [Google Scholar]
- 10. Calabrese M, Gallo P (2009) Magnetic resonance evidence of cortical onset of multiple sclerosis. Mult Scler 15:933–941. [DOI] [PubMed] [Google Scholar]
- 11. Calabrese M, Rinaldi F, Grossi P, Gallo P (2011) Cortical pathology and cognitive impairment in multiple sclerosis. Exp Rev of Neurother 11:425–432. [DOI] [PubMed] [Google Scholar]
- 12. Calabrese M, Poretto V, Favaretto A, Alessio S, Bernardi V, Romualdi C et al (2012) Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 135:2952–2961. [DOI] [PubMed] [Google Scholar]
- 13. Calabrese M, Favaretto A, Martini V, Gallo P (2013) Grey matter lesions in MS From histology to clinical implications. Prion 7:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calabrese M, Magliozzi R, Ciccarelli O, Geurts JJG, Reynolds R, Martin R (2015) Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci 16:147–158. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 15. Castellaro M, Magliozzi R, Palombit A, Pitteri M, Silvestri E, Camera V et al (2017) Heterogeneity of cortical lesion susceptibility mapping in multiple sclerosis. Am J Neuroradiol 38:1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chard D, Miller D (2009) Grey matter pathology in clinically early multiple sclerosis: evidence from magnetic resonance imaging. J Neurol Sci Elsevier B.V. 282:5–11. [DOI] [PubMed] [Google Scholar]
- 17. Daams M, Geurts JJG, Barkhof F (2013) Cortical imaging in multiple sclerosis: recent findings and “grand challenges”. Curr Opin Neurol 26:345–352. [DOI] [PubMed] [Google Scholar]
- 18. Dawson JW (1916) The histology of disseminated sclerosis. Trans R Soc Edinburgh 50:517–740. [Google Scholar]
- 19. Deluca GC, Alterman R, Martin JL, Mittal A, Blundell S, Bird S et al (2013) Casting light on multiple sclerosis heterogeneity: the role of HLA‐DRB1 on spinal cord pathology. Brain 136:1025–1034. [DOI] [PubMed] [Google Scholar]
- 20. De Stefano N, Matthews PM, Filippi M, Agosta F, De Luca M, Bartolozzi ML et al (2003) Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 60:1157–1162. [DOI] [PubMed] [Google Scholar]
- 21. Dutta R, Trapp BD (2007) Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 130(Pt 10):2566–2576. [DOI] [PubMed] [Google Scholar]
- 22. Farina G, Magliozzi R, Pitteri M, Reynolds R, Rossi S, Gajofatto A et al (2017) Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: a combined CSF and MRI study. J Neuroinflammation 14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Filippi M, van den Heuvel MP, Fornito A, He Y, Hulshoff Pol HE, Agosta F et al (2013) Assessment of system dysfunction in the brain through MRI‐based connectomics. Lancet Neurol 12:1189–1199. [DOI] [PubMed] [Google Scholar]
- 24. Filippi M, Evangelou N, Kangarlu A, Inglese M, Mainero C, Horsfield MA et al (2014) Ultra‐high‐field MR imaging in multiple sclerosis. J Neurol Neurosurg Psychiatry 85:60–6. [DOI] [PubMed] [Google Scholar]
- 25. Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA et al (2008) Gray matter atrophy is related to long‐term disability in multiple sclerosis. Ann Neurol 64:247–254. [DOI] [PubMed] [Google Scholar]
- 26. Frischer JM, Bramow S, Dal‐Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M et al (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132(Pt 5):1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geurts JJG, Bö L, Pouwels PJW, Castelijns JA, Polman CH, Barkhof F (2005) Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. Am J Neuroradiol 26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 28. Geurts JJG, Stys PK, Minagar A, Amor S, Zivadinov R (2009) Gray matter pathology in (chronic) MS: modern views on an early observation. J Neurol Sci 282:12–20. [DOI] [PubMed] [Google Scholar]
- 29. Gilmore CP, Deluca GC, Bö L, Owens T, Lowe J, Esiri MM et al (2009) Spinal cord neuronal pathology in multiple sclerosis. Brain Pathol 19:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giorgio A, De Stefano N (2010) Cognition in multiple sclerosis: relevance of lesions, brain atrophy and proton MR spectroscopy. Neurol Sci 31(SUPPL. 2):S245–S248. [DOI] [PubMed] [Google Scholar]
- 31. Granberg T, Fan Q, Treaba CA, Ouellette R, Herranz E, Mangeat G et al (2017) In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain 140:2912–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haider L, Zrzavy T, Hametner S, Höftberger R, Bagnato F, Grabner G et al (2016)The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139(Pt 3):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R (2010) Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J Neuropathol Exp Neurol 69:1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T (1999) Cortical lesions in multiple sclerosis. Brain 122:17–26. [DOI] [PubMed] [Google Scholar]
- 35. Kilsdonk ID, De Graaf WL, Soriano AL, Zwanenburg JJ, Visser F, Kuijer JPA et al (2013) Multicontrast MR imaging at 7T in multiple sclerosis: highest lesion detection in cortical gray matter with 3D‐FLAIR. Am J Neuroradiol 34:791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kilsdonk ID, Jonkman LE, Klaver R, Van Veluw SJ, Zwanenburg JJM, Kuijer JPA et al (2016) Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post‐mortem verification study. Brain 139(Pt 5):1472–1481. [DOI] [PubMed] [Google Scholar]
- 37. Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128(Pt 11):2705–2712. [DOI] [PubMed] [Google Scholar]
