Abstract
Background
The recent outbreak of Zika virus (ZIKV) infection and the associated increased prevalence of microcephaly in Brazil underline the impact of viral infections on embryo fetal development. The aim of the present study is to provide a detailed clinical and histopathological study of the fetal disruption caused by the ZIKV, with a special focus on the associated neuropathological findings.
Methods
A detailed feto‐placental examination, as well as neuropathological and neurobiological studies were performed on three fetuses collected after pregnancy termination between 22 and 25 weeks of gestation (WG), because brain malformations associated with a maternal and fetal ZIKV infection was diagnosed.
Results
In all three cases, the maternal infection occurred during the first trimester of pregnancy. A small head was observed on the ultrasound examination of the second trimester of pregnancy and led to the diagnosis of ZIKV fetopathy and pregnancy termination. The fetal histopathological examination was unremarkable on the viscera but showed on the testis an interstitial lymphocytic infiltrate. The placenta contained a Hofbauer cells hyperplasia with signs of inflammation. Neuropathological findings included a meningoencephalitis and an ex vacuo hydrocephalus. Immunohistochemical studies showed the presence of T lymphocytic and histiocytic meningitis associated with an abundant cerebral astroglial and macrophagic reaction. In situ hybridization demonstrated, abundant ZIKV particles within the cerebral parenchyma mainly in the ventricular/subventricular zone and in the cortical plate. In addition massive cells death and endoplasmic reticulum damage were present.
Conclusion
The present study reports on the clinical and histopathological findings observed in three fetuses infected by the ZIKV. It emphasizes the severity of brain damages and the minimal visceral and placental changes observed upon ZIKV infection. This confirms the selective neurotropism of ZIKV. Finally, it allows us to describe the cascade of multifactorial developmental defects leading to microcephaly.
Keywords: ER stress, fetus, meningoencephalitis, micrencephaly, microcephaly, ZIKV infection.
Introduction
ZIKA virus (ZIKV) is an arbovirus belonging to the Flavivirus genus (Flaviviridae family) mainly transmitted by Aedes aegypti mosquitoes. It was originally isolated from a sentinel primate in Uganda in 1947 5, 12, 22, 24. Since 2007, ZIKV infection spreads to Micronesia (2007), French Polynesia (2013), and more recently to South America, notably to Brazil (2015–2016) 3, 5, 12. In humans, ZIKV is known to cause a mild febrile disease, headache, arthralgia and myalgia, a maculopapular rash, and Guillain‐Barre syndrome. The recent emergence of ZIKV in Brazil has been associated with an acute and significant increased prevalence of congenital microcephaly in the 2015–2016 period 5, 6, 22. Cauchemez et al estimated that the number of microcephaly cases associated with ZIKV was 95 per 10 000 women infected during the first trimester 9. In this context, WHO declared on 1 February 2016 that ZIKV infection was a public health emergency of international concern 39.
This large outbreak has revealed that, beside mosquito transmission, ZIKV is also transmitted through hematogenic and sexual routes 5, 9, 24. Unlike other mosquito‐borne Flaviviruses, ZIKV persists in human fluids for up to 6 months 24. Fetal contamination is considered to be hematogenic. The diagnosis of fetal infection is based on ZIKV RNA detection in the amniotic fluid of pregnant women, having a positive result on blood testing (serologic and/or RT‐PCR testing) 12.
Affected newborns present with a syndrome characterized by a small head (microcephaly) with joint deformities (arthrogryposis). Brain imaging usually detects a small brain (micrencephaly) with ventriculomegaly, a malformed cortex, an abnormal corpus callosum, and diffused calcifications in the subcortical parenchyma and the thalamus 18, 22, 31. Despite the extent of the ZIKV fetal epidemics and the WHO recommendation, postmortem studies are still scarce. Indeed, only a small number of postmortem studies have been reported in infants who may have suffered from additional stress due to delivery or resuscitation attempts 11, 12, 22. In unborn babies, description of visceral and brain anomalies is usually based on ultrasound findings alone, whereas histopathological and neurobiological examinations are instrumental to describe in details the spectrum of lesions associated with the ZIKV infection.
The aim of the present report is precisely to better characterize the spectrum of fetal disruption caused by the ZIKV infection, by providing autopsy results with detailed visceral histopathological, neuropathological, and complementary studies including immunohistochemical and neurobiological investigations.
Material and Methods
Population
The study concerns three fetuses collected during the January–December 2016 period and autopsied in our laboratory after a pregnancy termination was performed because brain malformations were diagnosed in a context of ZIKV maternal infection.
ZIKV infection
The detection of the ZIKV was performed using RT‐PCR (RealStar® Zika Virus RT PCR kit 1.0 Altona, Joué‐les‐Tours, France) following manufacturer instructions. RNA was extracted from 200 μL of maternal plasma, amniotic fluid, and fetal blood or from fetal tissues and 8 placental biopsy samples using the NucliSens® EasyMag® kit following manufacturer instructions (BioMérieux, Marcy L’étoile, France). Elution of extracted RNA was done in 100 μL of elution buffer. The kit did not include quantification standards but allowed to perform a semi‐quantitative estimation of the results based on the cycle threshold value (CT) obtained for each sample. Samples with a CT value between 35–40, 30–34, 25–29 and <25 were graded, respectively, as low positive (+), moderately positive (++), highly positive (+++), and very highly positive (++++).
Fetal autopsy
Fetal autopsy was performed after parental consent, following the guidelines of the French Society of Foetopathology (SOFFOET). It included fetal photographs and X‐rays, external macroscopic examination, complete autopsy with visceral sampling for histopathological studies. Macroscopic analysis and sampling of the placenta, membranes, and cord were performed after formalin fixation. Complementary samplings for viral, electron microscopy, genetic, and neurobiological studies were performed on fresh central nervous system.
Biometrical parameters were compared with published normograms established according to the gestational age 17. In Case 1, cerebral hemispheres were separated, one frozen at −80° and the other fixed in formalin zinc solution. In Cases 2 and 3, brains were fixed in toto after samples from the frontal and occipital horns were frozen at −80°. For histopathological studies, specimens were taken from frontal, parietal, temporal, and occipital lobes, deep brain nuclei, brainstem, cerebellum, spinal cord, eyes, and muscles. Histological preparations were stained with H&E and immuno‐stained according to standard protocols using a panel of antibodies to explore the inflammatory response (CD3, CD20, CD45, CD68), the endothelial cells (CD34), and brain reactive astrocytosis (GFAP).
