Abstract
Objectives
To evaluate the effect of orthodontic appliances on physicochemical, biochemical, and oxidative stress changes in salivary parameters during treatment.
Materials and Methods
A cohort study was conducted with 112 healthy patients. Salivary samples were taken at baseline, 1 month, and 9 months after placement of the orthodontic appliances used in treatment.
Results
A statistically significant difference was observed in certain examined salivary parameters, including enzymes, electrolytes, and oxidative stress markers.
Conclusions
The use of aligners had a lower prevalence of disturbing salivary parameters. Orthodontist must consider these changes to prevent the occurrence of white spot lesions.
Keywords: Orthodontic appliances, Oxidative stress, Salivary enzymes, Electrolytes
INTRODUCTION
A healthy mouth and esthetic teeth are not only important for a person's self-esteem but also because they reflect a person's general state of health. The interest in scientific studies related to dentistry, especially in the orthodontic field, has grown over the past 50 years with the advent of new analysis techniques concerning biological samples, notably saliva.1
Saliva is a valuable biological fluid essential to overall well-being and is implicated in a wide variety of biological processes essential to the proper effectiveness of oral functions. It preserves oral health by participating in the body's oral defense and maintaining ecological balance.2–4 The placement of orthodontic appliances introduces a new material in the oral cavity.
Orthodontic biomaterials influence the oral environment and have a complex interaction with different components. In addition, their impact on various salivary parameters is not yet elucidated in a tangible way, despite the current innovations of orthodontic biomaterials and the characterization of tissue-material interactions.5 Previous investigations did not determine the specific correlation between the placement of orthodontic appliances and biological and clinical outcomes.6
The pH of saliva, calcium level, and oxidative status can be affected during orthodontic treatment, leading to enamel decalcification that is clinically revealed by the appearance of white spot lesions (WSLs), especially when associated with poor hygiene.7 In this framework, the purpose of this study was to investigate variations in salivary parameters, notably changes in the physicochemical, biochemical, and oxidative stress parameters related to the type of orthodontic appliances used.
MATERIALS AND METHODS
Study Population
In this prospective clinical study, the sample-size calculation was based on a 5% type 1 error and 80% power analysis. Thus, at least 93 participants were needed. To account for nonrespondents and dropouts, 20% were added.
The study protocol was approved by the ethical committee (no. 35220228) of the Faculty of Dental Medicine, University of Monastir, Monastir, Tunisia. Informed consent was signed by all patients or their legal representatives after explaining the protocol and study objective. The basic ethical principles of the 2013 Helsinki Declaration were applied.
Potential participants were patients examined during 1 year (2018) (n = 321) who routinely presented to the Monastir orthodontic department. Of these,112 healthy young patients were selected who met the inclusion criteria. The enrolled patients were between 10 and 20 years old with good oral hygiene and were free of periodontal/mucosal disease or active caries. Any patient showing a pocket depth >3 mm was excluded. Patients who had been taking medication within the previous 3 months, were pregnant, consumed tobacco or alcohol, or had had previous orthodontic treatment were also excluded from the study.
All participants used the same concentration of fluoride-containing toothpaste (1450 ppm). No supplementary fluoride was used during the study period.
Orthodontic Data
After clinical and radiographic examination, the orthodontic diagnosis was established for the patients enrolled in the study (n = 112). The patients were divided into the following three groups (Figure 1):
Figure 1.