- 38. Lassmann H, Brück W, Lucchinetti CF (2007) The immunopathology of multiple sclerosis: an overview. Brain Pathol 17:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leech S, Kirk J, Plumb J, McQuaid S (2007) Persistent endothelial abnormalities and blood‐brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol 33:86–98. [DOI] [PubMed] [Google Scholar]
- 40. Lucchinetti CF, Popescu BFG, Bunyan RF, Moll NM, Roemer SF, Lassmann H et al (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365:2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Magliozzi R, Columba‐Cabezas S, Serafini B, Aloisi F (2004) Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle‐like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol 148:11–23. [DOI] [PubMed] [Google Scholar]
- 42. Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M et al (2007) Meningeal B‐cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130(Pt 4):1089–1104. [DOI] [PubMed] [Google Scholar]
- 43. Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B et al (2010) A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68:477–493. [DOI] [PubMed] [Google Scholar]
- 44. Magliozzi R, Serafini B, Rosicarelli B, Chiappetta G, Veroni C, Reynolds R et al (2013) B‐cell enrichment and epstein‐barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J Neuropathol Exp Neurol 72:29–41. [DOI] [PubMed] [Google Scholar]
- 45. Magliozzi R, Howell OW, Nicholas R, Cruciani C, Castellaro M, Romualdi C et al (2018) Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol 83:739–755. [DOI] [PubMed] [Google Scholar]
- 46. Mainero C, Louapre C (2015) Erratum: meningeal inflammation in multiple sclerosis: the key to the origin of cortical lesions. Neurology 85:12–13. [DOI] [PubMed] [Google Scholar]
- 47. Peterson JW, Bö L, Mörk S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400. [DOI] [PubMed] [Google Scholar]
- 48. Pitteri M, Romualdi C, Magliozzi R, Monaco S, Calabrese M (2017) Cognitive impairment predicts disability progression and cortical thinning in MS: an 8‐year study. Mult Scler 23:848–854. [DOI] [PubMed] [Google Scholar]
- 49. Ransohoff RM, Kivisäkk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. [DOI] [PubMed] [Google Scholar]
- 50. Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O (2011) The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol 122:155–170. [DOI] [PubMed] [Google Scholar]
- 51. Rovaris M, Comi G, Filippi M (2006) MRI markers of destructive pathology in multiple sclerosis‐related cognitive dysfunction. J Neurol Sci 245:111–116. [DOI] [PubMed] [Google Scholar]
- 52. Schmierer K, Parkes HG, So P‐W, An SF, Brandner S, Ordidge RJ et al (2010) High field (9.4 Tesla) magnetic resonance imaging of cortical grey matter lesions in multiple sclerosis. Brain 133(Pt 3):858–867. [DOI] [PubMed] [Google Scholar]
- 53. Seewann A, Vrenken H, Kooi E, van der Valk P, Knol DL, Polman CH et al (2011) Imaging the tip of the iceberg: visualization of cortical lesions in multiple sclerosis. Mult Scler 17:1202–1210. [DOI] [PubMed] [Google Scholar]
- 54. Seewann A, Kooi E‐J, Roosendaal SD, Pouwels PJW, Wattjes MP, van der Valk P et al (2012) Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology 78:302–308. [DOI] [PubMed] [Google Scholar]
- 55. Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F (2004) Detection of ectopic B‐cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sethi V, Yousry TA, Muhlert N, Ron M, Golay X, Wheeler‐Kingshott C et al (2012) Improved detection of cortical MS lesions with phase‐sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry 83:877–882. [DOI] [PubMed] [Google Scholar]
- 57. Simon B, Schmidt S, Lukas C, Gieseke J, Träber F, Knol DL et al (2010) Improved in vivo detection of cortical lesions in multiple sclerosis using double inversion recovery MR imaging at 3 Tesla. Eur Radiol 20:1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stadelmann C, Albert M, Wegner C, Brück W (2008) Cortical pathology in multiple sclerosis. Curr Opin Neurol 21:229–234. [DOI] [PubMed] [Google Scholar]
- 59. Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285. [DOI] [PubMed] [Google Scholar]
- 60. Van Horssen J, Dijkstra CD, De Vries HE (2007) The extracellular matrix in multiple sclerosis pathology. J Neurochem 103:1293–1301. [DOI] [PubMed] [Google Scholar]
- 61. Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL et al (2007) Altered glutamate reuptake in relapsing‐remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J Neuropathol Exp Neurol 66:732–739. [DOI] [PubMed] [Google Scholar]
- 62. Wattjes MP, Lutterbey GG, Gieseke J, Träber F, Klotz L, Schmidt S et al (2007) Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR Am J Neuroradiol 28:54–59. [PMC free article] [PubMed] [Google Scholar]
- 63. Wattjes MP, Barkhof F (2009) High field MRI in the diagnosis of multiple sclerosis: high field‐high yield? Neuroradiology 51:279–292. [DOI] [PubMed] [Google Scholar]
- 64. Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM (2006) Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 67:960–967. [DOI] [PubMed] [Google Scholar]
- 65. Zivadinov R, Heininen‐Brown M, Schirda CV, Poloni GU, Bergsland N, Magnano CR et al (2012) Abnormal subcortical deep‐gray matter susceptibility‐weighted imaging filtered phase measurements in patients with multiple sclerosis. A case‐control study. Neuroimage 59:331–339. [DOI] [PubMed] [Google Scholar]
- 66. Zivadinov R, Jakimovski D, Gandhi S, Ahmed R, Dwyer MG, Horakova D et al (2016) Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Exp Rev Neurother 16:777–793. [DOI] [PubMed] [Google Scholar]