Neurobiological study
Cryosectioning
Samples from cerebral hemispheres, fixed 2 weeks in zinc formalin solution were cryoprotected (30% sucrose in PBS o.n.) before embedding in NEG‐50 Blue (Thermo Scientific) for cryosectioning (Leica) (20μm) onto slides (SuperFrost Plus, 326 VWR International).
RNA in situ hybridization (ISH)
Non‐radioactive RNA in situ hybridizations using digoxigenin‐labeled sense and antisense riboprobes for hCHOP and ZIKV mRNA were performed on frozen fresh cerebral hemispheric sections as described previously 8. The templates were: (i) a PCR fragment amplified from hCHOP complete sequence (cDNA clone MGC:4154, IMAGE:330545 from Source Bioscience LifeSciences) and cloned in a PCR‐II TOPO vector (Thermo Fisher Scientific) according to manufacturer instructions; (ii) a PCR fragment amplified from the ZIKV genome (GCF_000882815.3). The following primers were used to amplify the 3′UTR region of hCHOP and ZIKV cDNAs:
CHOP.FW 5′‐AATCTTCACCACTCTTGACCCTGC ‐3′,
CHOP.REV 5′‐CTTTTGTCTACTCCAAGCCTTCCC ‐3′,
ZIKV.FW 5′‐AGGTGAAGCACGGAGATCTAGAAG ‐3′,
ZIKV.REV 5′‐CCACTAACGTTCTTTTGCAGACAT ‐3′.
The riboprobes were synthetized using the DIG labeling mix (Roche) according to manufacturer instructions.
Transmission Electron Microscopy (TEM)
Samples were fixed for 24 hours at 4°C in 2.5% glutaraldehyde in Sorensen's buffer 0.1M. After several washes in Sorensen's buffer, samples were post‐fixed at 4°C with 2% osmium tetroxide in Sorensen's buffer 0.1M for 60 min, then washed in deionized water, dehydrated at room temperature through a graded ethanol series (70%, 96%, and 100%), and embedded in Epon for 48 hours at 60°C. Ultrathin 70‐nm sections were obtained using an ultra‐microtome (Reichert Ultracut E) equipped with a diamond knife (Diatome). The sections were mounted on copper grids coated with collodion and contrasted with uranyl acetate and lead citrate for 15 minutes each. The ultrathin sections were observed under a JEM‐1400 transmission electron microscope (Jeol) at 80 kV and photographed with an 11 MegaPixel bottom‐mounted TEM camera system (Quemesa, Olympus). The images were analyzed using RADIUS software (Olympus).
Immunofluoresence
This was performed as described previously 38. Briefly antigen retrieval (Dako Target Retrieval Solution,) was performed at 95°C for 15 minutes, prior to incubation with primary antibodies. The primary antibody was an anti‐NS1 from Dengue Virus conjugated with Alexa‐546 (1:500, isolated by Marie Flamand). Nuclei were counterstained with Hoechst (1:5000, Thermo Fisher Scientific) and mounted in Mowiol (SIGMA) solution.
Imaging
Immunofluorescence pictures were visualized using the Nikon A1 confocal microscope or a Carl Zeiss LSM710 confocal microscope. In situ hybridizations pictures were visualized using an Olympus AX70 PROVIS microscope.
Ethics
A parental consent was signed by the parents to participate to this research.
All procedures were approved by the ethics committee (Agence de BioMedecine, approval: PFS 15‐009).
Results
Here, we report on the clinical and histopathological findings observed in three fetuses ascertained after pregnancy termination in the context of a maternal ZIKV infection acquired during the first trimester of pregnancy (Table 1).
Table 1.
Clinical, microbiological, and histopathological data of fetuses infected by ZIKV
| Case No | WG at PI | US features at amniocentesis | WG at Amnio‐Centesis/or blood sampling | PCR in maternal blood at amnio‐centesis | PCR in AF | PCR in fetal blood | WG at TOP | Sex | Macroscopic visceral anomalies | Macroscopic anomalies of fetal brain at TOP | Microscopic anomalies of fetal brain | Anti CD68 staining in fetal brain | Anti CD3 staining in fetal brain | PCR in fetal brain | Microscopic anomalies of other organs | Macroscopic anomalies of the placenta | Microscopic anomalies of the placenta | Zika PCR in placenta (% positive biopsies) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | Moderate ventriculomegaly | 23 | + | +++ | NA | 25 | F | Hepatomegaly | Microcephaly (<5th percentile) | Arachnoiditis | +++ | ++ | ++++ | No | Hypotrophic (<5th percentile) | Acute chorioamnio‐nitis | ++ (75%) |
| Ependymitis | ||||||||||||||||||
| Splenomegaly | Hyperplasia of Hofbauer cells | |||||||||||||||||
| Ventriculomegaly | Vasculitis | |||||||||||||||||
| Truncal vessels lesions | ||||||||||||||||||
| Atrophy of corpus callosum | Thymic hypertrophy | Thin corpus callosum | Necrosis | |||||||||||||||
| Cerebral cortex alteration | ||||||||||||||||||
| 2 | 9 | IUGR | 22 | + | +++ | + | 22 | M | IUGR (<5thpercentile) | Microcepahly (<5th percentile) | Arachnoiditis | +++ | ++ | ++++ | Lymphocytic infiltrate of testes | No | Chronic villitis | + (35%) |
| Dysmorphic lateral ventricule | Ependymitis | |||||||||||||||||
| Hyperplasia of Hofbauer cells | ||||||||||||||||||
| Triventriculomegaly | Vasculitis | |||||||||||||||||
| Hypotrophy of corpus callosum | Truncal vessels lesions | |||||||||||||||||
| Necrosis | ||||||||||||||||||
| Cerebral cortex alteration | ||||||||||||||||||
| Adrenal hypoplasia | ||||||||||||||||||
| 3 | 8 | Hepatic calcifications | 20 | + | ++ | + | 22 | M | No | Microcephaly (<5th percentile) | +++ | ++ | ++ | Lymphocytic infiltrate of testes | Hypotrophic (<5thpercentile) | Few neutrophils in intervillous space | +(100%) | |
| Atrophy of cerebral hemispheres | ||||||||||||||||||
| Hyperplasia of Hofbauer cells | ||||||||||||||||||
| Microcephaly (<5th) | ||||||||||||||||||
| Triventriculomegaly | Truncal vessels lesions | |||||||||||||||||
| Agenesis of the corpus callosum | ||||||||||||||||||
| Colpocephaly | ||||||||||||||||||
| Atrophy of corpus callosum |
Zika PCR results are given in semi‐quantitative ranges.
