Non‐small‐cell lung cancer (NSCLC) is characterized by aggressive clinical course including frequent occurrence of distant metastases. Central nervous system (CNS) metastases are diagnosed in 20%–40% of NSCLC patients and they are considered as a pharmacological sanctuary lesions for most cytotoxic agents. Molecularly targeted therapies have shown relatively high activity in CNS metastases in patients harboring “drugable” abnormalities in EGFR or ALK genes. Especially promising activity of the second‐generation ALK inhibitors (alectinib, ceritinib) and sequential or concurrent application of EGFR/ALK TKIs and WBRT/stereotactic radiotherapy has been postulated 4, 6, 8.
To date the knowledge on the effectiveness of molecularly targeted therapies in NSCLC patients with CNS metastases is relatively scarce. The patients with untreated CNS metastases are excluded from recent clinical trials investigating new therapies in lung cancer 4, 8. Therefore, we retrospectively assessed the spectrum of “drugable” abnormalities in 10 genes, in CNS metastases of NSCLC (145 FFPE tissue samples—45 females and 100 males; median age 60 ± 8.8 years; PS = 0 or 1; all patients chemotherapy and TKI naïve), and determined the relationship between molecular status and clinical characteristics of our patients. The studied group was heterogeneous in terms of histopathology (80 adenocarcinoma, 29 squamous cell carcinoma, 22 large cell cancer and 14 not‐otherwise‐specified NSCLC patients) and smoking status (73 current‐smokers, 21 former smokers, 36 non‐smokers).
The molecular profile of selected mutations was assessed using different molecular methods. Mutations in EGFR (exons 18–21), KRAS (codons: 12; 13; 61), NRAS (codons: 12; 61), BRAF (codon: 600), PTEN (codon: 233) and AKT1 (codon: 17) genes were analyzed with commercially available kits certified for in vitro diagnostic (Entrogen, Woodland Hills, California, USA) or TaqMan probes for research use only (Applied Biosystem, Carlsbad, California, USA). To analysis mutation in, PIK3CA (codons: 542; 545; 1047), MEK1 (codons: 56; 57; 67), HER2 (exon 20) and DDR2 (codon: 768) genes we used originally designed methods which based on allele‐specific PCR (ASP‐PCR) and high‐resolution melting PCR (HRM‐PCR). Moreover, direct sequencing and multi‐temperature single strand conformation polymorphism (MSSCP) techniques were used to confirm results obtained by originally designed methods. ALK abnormal protein was determined using automated immunohistochemistry with Positive Rabbit Monoclonal Antibody D5F3 (Ventana, Tucson, Arizona, USA) according to manufacturer instructions 9. To confirm ALK gene rearrangement we used FISH technique with Vysis ALK Break Apart FISH Probe Kit (Abbot Molecular, Des Plaines, Illinois, USA). The criteria of FISH analysis were in accordance with FDA guidelines 10. In 30 patients, the material was simultaneously available from primary and metastatic NSCLC tumors.
We identified at least one abnormality in 59 cases (41%): KRAS—21.4% (31/145), EGFR—6.2% (9/145), ALK—4.8% (7/145), DDR2—2.1% (3/145), PIK3CA—2.1% (3/145), NRAS—1.4% (2/145) and HER2, AKT1, PTEN, MEK1 respectively in 0.7% of patients (1/145) (Figure 1A). Coexistence of two mutations (KRAS and DDR2) was found in one patient. Mutations were significantly more frequently observed in adenocarcinoma compared to other histologic types of NSCLC (P = 0.001; χ2 = 15.9; Figure 1B,C) and in non‐smokers compared to former/current smokers (P = 0.034; χ2 = 11.39, Figure 1D,E). The mOS of patients with mutations was insignificantly longer than in patients with wild‐type of analyzed genes (16 vs. 11.7 months; P = 0.084; HR = 1.36). Cox multivariate logistic regression demonstrated that the factors significantly prolonging patients' survival were younger age (<60 years) and mutations' presence (overall model: P = 0.0459; χ2 = 6.161). In 30 matched primary NSCLC tumors, 23% had EGFR and 7% had KRAS mutations. Heterogeneity between primary tumor and CNS metastases concerned only KRAS mutations. In five cases KRAS gene mutations were identified in both specimens, in one case only in primary tumor and in one case only in CNS metastases.
Figure 1.