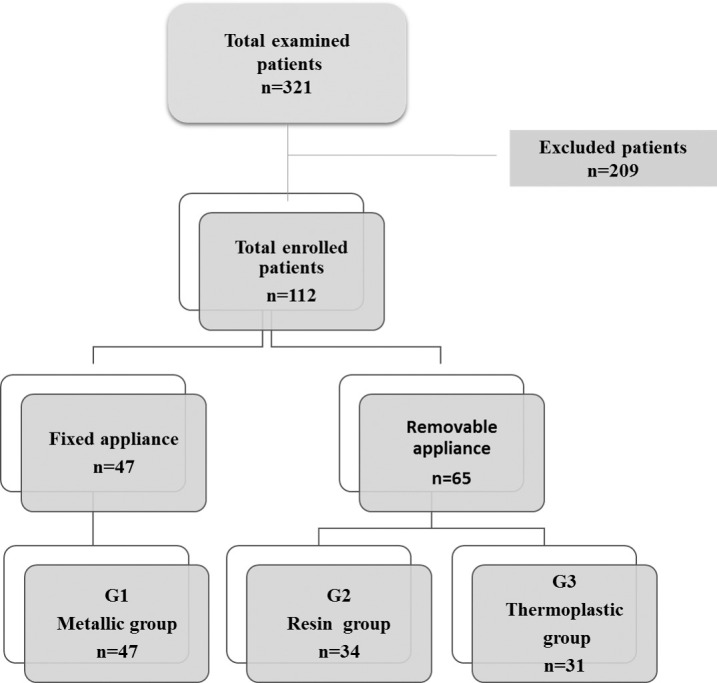
Flow chart of the study.
Group 1 (G1): patients (n = 47) wearing metallic fixed appliances (Forestadent, Pforzheim, Germany)
Group 2 (G2): patients (n = 34) wearing a resin activator of Andresen type II (Major Ortho, Moncalieri, Italy)
Group 3 (G3): patients (n = 31) wearing thermoplastic aligners (Invisalign, Juàrez, Chih, Mexico)
Sampling
Three salivary samples were taken from each participant at three different time points:
T0: Before orthodontic appliance placement
T1: One month after orthodontic appliance placement
T2: Nine months after orthodontic appliance placement
The samples were taken in the dental chair between 9 and 12 AM, two hours away from any food intake. Participants were asked to rinse with water for 30 seconds. To stimulate salivary flow, they were asked to chew a piece of paraffin for 1 minute. Then, the stimulated saliva was collected during 5 minutes in sterilized and preweighed tubes.
Physicochemical Parameter Exploration
Volume and salivary flow assay.
The tubes were weighed again using an analytical balance. Salivary volume was determined according to the following formula:
Salivary volume (mL) = (Full tube weight – Empty tube weight) / Salivary ρ (salivary ρ is the density of saliva that is equivalent to 1 g/mL8).
Salivary flow was then calculated according to the following formula:
Salivary flow rate (mL/min) = (Salivary volume) / (Collection duration)
Salivary pH measurement.
Salivary pH was measured with an electronic pH meter with a sensitivity of 0.01. Each measure was repeated three times.
Buffering capacity determination.
Saliva buffer capacity was measured according to the Ericsson method with some modifications: 1.5 mL of HCl (5 mmol/L) was added to 0.5 mL of saliva, and the mixture was homogenized with a stirrer for 30 seconds. After 10 minutes, a second pH measurement was performed8.
Biochemical Parameter Measurement
The studied salivary parameters (electrolytes, salivary enzymes, and substrate) were analyzed by an automated biochemistry analyzer (Cobas 6000, Roche Diagnostics, Mannheim, Germany) using human diagnostic kits (Roche Diagnostics, Mannheim, Germany).9 Each measure was repeated three times, and the means were taken.
Oxidative Stress
Trolox equivalent antioxidant capacity (TEAC) assay.
The TEAC assay was carried out according to the method of Re et al.10 It is based on the ability of antioxidant molecules to quench the long-lived 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation, a blue/green chromophore with a characteristic absorption at 734 nm, in comparison with that of Trolox, a water-soluble vitamin E analog.
Lipid peroxidation.
The evaluation of lipid peroxidation was performed according to the method of Ohkawa et al.11 based on the generation of malondialdehyde (MDA), the end product of lipid degradation.
Clinical Investigation of White Spot Lesions
Each tooth was examined at baseline and then after 1 month and 9 months. Three investigators evaluated and counted any obvious WSLs using visual evaluation of all buccal surfaces. Light is reflected differently from demineralized enamel surfaces compared with the adjacent enamel, giving rise to a chalky white appearance. In case of disagreement, a consensus was reached.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (IBM SPSS Statistics Version 24 for Windows 64 bits, SPSS Inc, Chicago, Ill). A χ2 test was used to compare percentages within groups. The analysis of variance was verified by the Fisher test. For quantitative variables, the Wilcoxon test was used for intragroup comparison (comparing t0, t1, and t2); The Mann-Whitney test was used for pairwise comparison between independent groups, and Kruskal-Wallis was used to compare the three groups. The confidence interval was set at 95%, and the difference was considered significant if P < .05.