PI = Zika primary infection; WG = weeks of gestation; F = female, M = male, AF = amniotic fluid; US = ultrasounds; IUGR = Intra‐uterine growth retardation; NA = none applicable; ND = not done.
Clinical data
In all three fetuses, the mother, living, respectively, in Martinique, Venezuela, and Guadeloupe presented an episode of fever and a skin rash evocative of ZIKV infection during the first trimester of pregnancy (13 weeks of gestation (WG) in case 1; 9 WG in case 2, and 8 WG in case 3), In each case, the maternal ZIKV infection was confirmed by blood RT‐PCR. The first trimester obstetrical ultrasound examination was unremarkable in all three cases. The second trimester ultrasound examination showed, respectively, in Case 1 at 22 WG, a moderate ventriculomegaly, calcified ventricular walls and hypoplasia of the corpus callosum; in Case 2, at 21 WG growth retardation; and microcephaly with deformed lateral ventricles and in Case 3 at 20 WG hepatic calcifications and cerebral anomalies including microcephaly, a misplaced fourth ventricle, a corpus callosum agenesis, enlarged occipital ventricles with hyperechogenic walls. Amniotic fluid RT‐PCR for ZIKV infection was highly positive (+++) in Cases 1 and 2 and moderately positive (++) in Case 3. RT‐PCR test for ZIKV detection on fetal blood performed in Cases 2 and 3 showed a low positivity (+) in both cases. Termination of pregnancy was performed at 25 WG in Case 1 and at 22 WG in Cases 2 and 3.
Fetal examination
External macroscopic examination showed in Case 1 a female eutrophic fetus (weight: 831 g, 50th percentile for term), in Case 2 a male fetus with growth retardation (weight: 341 g, 5th percentile for term) and in Case 3 a male eutrophic fetus (weight: 374 g, 10thpercentile for term). All fetuses presented a small head. None had joint deformity.
Visceral examination
Hepatomegaly (enlarged liver weight: 63 g, >95th percentile for term), splenomegaly (enlarged spleen weight: 11.6 g, >95th percentile for term) and thymus hypertrophy (thymus weight: 6 g, >95th percentile for term) were observed in Case 1. In Case 2, adrenal gland hypoplasia (adrenals weight: 0.4 g, <5th percentile for term) was observed. No internal anomaly was noted in Case 3. Microscopic examination showed no major visceral changes, except an interstitial lymphocytic infiltrate (confirmed by anti‐CD45 immunohistochemistry) in the testis of both male fetuses (Fig. 1A and 1B).
Figure 1.
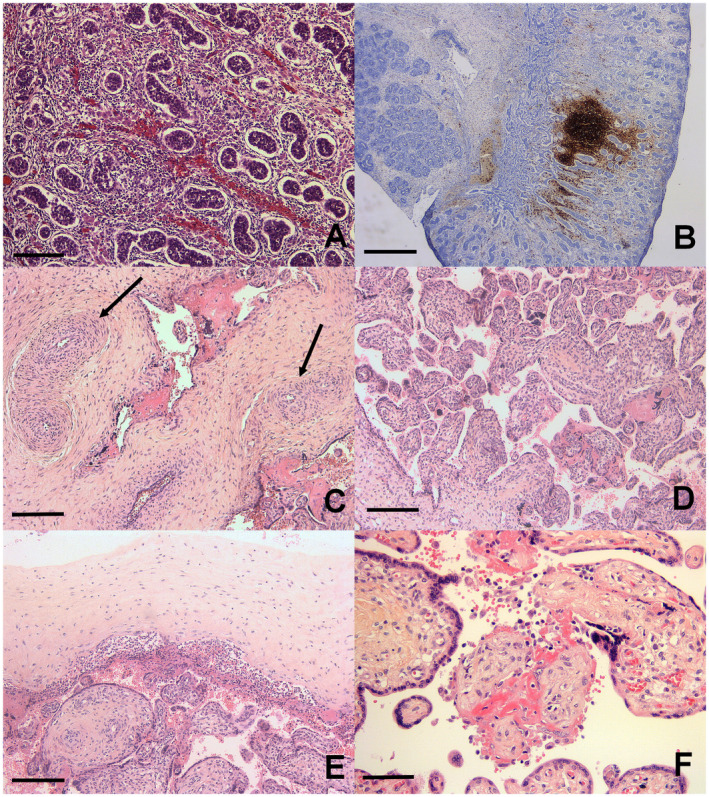
Testes and placental analysis. A. Histological section: interstitial lymphocytic infiltrate in testis. B. Anti CD20 immunohistochemistry: interstitial nodular lymphocytic infiltrate in testis. C. Fibromuscular hypertrophy of truncal vessels (arrows). D. Hofbauer cells hyperplasia. E. Acute chorioamnionitis. F. Chronic villitis. H&E, scale bar: 400 μm (B), 80 μm (A, D, E), 40 μm (C, F).
Neuropathological examination
Gross examination showed small cerebral hemispheres with an hypoplastic brainstem and cerebellum, covered with congestive, and thickened meninges, weighing below <5th percentile for age in Cases 1 and 2 (47.3 g in Case 1, 39.8g in Case 2), and at the 10th percentile in Case 3 (47.5g). Coronal sections of cerebral hemispheres showed various degrees of ventricular dilatation and corpus callosum anomalies (thin in Case 1, short in Case 2, and absent in Case 3) (Fig. 2A, 2B, and 2C). In Case 1, the cerebral mantle was reduced and contained a rim of subcortical mineralized material. Deep brain nuclei were small. In addition, Case 2 presented diffuse cortical hemorrhagic lesions. In Case 3, the cerebral mantle was well preserved, despite some hemorrhagic spots.
Figure 2.