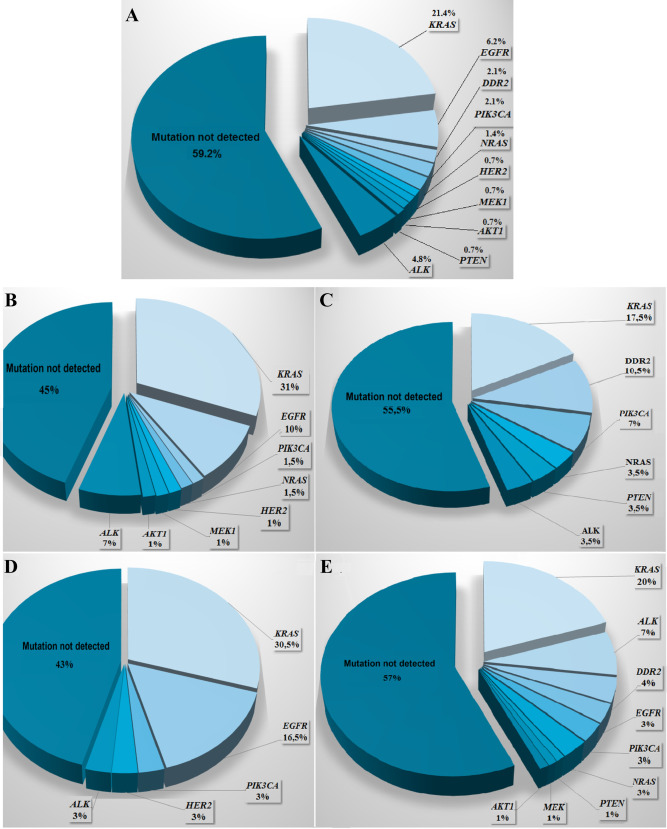
General prevalence of “drugable” mutations in CNS metastases of NSCLC (A) and prevalence of “drugable” mutations in CNS metastases of NSCLC according to histological type of cancer (B. adenocarcinoma, C. squamous‐cell carcinoma) or patients' smoking status (D. never smokers, E. smokers).
Most of our observations are in concordance with large epidemiological data from studies focusing on primary tumors of NSCLC. Barlesi et al indicated the presence of driver mutations in six examined genes in 50% out of 17 664 European and Caucasian patients. Prevalence of analyzed mutations was as follows: KRAS mutations in 29% (4894/17 001) of cases, EGFR mutations in 11% (1947/17 706) of cases, ALK rearrangement in 5% (388/8134) of cases, BRAF mutations in 2% (262/13 906) of cases, PIK3CA mutations in 2% (252/10 678) of cases and HER2 mutations in 1% of patients (98/11 723). The presence of a genetic alterations was significantly associated with longer duration of response to both first‐ and second‐line of treatment, as well as with longer first‐line PFS and with longer mOS. Cox multivariate analysis confirmed that the presence of ALK rearrangements and EGFR and HER2 genes mutations had a favorable effect on prognosis 1. Kris et al reported the driver mutations in 64% (466/733) of American patients with adenocarcinoma. The analysis included ten genes and spectrum of particular disorders was fallowing: KRAS mutations in 25% (182/733) of cases, EGFR activation mutations in 17% (122/733) of cases, ALK rearrangements in 8% (57/733) of cases, HER2 mutations in 3% (19/733) of cases, BRAF mutations in 2% (16/733) of cases; PIK3CA mutations in <1% (6/733) cases; MET amplification in <1% (5/733) of cases; NRAS mutations in <1% (5/733) of cases; MEK1 mutation in <1% (1/733) of cases. The authors have not identified a mutation in AKT1 gene. Moreover, they noted the co‐existence of two or more mutations in 3% (24/733) of cases. PIK3CA gene mutations commonly overlapped with others genetic abnormalities. Kris et al observed significantly longer mOS in patients with molecular abnormalities who received targeted therapies compared to such patients treated with chemotherapy (3.5 years vs. 2.4 years, respectively, P = 0.006). Survival of patients with genetic alteration treated with chemotherapy and patients without molecular abnormalities was similar 5. Although our studied group was less numerous, the overall frequency of driver mutations and their spectrum have not shown substantial diversity from Barlesi and Kris results. However, according to our results, presence of the driver mutations may be good prognostic factor in NSCLC patients treated with neurosurgery—independently of further chemotherapy or radiotherapy or best supportive care procedures (patients were not treated with molecularly targeted therapy).