RESULTS
Included in this study were 112 patients, 55 women (49.1%) and 57 men (50.9%), with a mean age of 13 years ± 3 months. Patients' eating habits and dental hygiene are illustrated in Table 1.
Table 1. .
Sample Characteristics
| Fixed Appliance (n = 47) |
Removable (n = 65) |
||||||
| G1: Metallic Group (n = 47) |
G2: Resin Group (n = 34) |
G3: Thermoplastic Group (n = 31) |
P Value (G1 to G2) |
P Value (G1 to G3) |
P Value (G2 to G3) |
P Value |
|
| Mean age | |||||||
| 15.16 | 13.16 | 16.75 | .56 | .688 | .35 | .197 | |
| ±3 years | ± 2 years | ± 2 years | |||||
| Gender | |||||||
| Girls (n) | 22 | 16 | 17 | .571 | .517 | .278 | .548 |
| Boys (n) | 25 | 18 | 14 | ||||
| Frequency of meals per day | |||||||
| Two to three times | 32 | 19 | 19 | .333 | .394 | .899 | .53 |
| Up to three times | 15 | 15 | 12 | ||||
| Consumption of carbohydrates | |||||||
| Rare | 15 | 7 | 6 | .016a | .067 | .912 | .081 |
| Moderated | 23 | 22 | 20 | ||||
| Excessive | 9 | 5 | 5 | ||||
| Brushing frequency (per day) | |||||||
| One time | 12 | 7 | 8 | .748 | .4 | .733 | .752 |
| Two times | 10 | 14 | 9 | ||||
| Three times | 25 | 13 | 14 | ||||
Significant P value following χ2.
Table 2 shows the salivary physicochemical, biochemical, and oxidative stress parameters. Medians and ranges of the measurements were explored and statistically analyzed. Overall comparisons among the three groups did not reveal significant differences.
Table 2. .
Comparison of Salivary Parameters Among Fixed and Removable Appliancesa,b
| Fixed Appliance (n = 47) |
|||
| Metallic (n = 47) | |||
| T0 |
T1 |
T2 |
|
| Saliva physicochemical parametersb | |||
| Flow rate | 1.32 [0.99–1.52] | 1.36 [0.98–1.47] | 1.18 [0.82–1.51] |
| pH | 7.15 [6.92–7.4] | 7.14 [6.78–7.31] | 7.03 [6.93–7.09] |
| Buffer capacity | 4.52 [3.94–4.69] | 4.45 [3.75–4.56] | 4.55 [3.84–4.87] |
| Saliva biochemical parameters electrolytes | |||
| Potassium (mmol/L) | 17.43 [16.63–21.02] | 15.90 [12.94–18.27]c | 16.56 [14.79–20.44] |
| Chlorine (mmol/L) | 15.2 [12.8–21.2] | 14.3 [11.5–20.1] | 11.8 [10.2–16.9]e |
| Calcium (mmol/L) | 0.39 [0.11–0.82] | 0.44 [0.44–1.14]c | 0.55 [0.24–1.18]d,e |
| Phosphorus (mmol/L) | 2.82 [0.86–4.47] | 3.96 [0.65–5.12]c | 4.43 [1.73–4.75]d,e |
| Salivary substrates | |||
| Total proteins (mmol/L) | 405.6 [194.1–521.0] | 347.0 [140.2–596.0]c | 371.0 [176.0–698.0] |
| Albumin (mg/L) | 71.73 [53.41–108.89] | 48.19 [20.67–145.60]c | 46.56 [22.81–163.87] |
| Salivary enzymes | |||
| LDH (UI/L) | 69 [17–158] | 78 [21–163]c | 70 [17–148]e |
| ALAT (UI/L) | 3.3 [1.0–7.0] | 4.5 [1.0–6.4]c | 2.9 [1.0–7.5]e |
| ASAT (UI/L) | 15.0 [12.6–37.5] | 16.3 [3.9–27.1]c | 14.7 [4.3–34.0]e |
| Amylase (UI/L) | 59,644 [39,304–91,937] | 68,170 [43,819–110,753]c | 78,790 [49,586–125,148]d,e |
| Lipase (UI/L) | 0.23 [0.1–1.0] | 0.37 [0.1–1.4]c | 0.68 [0.1–1.8]d,e |
| Oxidative stress parameters | |||
| TEAC (mmol Trolox/mL) | 0.631 [0.394–0.712] | 0.630 [0.550–0.722] | 0.419 [0.323–0.519]d,e |
| Uric acid (mmol/L) | 77 [52–84] | 69 [51–71] | 61 [48–80]d |
| MDA (nmol/mg total proteins) | 0.436 [0.418–0.571] | 0.418 [0.396–0.462] | 0.474 [0.451–0.499] |
ALAT indicates alanine aminotransferase; ASAT, aspartate aminotransferase; G1, group 1; G2, group 2; G3, group 3; LDH, lactate dehydrogenase; MDA, malondialdehyde; T0, before orthodontic appliance placement; T1; one month after the orthodontic appliance placement; T2, 9 months after orthodontic appliance placement; TEAC, Trolox equivalent antioxidant capacity.