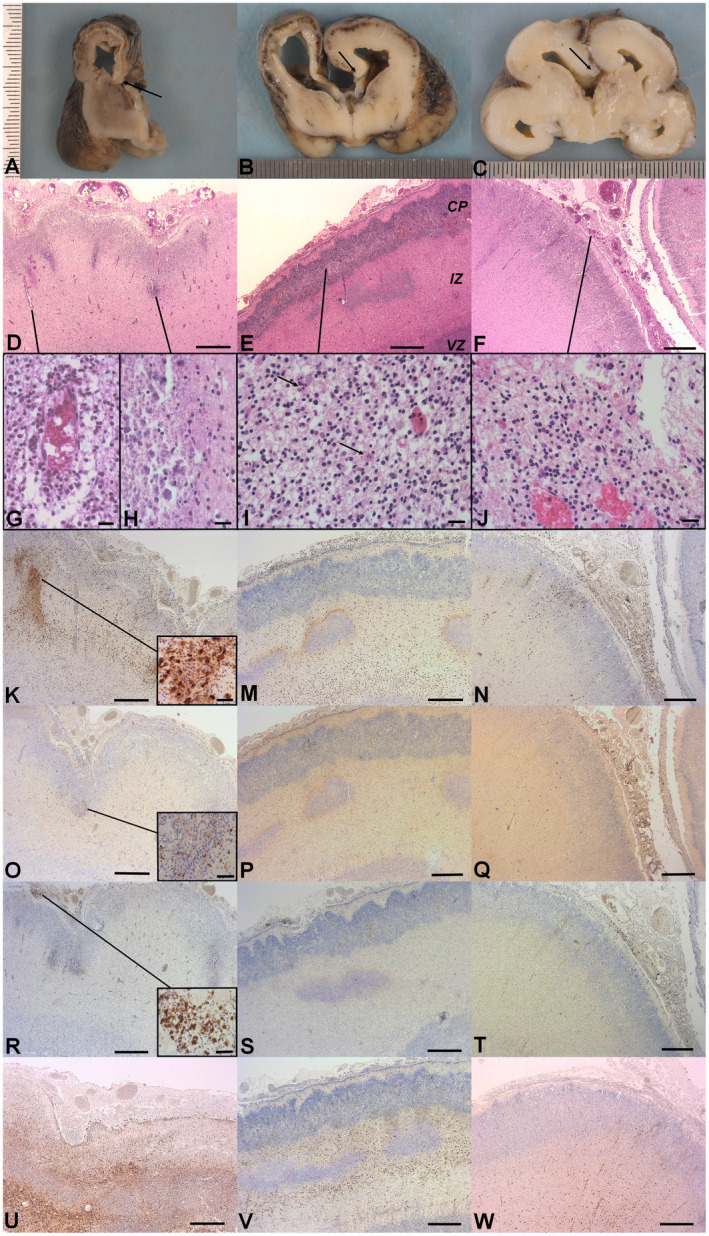
Neuropathological findings. Gross examination. A. Case 1: Coronal section of the right cerebral hemisphere showing: the lateral ventricle dilatation, the thin cerebral mantle, the cortical plate is separated from the underlying intermediate zone by a rim of mineralized tissue, thin corpus callosum (arrow), reduced deep brain nuclei. B. Case 2: Coronal section of cerebral hemispheres showing: triventricular dilatation, short corpus callosum (arrow), diffuse cortical hemorrhagic lesions. C. Case 3: Coronal section of cerebral hemispheres showing: triventricular dilatation and absent corpus callosum (arrow), focal cortical hemorrhagic lesion. Histological findings. D. Case 1: The cerebral mantle (H&E): Note diffuse inflammatory infiltrate in the arachnoid, associated to ependymal lesions and necrotic zones at the interface between the cortical plate (CP) and the intermediate zone (IZ). E. Case 2: The cerebral mantle (H&E): Note diffuse inflammatory infiltrate in the arachnoid, associated to ependymal lesions and necrotic zones at the interface between the CP and the IZ. CP: cortical plate, IZ: intermediate zone, VZ: ventricular zone. F. Case 3: The cerebral mantle (H&E): Note diffuse inflammatory infiltrate in the arachnoid, associated to ependymal lesions. G. Vasculitis in case 1 (high magnification, H&E). H. Calcified necrotic zone in case 1 (high magnification, H&E). I. Cerebral cortex case 2 (high magnification, H&E): Neuronal rarefaction, apoptotic bodies (arrow). J. Case 3: Inflammatory infiltrate in the arachnoid composed of numerous macrophages and lymphocytes (H&E). Immunohistochemistry. K. M. N. Anti CD68: macrophagic reaction in the wall of cerebral hemisphere, mainly around the necrotic zones in case 1 (magnified field) and 2. O. P. Q. Anti CD3: T lymphocytes are less numerous than macrophages but are also found in all cerebral areas and mainly around necrotic zones (magnified field). R. S. T. Anti CD20: few lymphocytes marked in the arachnoid (magnified field). U. V. W. Anti GFAP: hyperplastic and hypertrophic astrocytes in cerebral parenchyma. Scale bar: 400 μm (D, E, F, K, M, N, O, P, Q, R, S, T, U, V, W), 40 μm (G, H, I, J, magnified fields K, O and R).
In all three cases, the histopathological study found a diffuse arachnoiditis with ependymitis and vasculitis (Fig. 2D, 2E and 2F). Arachnoiditis was characterized by thickened meninges, filled with numerous inflammatory cells, composed mainly of macrophages and T lymphocytes (CD3+) (Fig. 2J). Vasculitis was revealed by the presence of swollen endothelial cells surrounded by active microglia (macrophages) and astrocytes (Fig. 2G). An additional spectrum of parenchymal lesions was observed involving the whole hemispheric wall namely the cortical plate (CP), the intermediate, and the ventricular zones (IZ, VZ). The CP lesions consisted in a loss of lamination with radial glia disruption, focal polymicrogyria, neuronal loss, chromatin fragmentation with numerous apoptotic residues and mineralization (Fig. 2I). In Cases 1 and 2, these were associated with foci of parenchymal destruction (necrosis) often mineralized, mainly present in the subcortical zone at the interface between the CP and the IZ (Fig. 2H). In Case 1, necrotic zones were larger than in Case 2. In Case 3, no necrotic lesions were observed. In cases 1 and 2, the IZ contained an intense macrophagic and astrocytic reaction. The VZ displayed signs of ependymal abrasion and congestion of the ganglionic eminences. The sub‐ventricular region was filled with astrocytes and macrophages and numerous apoptotic residues as well as the deep brain nuclei. The number of callosal fibers and longitudinal tracts were severely reduced, mainly in Cases 1 and 2. The brainstem was small because the longitudinal and transversal tracts were poorly developed. In addition, it contained numerous damaged neurons. In the cerebellum, the width of the external and the internal granular layers were reduced. The neurons were shrunken and contained a fragmented chromatin (karyorrhexis). Macrophages and numerous hypertrophic astrocytes were present. In the spinal cord, the astrocytic and macrophagic reaction was mild and neurons were spared. The longitudinal tracts were missing.