Villalva et al reported incidence of “drugable” mutations in CNS metastases of NSCLC (44.2%; 34/77). They diagnosed KRAS mutations in 39% (30/77) of samples; EGFR mutations in 3.9% (3/77) of samples and ALK rearrangement in 7.7% (1/13) of samples. The authors found no mutation in BRAF and HER2 genes. Moreover, mutations were significantly more frequently observed in female than in male patients (71% vs. 32%, P = 0.002). However, they found similar mutational profile in different histopathological types of NSCLC 7. Our studied group was more heterogeneous in terms of histopathology and analysis included five additional genes that allowed to indicate presence of rare mutation in DDR2; PTEN, AKT1, NRAS and MEK1. Moreover, we have shown a significant relationship between the mutational profile of CNS metastases and histological type of NSCLC as well as smoking status of patients.
Recently, the The Cancer Genome Atlas (TCGA) Research Network showed discrepancies between oncogenic pathways involved in pathogenesis of adenocarcinoma and squamous‐cell carcinoma which arise from variability in smoking habits 2, 3. Molecular profiling of AD indicated mutations in 75.6% (174/230) of analyzed tumors and 62% of detected mutations showed oncogenic value. TCGA observed mutations in commonly mutated oncogenes like KRAS (32%), EGFR (11%), BRAF (7%), MET (4.5%). In oncogenic negative tumors, TCGA indicated presence of uncommon mutations in tumor suppressor genes: TP53, KEAP1, NF1 and RIT1 3. Molecular profiling of SCC indicated that 96% of tested tumors (171/178) harbored some genetic alterations. However, the mutational landscape of SCC was different than in AC and commonly included aberrations in following genes: TP53, CDKN2A, PTEN, PIK3CA, KEAP1, MILL2, HLA‐A, NFE2L2, NOTCH1 and RB1. TCGA reported that large number of potentially oncogenic mutations were observed in following pathways: PIK3CA (16%), EGFR/HER (15%), PTEN (15%), FGFR (12%) and RAS/RAF (11%) 2. In our study, we also showed discrepancies in molecular landscape of CNS metastases from adenocarcinoma and squamous‐cell carcinoma as well as from smokers and non‐smoking patients (Figure 1B–E).
Our analysis confirmed that driver mutations could be observed and detected in CNS metastases of NSCLC that confirmed usefulness of CNS metastatic tissue for molecular testing in qualification to molecularly targeted therapies. Because of molecular tumor heterogeneity between corresponding cancer lesions the primary tumor tissue sample cannot always be considered as representative for all cancer sites. Simultaneous analysis of molecular profile in primary NSCLC and CNS metastatic sites should be commonly used because it may broaden therapeutic choices in group of patients with distant metastases of NSCLC.
Conflict of Interest
The authors declare no conflict of interest.
The study was approved by the Ethics Committee of the Medical University of Lublin, Poland (No. KE‐0254/86/2013).
References
- 1. Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H et al (2016) Routine molecular profiling of patients with advanced non‐small‐cell lung cancer: results of a 1‐year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 387:1415–1426. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Genome Atlas Research Network (2012) Comprehensive genomic characterization of squamous cell lung cancers. Nature 489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen YM (2013) Usage of EGFR‐TKI and WBRT in NSCLC patients with brain metastases. Ann Palliat Med 2:108–110. [DOI] [PubMed] [Google Scholar]
- 5. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II et al (2014) Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takei H, Rouah E, Ishida Y (2016) Brain metastasis: clinical characteristics, pathological findings and molecular subtyping for therapeutic implications. Brain Tumor Pathol 33:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Villalva C, Duranton‐Tanneur V, Guilloteau K, Burel‐Vandenbos F, Wager M, Doyen J et al (2013) EGFR, KRAS, BRAF, and HER‐2 molecular status in brain metastases from 77 NSCLC patients. Cancer Med 2:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang TJ, Wu AJ (2016) Cranial irradiation in patients with EGFR‐mutant non‐small cell lung cancer brain metastases. Transl Lung Cancer Res 5:134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VENTANA ALK (D5F3) CDx assay interpretation guide for non‐small cell lung carcinoma (NSCLC). Available at: http://alkihc.com (accessed 04 October 2017).
- 10. Malik SM, Maher VE, Bijwaard KE, Becker RL, Zhang L, Tang SW et al (2014) U.S. Food and Drug Administration approval: crizotinib for treatment of advanced or metastatic non‐small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res 20:2029–2034. [DOI] [PubMed] [Google Scholar]