All data were presented as median interquartile range.
T1 is statistically significant compared with T0 as calculated by Wilcoxon test.
T2 is statistically significant compared with T0 T0 as calculated by Wilcoxon test.
T2 is statistically significant compared with T1 as calculated by Wilcoxon test.
G1 is statistically significant compared with G2 as calculated by Mann-Whitney test.
G1 is statistically significant significant compared with G3 as calculated by Mann-Whitney test.
Table 2. .
Extended
| Removable Appliance (n = 65) | |||||
| Resin (n = 34) |
Thermoplastic (n = 31) |
||||
| T0 |
T1 |
T2 |
T0 |
T1 |
T2 |
| Saliva physicochemical parametersb | |||||
| 1.19 [0.83–1.32] | 1.14 [0.85–1.91] | 1.15 [0.78–1.52] | 1.17 [0.61–1.63] | 1.47 [0.67–1.70] | 1.36 [0.61–1.76] |
| 7.07 [6.97–7.11] | 7.18 [6.72–7.20] | 7.07 [6.77–7.24] | 7.21 [6.74–7.48] | 7.14 [6.66–7.26] | 7.10 [6.92–7.17] |
| 4.01 [3.87–4.13] | 4.32 [3.84–4.86] | 4.03 [3.76–4.15] | 4.51 [3.93–4.59] | 4.19 [3.92–4.61] | 3.99 [3.66–4.35] |
| Saliva biochemical parameters electrolytes | |||||
| 17.81 [15.03–22.11] | 15.86 [12.71–20.57] | 17.15 [14.84–21.08] | 17.49 [15.54–23.10] | 15.04 [13.43–21.17] | 18.42 [16.63–23.32] |
| 14.9 [11.0–20.9] | 13.9 [10.2–21.4] | 13.4 [12.0–22.4] | 14.2 [13.2–19.0] | 15.0 [12.1–16.2] | 13.2 [12.0–18.1]g |
| 0.32 [0.12–0.92] | 0.40 [0.28–1.02]c | 0.47 [0.33–1.07]d,e,f | 0.37 [0.15–0.68] | 0.41 [0.27–0.70]c | 0.46 [0.35–1.08]d,e,g |
| 3.05 [1.49–5.87] | 3.48 [1.91–6.58]c | 3.66 [1.16–4.71]d,e,f | 2.99 [1.45–4.49] | 3.11 [1.99–5.24]c | 3.21 [1.78–5.20]d,e,g |
| Salivary substrates | |||||
| 360.7 [209.8–517.0] | 256.9 [198.7–486.6]c,f | 317.0 [123.0–650.0] | 324.2 [118.1–573.0] | 254.0 [149.0–574.2]c,g | 377.0 [163.0–563.0] |
| 58.95 [23.10–142.84] | 43.27 [20.21–165.14]c,f | 64.86 [21.61–125.32]d | 70.10 [36.68–131.20] | 47.86 [20.34–109.86]c | 53.05 [30.76–119.55] |
| Salivary enzymes | |||||
| 61 [15–153] | 76 [17–123]c,f | 64 [14–84]e | 70 [29–124] | 74 [16–121]c | 72 [13–130]e |
| 3.4 [1.0–6.0] | 4.2 [1.0–7.2]c | 3.1 [1.0–5.8]e | 2.1 [1.0–5.0] | 2.6 [1.6–4.7]c | 2.1 [1.2–3.0]e |
| 11.7 [3.2–44.4] | 14.1 [3.0–25.5]c,f | 12.3 [4.1–30.4]e | 8.5 [3.4–62.3] | 11.0 [5.1–40.9]c | 9.1 [6.6–50.8]e |
| 54,490 [20,489– 902,415] | 68,424 [25,567– 101,599]c | 78,066 [34,309– 112,599]d,e | 53,649 [21,426– 80,853] | 65,582 [32,352– 141,127]c | 72,320 [29,006– 135,014]d,e |
| 0.30 [0.1–0.8] | 0.51 [0.3–1.2]c | 0.72 [0.1–1.6]d,e | 0.19 [0.1–0.6] | 0.41 [0.1–1.9]a | 0.80 [0.4–1.2]d,e |
| Oxidative stress parameters | |||||
| 0.645 [0.561–0.742] | 0.658 [0.535–0.776] | 0.397 [0.216–0.541]d,e | 0.627 [0.591–0.857] | 0.661 [0.455–0.862] | 0.648 [0.473–0.763] |
| 85 [58–91] | 78 [53–81] | 71 [60–80]d | 84 [54–94] | 78 [49–80] | 82 [65–89] |