In all three cases, the immunohistochemical study on fixed cerebral tissues showed various degrees of positive responses (Table 2). Anti‐CD68 antibody confirmed the excess of a macrophagic response mainly in the IZ and the VZ (Fig. 2K, 2M, and 2N). Anti‐CD3 immunostaining showed abundant T lymphocytes (Fig. 2O, 2P and 2K). A small number of B lymphocytes (marked by anti‐CD20 antibody) was found in the arachnoid (Fig. 2R, 2S and 2T). The GFAP (Glial Fibrillary Acidic Protein) antibody confirmed the astroglial nature of the gliosis found mainly in the vicinity of necrotic regions in the subventricular and in the intermediate zones (Fig. 2U, 2V and 2W). Anti‐CD34 immunostaining confirmed the endothelial nature of the turgescent cells in the vessels wall.
Table 2.
Severity of neuropathological lesions on histological sections and intensity of astrocytosis, inflammation, and viral infection in different brain territories in the three cases
| Case | Territories | NP lesions H&E | Astrocytosis IHC | Inflammation IHC | Viral infection ISH |
|---|---|---|---|---|---|
| 1 | Cortical plate | High | Mild | Mild | High |
| Subcortical zone | High | High | High | Low | |
| Intermediary zone | High | High | High | Low | |
| Ventricular zone | Mild | Mild | Mild | High | |
| Brainstem/Cerebellum | Mild | Mild | Mild | NA | |
| Spinal cord | Very low | Low | Very low | NA | |
| 2 | Cortical plate | High | Mild | Mild | High |
| Subcortical zone | High | High | Mild | Low | |
| Intermediary zone | High | High | High | Low | |
| Ventricular zone | Mild | Mild | Mild | High | |
| Brainstem/Cerebellum | Low | Mild | Mild | NA | |
| Spinal cord | Low | Mild | Low | NA | |
| 3 | Cortical plate | High | Low | Mild | High |
| Subcortical zone | Mild | Mild | Mild | Low | |
| Intermediary zone | Mild | Mild | Mild | Low | |
| Ventricular zone | Low | Low | Mild | High | |
| Brainstem/Cerebellum | Mild | Mild | Mild | NA | |
| Spinal cord | Low | Mild | Low | NA |
NP lesions: Neuropathological lesions; H&E: Hematein and Eosin; IHC: Immunohistochemistry; ISH: In situ Hybridization; NA = not applicable.
The severity of neuropathological lesions on histological sections and the intensity of astrocytosis, inflammation and viral infection in the different brain territories in the three cases are summarized in Table 2.
Brain ZIKV infection
A RT‐PCR analysis performed on fresh brain tissues showed, respectively, high positivity (++++) in Cases 1 and 2 and moderate positivity (++) in Case 3. In addition the presence of the ZIKV infection was investigated using in situ hybridization (ISH). The study using an antisense riboprobe against the mRNA of ZIKV (Case 1; Fig. 3A–H, supporting information figure S1) and hCHOP, which is a marker of ZIKV‐induced stress in the endoplasmic reticulum of neurons and progenitor cells 15 (Table 2). It showed ZIKV‐infected cells with a columnar organization in the cortical plate (Fig. 3A, 3B), as well as in the ventricular and subventricular zones (Fig. 3E–H–3I–3L). Moreover, nonstructural protein 1 of ZIKV was detected by immunolabeling of cortical cells in Case 1 (Fig. 3M‐P). Interestingly, the ultrastructural analysis of ZIKV‐infected VZ showed signs of endoplasmic reticulum (ER) stress. At the ultrastructural level, and as expected in cells undergoing ER stress 20, 27 we observed that ZIKV‐infected cells exhibited enlarged ER and several autophagosomes containing 50‐nm electron‐dense ZIKV particles (Fig. 3Q–S, supporting information figure S2).
Figure 3.
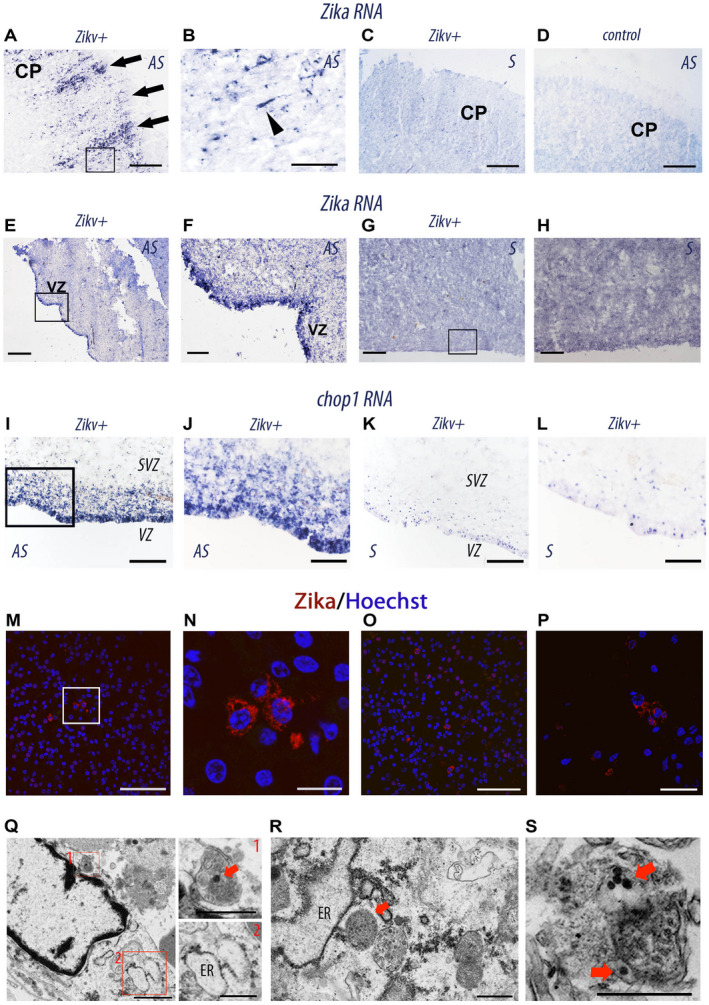
Histological and cellular analyses of ZIKV‐infected brains. A–L, in situ hybridization on coronal sections from human fetus ZIKV‐infected (Zikv+, case 1 A–C, E–H, and I–L) ZIKV‐uninfected (D,control) with antisense (AS) and sense (S) RNA probes of Zikv (A–H) and chop (I–L). B, F, and H are the magnifications of insets in A, E, and G, respectively. CP, cortical plate; VZ, ventricular zone. The black arrowhead indicates a single infected bipolar neuron migrating to the CP. M–P Immunolabeling of coronal sections from (case 1) ZIKV‐infected fetal brain with an antibody specific for NS1 (non‐structural protein 1 expressed by flaviviruses). M, O, P represent different dorsal regions of the cerebral cortex of case1 ZIKV‐infected fetal brain. N is a magnification of the insets in M. NS1 is in Red and nuclei are labeled by Hoechst (blue). Q–S, transmission electronic micrographs of frontal cortical sample from the brain of the (case 1) ZIKV‐infected fetus showing a cell with an autophagosome with an electron‐dense 50‐nm ZIKV particle (red square 1) and enlarged ER (red square 2) (Q), another one with enlarged ER and autophagosomes with ZIKV particles (R, S). Red arrows point ZIKV particles. Scale bar: 150 μm (A, C, D), 40 μm (B), 250 μm (E, G, H), 50 μm (F, J, L, M, O), 100 μm (I, K), 10μm (N), 20μm (P), 1 μm (Q, left), 500 nm (Q, right; R; S).
Placental examination
Placental examination showed a normotrophic placenta in Case 2 (weight: 138 g, 25th percentile for term) and a hypotrophic placenta in Cases 1 and 3 (weight: 142 g in Case 1 and 62 g in Case 3, <5th percentile for term). In all three cases, truncal vessels presented fibromuscular hypertrophy causing a narrowing of the lumen (Fig. 1C) and an excess (hyperplasia) of Hofbauer cells (Fig. 1D), confirmed by CD68 expression. An inflammatory infiltrate was noted in all three cases: an acute chorioamnionitis and funisitis in Case 1 (Fig. 1E), a chronic villitis in Case 2 (Fig. 1F), and a small number of neutrophils in the intervillous space in Case 3. Immature chorionic villi were observed in Case 2 and fibrous chorionic villi were observed in Case 3. The RT‐PCR test for ZIKV infection performed on placental tissue was moderately positive in 75% in Case 1, low positive in 35% in Case 2, and low positive in 100% in Case 3.
Discussion
On 1 February 2016, WHO declared the ongoing ZIKV epidemics as a public health emergency of international concern 39. This stimulated numerous clinical and biological investigations which demonstrated the fetoplacental transmission of ZIKV infection and confirmed the existence of ZIKV‐associated congenital brain malformations 18, 22, 31. However, despite the poor outcome and high mortality associated with ZIKV fetopathy and the WHO recommendation for postmortem investigations, neuropathological studies remain scarce. The present study allowed evaluating the impact of ZIKV infection on unborn babies avoiding thus the additional pathological changes caused by delivery stress and/or by resuscitation attempts. Importantly, the present study highlights the severity of the brain damages in the absence of major visceral and placental lesions, underlining the selective neurotropism of ZIKV. In addition, it allowed the description of a yet unreported involvement of fetal testis in ZIKV infection. This may have long‐term implications in adults infected in utero with ZIKV.
ZIKV‐induced Central Nervous System (CNS) anomalies
On brain imaging, CNS anomalies include microcephaly with ventriculomegaly and anomalies of the corpus callosum 3, 5, 7, 9, 11, 22, 25, 33. Microcephaly is usually reported as an isolated finding or in association with arthrogryposis 11. In the present series of ZIKV fetopathies examined after pregnancy termination for severe brain malformations diagnosed during the second trimester of pregnancy, spinal cord motor neurons were not affected and arthrogryposis was absent. Our neuropathological study allowed us to observe brain damages producing an ex vacuo hydrocephalus in addition to a diffuse neuronal loss resulting from an ER stress caused by the ZIKV infection.
Ex vacuo hydrocephalus
In all three, cases the present study founds a meningoencephalitis associated with diffuse arachnoiditis, ependymitis, and vasculitis. These changes are not specific of ZIKV infection. They have been reported upon other viral infection such as the cytomegalovirus infection 13, 22, 36. Vasculitis was diffuse and characterized by endothelial cells swelling surrounded by active astrocytes and macrophages. Parenchymal damages involved the entire cerebral hemispheric wall including the CP, the IZ, and the VZ. In the CP, major findings included a loss of radial lamination associated with disruption of the radial glia, the presence of focal polymicrogyria, and a diffuse loss of neurons. In the IZ, necrotic lesions were found mainly in the subcortical region at the interface with the CP and in the vicinity of damaged vessels, as described previously 33. Astrocytosis (GFAP+) and a macrophagic reaction (CD68+) were observed throughout the cerebral hemispheres, namely in the subcortical and intermediary zones. The extent of parenchymal damages associated with the loss of callosal fibers and longitudinal tracts caused the cerebral atrophy and the ventricular enlargement leading to an ex‐vacuo hydrocephalus, as observed in other congenital brain infections and in hypoxic–ischemic damages 13, 29, 36. The adrenal gland atrophy might result from the disruption of the hypothalamic and pituitary axis.