| 0.449 [0.386–0.470] | 0.429 [0.401–0.498] | 0.490 [0.381–0.499] | 0.517 [0.413–0.523] | 0.459 [0.369–0.467] | 0.487 [0.394–0.491] |
Physicochemical Parameters
The flow rate, pH, and buffer capacity did not significantly change between the three-time points, regardless of the appliance type used during orthodontic treatment.
Biochemical Parameters
Regarding the electrolyte measurements, potassium significantly decreased after 1 month (T1) compared with T0 in G1. However, no significant difference was observed after 9 months (T2). Chlorine was continuously decreasing and was significantly different at T2 compared with T0. Additionally, there was a significant difference in chlorine levels between G1 and G3 at T2.
Potassium showed no significant changes during treatment in G2 or G3. Calcium and phosphorus significantly increased during treatment. This increase was more accentuated in G1. Additionally, there was a significant decrease in salivary substrate rates (total proteins and albumin) at T1 independent of appliance type.
Evaluation of salivary enzymes revealed that lactate dehydrogenase (LDH), alanine aminotransferase (ALAT), and aspartate aminotransferase (ASAT) were significantly increased at T1 compared with T0 and were significantly different between G1 and G2. Then, they decreased by T2, reaching almost baseline values, whereas amylase and lipase were continuously and significantly increasing during the 9 months. This increase was more accentuated for G3.
Oxidative Stress
As shown in Table 2, there was no significant change in TEAC after 1 month or after 9 months (T2) for G3. However, while there were no significant differences at T1, TEAC was significantly decreased at T2 compared with T0 for G1 and G2.
Uric acid level, another marker of oxidative stress, showed a significant decrease at T2 compared with T0 in G1 and G2. Nevertheless, uric acid concentration remained unchanged during treatment for G3.
No significant induction of MDA was observed for any group during treatment, indicating that there was no severe damage leading to the peroxidation of lipids in saliva.
Clinical Outcomes: WSLs
Of all the patients treated with orthodontic appliances, 19% developed WSLs. The percentage of WSLs was about 29% in G1, followed by 16% in G2 and 7% in G3. The comparison among the three groups showed significant differences (P = .027). However, regarding the pairwise comparison, there was only a statistically significant difference between G1 and G3: P = .114 (comparing G1 and G2), P = .013 (comparing G1 and G3), and P = .284 (comparing G2 and G3). Orthodontic patients wearing fixed appliances were the most likely to develop WSLs, which were located around the brackets.