Deficit in cortical neurons
The prevailing view is to link ZIKV associated microcephaly to a deficit in cortical neurons 10, 21, 35. Several neurobiological studies did underline the increase of cells death and the impaired cell cycle leading to a decreased neural progenitor cell proliferation, causing a decrease in the number of cortical neurons leading to microcephaly. In in utero experimentally infected mouse embryos, cortical progenitor cells are selectively targeted in vivo by the ZIKV. This infection leads to induction of an ER stress 15. Our neurobiological investigations using RT‐PCR, immunohistochemistry (IHC), in situ hybridization (ISH), and transmission electron microscopy (TEM) allowed us to reveal the existence of these cellular damages and the presence of viral particles mainly in the ventricular, the sub‐ventricular zones and in the cortical plate, where reside, respectively, the basal progenitor cells, the apical progenitor cells and the neurons (Table 2). Interestingly, in the present study, we detected the presence of ZIKV replicative RNA in the neurons, as described previously 4. In addition, we found an extensive neuronal loss as well as numerous ZIKV‐infected neurons showing chromatin changes and surrounded by apoptotic residues. Finally, our description of ZIKV‐associated ER stress leading to both decreased cortical progenitor cell proliferation and increased mature neuron cell death in the cerebral cortex is fully consistent with data documented previously in animal models 15, 21, 23, 35. Interestingly, accumulation of chop1 mRNAs was also detected in the subventricular zone, suggesting that ZIKV did infect intermediary cortical progenitor cells, causing apoptosis.
Thus, it appears that brain malformations observed in the fetal ZIKV infection result from a multifactorial cascade of pathological mechanisms involving the progenitor neural cells, the cortical neurons, and the endothelial cells 10, 24, 26, 29, 35.
Placental lesions
The histopathological study of the ZIKV‐infected placental tissue has been poorly described although it has been shown that ZIKV infects specifically human placental macrophages and trophoblasts 1, 29. As described previously by Rozenberg et al 30, we observed a hyperplasia of Hofbauer cells. But contrary to other studies, we showed in all three cases an inflammation: an acute chorioamniotis in Case 1, a chronic villitis in Case 2 and an acute intervillositis in Case 3 30, 37. Interestingly, the inflammation associated with the ZIKV infection appears to be less severe than the one observed upon cytomegalovirus infection 2.
In addition, in all three cases, we observed a fibromuscular hypertrophy of truncal vessels causing a lumen narrowing. This aspect has been described previously by Chimelli et al 11. It is also observed in rubella placental infection 14. Presumably lesions of truncal vessels could contribute to a generalized fetal hypoxia. The impact of the Hofbauer cells hyperplasia remains to be determined.
Visceral anomalies
Microscopic examination of viscera was unremarkable besides an interstitial lymphocytic infiltrate in the testis observed in both male fetuses. To our knowledge, no testis infiltrate has been reported in infected fetuses by the ZIKV. Nevertheless, the presence of this infiltrate in testis is consistent with data observed previously in experimentally infected mice by ZIKV 34. Actually, ZIKV was detected in the testis and epididymis of male mice, and the infiltrating inflammatory cells associated with the infection may worsen the injury of these tissues 16. In humans, Joguet et al have shown that replication‐competent ZIKV can be isolated from motile spermatozoa and that ZIKV infection causes semen alterations 19.
Microbiology
ZIKV RT‐PCR was positive in the fetal brain of all three fetuses. It was highly positive (++++) in the two first cases and only mildly positive (++) in the third one. Fetal brain lesions were not different except for the lack of necrosis in Case 3, where a lower viral replication was detected. Also, in Case 3, the concentration of ZIKV was lower in the amniotic fluid. This might result from either a milder ZIKV infection or an increased viral clearance in this fetus. Indeed, previous reports suggested that over time, ZIKV particles may be eliminated from the amniotic fluid or from the fetal blood, leading to RT‐PCR negative results during the third trimester 28, 32. The low sensitivity of ZIKV RT‐PCR observed in placental tissues during the second trimester has been reported previously 32. In the present study, RT‐PCR detected ZIKV mRNA in 35%–100% out of the 8 different placental biopsy samples. Therefore, increasing the number of biopsy samples might enhance the sensitivity of ZIKV detection in the placenta.
Conclusion
The present study extends the clinical and histopathological description of the fetal disruption caused by the ZIKV infection. For example, it allowed us to report on a so far undescribed finding in infected humans, namely the presence of a testis inflammation observed in both male fetuses. In addition, it underlined the severity of brain lesions and the minimal visceral and placental changes observed. Finally, it allowed us to discuss the cascade of multifactorial developmental defects leading to micrencephaly resulting from both a deficit of cortical neurons due to a progenitor cells loss caused by an ER stress and to an ex vacuo hydrocephalus due to perfusion failure.
Conflict of Interest
None.
Supporting information
Figure S1. Magnification of a fetal ZIKV+ brain slice hybridized with ZIKV sense riboprobe (see Figure 3C).
Figure S2. Transmission electronic micrographs of a non‐infected cell and of a ZIKV infected cell.
Acknowledgments
This project was funded in part by the European Union's Horizon 2020 Research and Innovation Program under ZIKAlliance Grant Agreement N° 734548 (to L.N. and M.L.) and by the LabEx IBEID, Institut Pasteur, and INSERM.