DISCUSSION
In this study, pH, buffer capacity, and flow rate did not show any changes resulting from an adaptive and protective response of saliva. Similar results were found by Sanpei et al.12 and Bonetti et al.13 Several findings focusing on fixed appliances revealed that flow rate and salivary pH significantly increased. However, buffer capacity remained unchanged.14 Other studies showed an increase in salivary flow, pH, and buffer capacity after 1 month of orthodontic treatment.15,16 In addition, Teixeira et al.17 noticed a mutual increasing concentration of bicarbonate and flow rate in saliva.
According to the literature, orthodontic appliances enhanced the salivary flow rate, thus increasing the protective effect against WSLs. The increase of salivary flow rate is considered a physiological response to the mechanical stimulation caused by the introduction of new appliances. WSLs can form due to salivary pH, flow rate and buffer capacity decline associated with poor oral hygiene.7,8
Additionally, the results showed a modification in several electrolyte rates, which may have been due to a disturbance in the ionic balance on tooth surfaces leading to WSLs. Orthodontic treatment can also produce gingival complications and aggravate existing lesions, which would affect salivary electrolytes. Previous studies revealed that electrolyte concentrations increased in severe periodontal disease. Sodium concentrations in saliva decreased as a result of the increased adrenocorticotropic hormone. Consequently, the change in sodium and potassium levels could be considered stress-response indicators.18
The calcium and phosphorus levels in saliva are recognized for their role in preserving the stability of calcification/decalcification on tooth surfaces. Calcium level is highly reliant on pH and salivary flow rate. It was found that people with low calcium concentrations in saliva accompanied by acid pH were more predisposed to demineralization than those with greater concentrations of calcium and phosphorus.19
The exposure of biomaterials to the oral environment causes a deposition of salivary components on their surfaces. Proteins or glycoproteins were among these deposits. This may explain the decrease in total proteins and albumin levels observed at T1. Ahn et al.20 revealed that several salivary proteins adhered to braces and had a significant role in the binding of oral bacteria. Steinberg and Eyal21 concluded that orthodontic appliances absorbed salivary proteins, leading to a decrease in their levels.
Changes in digestive enzyme activities (lipase and amylase) were demonstrated. Several studies have reported that such changes could be accompanied by taste sensitivity and dietary preference modifications and emotional stress.22 The increase in salivary enzymes was greater in G3, which could be due to accentuated dental movements because of the more frequent changes of the orthodontic appliances compared with fixed appliances. Similar findings were mentioned by De Almeida et al.6 and Campos et al.22
According to the current study, LDH, ASAT, and ALAT was decreased at T1 for the three groups, then increased at T2 to reach the initial values. This may reveal a biologic adaptation response. Adhitya et al.23 reported an increase in ASAT. Similarly, Husin et al.24 found a significant increase in LDH levels that was correlated with dental movements and the duration of orthodontic forces. To the contrary, Totan et al.25 found no significant changes in ALAT rates even when associated with periodontal disease.
Several investigations highlighted the role of transaminases (ALAT/ASAT) and uric acid in the initiation of inflammatory reactions.26 The salivary uric acid rate in the current study was significantly lower in G1 and G2 accompanied by lower TEAC, which could be due to oxidative stress in the oral cavity. In fact, uric acid is a physiological antioxidant parameter in saliva contributing to approximately 70% of the total antioxidant capacity27 and having the ability to chelate metals and react with biological oxidants. By contrast, there were no observed changes in uric acid level and TEAC in G3, suggesting that thermoplastic did not enhance oxidative stress during treatment, in comparison to G1 and G2.
Oxidative stress and its relationship to the appearance of WSLs was established previously in different studies. Tothova et al.28 concluded that salivary markers of oxidative stress were related to oral hygiene and periodontal and dental status. The decrease in TEAC was associated with poor oral hygiene, higher bleeding on probing index, and higher caries index.28 These findings were in agreement with the current results in which the appearance of WSLs observed after 9 months of treatment was associated with lower TEAC levels recorded at T2 in G1 and G2 compared with G3. The findings support the fact that removable appliances do not compromise oral hygiene and dental/periodontal status. Similarly, Chenin et al.29 specified that the appearance of WSLs could be prevented by the use of thermoplastic materials. Further studies are needed to confirm this hypothesis. Atuğ Özcan et al.30 did not show any changes in MDA rates after 1 month and 6 months of fixed appliance treatment.