References
- 1. Aagaard KM, Lahon A, Suter MA, Arya RP, Seferovic MD, Vogt MB et al (2017) Primary human placental trophoblasts are permissive for zika virus (ZIKV) replication. Sci Rep 7:41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benirschke K, Mendoza GR, Bazeley PL (1974) Placental and fetal manifestations of cytomegalovirus infection. Virchows Arch B Cell Pathol 16(2):121. [DOI] [PubMed] [Google Scholar]
- 3. Besnard M, Eyrolle‐Guignot D, Guillemette‐Artur P, Lastère S, Bost‐Bezeaud F, Marcelis L, et al (2016) Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill Bull Eur Sur Mal Transom Eur Common Dis Bull 21(13). [DOI] [PubMed] [Google Scholar]
- 4. Bhatnagar J, Rabeneck DB, Martines RB, Reagan‐Steiner S, Ermias Y, Estetter LBC, et al (2017) Zika virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis 23(3):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blázquez A‐B, Saiz J‐C (2016) Neurological manifestations of Zika virus infection. World J Virol 5(4):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brasil P, Pereira JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al (2016) Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 375(24):2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carvalho FHC, Cordeiro KM, Peixoto AB, Tonni G, Moron AF, Feitosa FEL, et al (2016) Associated ultrasonographic findings in fetuses with microcephaly because of suspected Zika virus (ZIKV) infection during pregnancy. Prenat Diagn 36(9):882–887. [DOI] [PubMed] [Google Scholar]
- 8. Cau E, Gradwohl G, Fode C, Guillemot F (1997) Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Dev Camb Engl 124(8):1611–1621. [DOI] [PubMed] [Google Scholar]
- 9. Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette‐Artur P, Eyrolle‐Guignot D, et al (2016) Association between Zika virus and microcephaly in French Polynesia, 2013‐15: a retrospective study. Lancet Lond Engl 387(10033):2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chavali PL, Stojic L, Meredith LW, Joseph N, Nahorski MS, Sanford TJ, et al (2017) Neurodevelopmental protein Musashi‐1 interacts with the Zika genome and promotes viral replication. Science 357(6346):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chimelli L, Melo ASO, Avvad‐Portari E, Wiley CA, Camacho AHS, Lopes VS, et al (2017) The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta Neuropathol 133(6):983. [DOI] [PubMed] [Google Scholar]
- 12. Driggers RW, Ho C‐Y, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, et al (2016) Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374(22):2142–2151. [DOI] [PubMed] [Google Scholar]
- 13. Eppes C, Rac M, Dunn J, Versalovic J, Murray KO, Suter MA, et al (2017) Testing for Zika virus infection in pregnancy: Key concepts to deal with an emerging epidemic. Am J Obstet Gynecol 216(3):209–225. [DOI] [PubMed] [Google Scholar]
- 14. Garcia AG, Marques RL, Lobato YY, Fonseca ME, Wigg MD (1985) Placental pathology in congenital rubella. Placenta 6(4):281–295. [DOI] [PubMed] [Google Scholar]
- 15. Gladwyn‐Ng I, Cordón‐Barris L, Alfano C, Creppe C, Couderc T, Morelli G, et al (2018) Stress‐induced unfolded protein response contributes to Zika virus‐associated microcephaly. Nat Neurosci 21(1):63–71. [DOI] [PubMed] [Google Scholar]
- 16. Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al (2016) Zika virus infection damages the testes in mice. Nature 540(7633):438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guihard‐Costa AM, Larroche JC, Droullé P, Narcy F (1995) Fetal biometry. Growth charts for practical use in fetopathology and antenatal ultrasonography. Introduction. Fetal Diagn Ther 10(4):211–278. [DOI] [PubMed] [Google Scholar]
- 18. Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, et al (2017) Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 317(1):59–68. [DOI] [PubMed] [Google Scholar]
- 19. Joguet G, Mansuy J‐M, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al (2017) Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 17(11):1200–1208. [DOI] [PubMed] [Google Scholar]
- 20. Laguesse S, Creppe C, Nedialkova DD, Prévot PP, Borgs L, Huysseune S, et al (2015) A dynamic unfolded protein response contributes to the control of cortical neurogenesis. Dev Cell 5:553–567. [DOI] [PubMed] [Google Scholar]
- 21. Li H, Saucedo‐Cuevas L, Regla‐Nava JA, Chai G, Sheets N, Tang W, et al (2016) Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell 19(5):593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melo AS de O, Aguiar RS, Amorim MMR, Arruda MB, Melo F de O, Ribeiro STC, et al (2016) Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol 73(12):1407–1416. [DOI] [PubMed] [Google Scholar]
- 23. Merfeld E, Ben‐Avi L, Kennon M, Cerveny KL (2017) Potential mechanisms of Zika‐linked microcephaly. Wiley Interdiscip Rev Dev Biol 6(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mladinich MC, Schwedes J, Mackow ER (2017) Zika virus persistently infects and is basolaterally released from primary human brain microvascular endothelial cells. mBio 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mlakar J, Korva M, Tul N, Popović M, Poljšak‐Prijatelj M, Mraz J, et al (2016) Zika virus associated with microcephaly. N Engl J Med 374(10):951–958. [DOI] [PubMed] [Google Scholar]
- 26. Noronha L de, Zanluca C, Azevedo MLV, Luz KG, Santos CNDD (2016) Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz 111(5):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26(24):9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pomar L, Malinger G, Benoist G, Carles G, Ville Y, Rousset D, et al Association between Zika virus and fetopathy: A prospective cohort study in French Guiana (2017) Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 49(6):729–736. [DOI] [PubMed] [Google Scholar]
- 29. Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, et al (2016) Zika virus infects human placental macrophages. Cell Host Microbe 20(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA (2017) Placental pathology of Zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch Pathol Lab Med 141(1):43–48. [DOI] [PubMed] [Google Scholar]
- 31. Sarno M, Aquino M, Pimentel K, Cabral R, Costa G, Bastos F, et al (2016) Progressive lesions of central nervous system in microcephalic fetuses with suspected congenital Zika virus syndrome. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 50(6):717–722. [DOI] [PubMed] [Google Scholar]
- 32. Schaub B, Vouga M, Najioullah F, Gueneret M, Monthieux A, Harte C, et al (2017) Analysis of blood from Zika virus‐infected fetuses: A prospective case series. Lancet Infect Dis 17(5):520–527. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz DA (2017) Autopsy and postmortem studies are concordant: pathology of Zika virus infection is neurotropic in fetuses and infants with microcephaly following transplacental transmission. Arch Pathol Lab Med 141(1):68. [DOI] [PubMed] [Google Scholar]
- 34. Sheng Z‐Y, Gao N, Wang Z‐Y, Cui X‐Y, Zhou D‐S, Fan D‐Y, et al (2017) Sertoli cells are susceptible to ZIKV infection in mouse testis. Front Cell Infect Microbiol 7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18(5):587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teissier N, Fallet‐Bianco C, Delezoide A‐L, Laquerrière A, Marcorelles P, Khung‐Savatovsky S, et al (2014) Cytomegalovirus‐induced brain malformations in fetuses. J Neuropathol Exp Neurol 73(2):143–158. [DOI] [PubMed] [Google Scholar]
- 37. van der Eijk AA, van Genderen PJ, Verdijk RM, Reusken CB, Mögling R, van Kampen JJA, et al (2016) Miscarriage associated with Zika virus infection. N Engl J Med 375(10):1002–1004. [DOI] [PubMed] [Google Scholar]
- 38. Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081. [DOI] [PubMed] [Google Scholar]
- 39. WHO . Zika virus and complications: 2016 Public Health Emergency of International Concern WHO. Available at: http://www.who.int/emergencies/zika-virus-tmp/en/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Magnification of a fetal ZIKV+ brain slice hybridized with ZIKV sense riboprobe (see Figure 3C).
Figure S2. Transmission electronic micrographs of a non‐infected cell and of a ZIKV infected cell.