CONCLUSIONS
Orthodontic treatment affects the oral environment when using fixed appliances.
The use of thermoplastic materials showed a lower chance of developing WSLs, likely due to the minimal impact on salivary parameters, notably the oxidative stress.
The orthodontist could consider these modifications when choosing the orthodontic appliance type.
ACKNOWLEDGMENTS
We thank Jihene Maatoug and Samia Dabou for help provided during the statistical analysis and Asma Kassab and Nebil Sakly for language revision.
REFERENCES
- 1.Dodds MWJ, Johnson DA, Yeh C-K. Health benefits of saliva: a review. J Dent. 2005;33:223–133. doi: 10.1016/j.jdent.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 3.Malathi N, Mythili S, Vasanthi HR. Salivary diagnostics: a brief review. ISRN Dent. 2014;2014:1–8. doi: 10.1155/2014/158786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang A, Sun H, Wang X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl Biochem Biotechnol. 2012;168:1718–1727. doi: 10.1007/s12010-012-9891-5. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Gupta A, Agarwal L. Role of saliva: an orthodontic perspective. Int J Oral Health Dent. 2016;2:126–131. [Google Scholar]
- 6.De Almeida PDV, Gregio A, Machado M, De Lima A, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9:72–80. [PubMed] [Google Scholar]
- 7.Benkaddour A, Bahije L, Bahoum A, Zaoui F. Orthodontics and enamel demineralization: clinical study of risk factors. Int Orthod. 2014;12:458–466. doi: 10.1016/j.ortho.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Dodawad BPP, Pyati R. Evaluation of flow rate, pH, buffering capacity, calcium, total proteins and total antioxidant capacity levels of saliva in caries free and caries active children: an in vivo study. Int J Clin Pediatr Dent. 2010;25:425–428. doi: 10.1007/s12291-010-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassena C, Casati M, Sala G, et al. Evaluation of a modified Roche enzymatic ammonia method for Roche Cobas 6000 (c501 Module) automated platform: when 5 μL improves performance. Clin Lab. 2016;62:2423–2428. doi: 10.7754/Clin.Lab.2016.160336. [DOI] [PubMed] [Google Scholar]
- 10.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 11.Ohkawa H, et al. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 12.Sanpei S, Endo T, Shimooka S. Caries risk factors in children under treatment with sectional brackets. Angle Orthod. 2010;80:509–514. doi: 10.2319/072909-431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonetti Alessandri G, Incerti Parenti S, Garulli G, Gatto MR, Checchi L. Effect of fixed orthodontic appliances on salivary properties. Prog Orthod. 2013;14:13. doi: 10.1186/2196-1042-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peros K, Mestrovic S, Anic-Milosevic S, Slaj M. Salivary microbial and nonmicrobial parameters in children with fixed orthodontic appliances. Angle Orthod. 2011;81:901–906. doi: 10.2319/012111-44.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lara-Carrillo E, Montiel-Bastida N-M, Sánchez-Pérez L, Alanís-Tavira J. Effect of orthodontic treatment on saliva, plaque and the levels of Streptococcus mutans and Lactobacillus. Med Oral Patol Oral Cir Bucal. 2010;15:1–15. doi: 10.4317/medoral.15.e924. [DOI] [PubMed] [Google Scholar]
- 16.Chang H, Walsh LJ, Freer TJ. The effect of orthodontic treatment HS Chang on salivary flow, pH, buffer capacity, and levels of mutans streptococci and lacto bacilli. Aust Orthod J. 1999;15:229. [PubMed] [Google Scholar]
- 17.Teixeira HS, Kaulfuss SMO, Ribeiro JS, Pereira B do R, Brancher JA, Camargo ES. Calcium, amylase, glucose, total protein concentrations, flow rate, pH and buffering capacity of saliva in patients undergoing orthodontic treatment with fixed appliances. Dent Press J Orthod. 2012;17:157–161. [Google Scholar]
- 18.Monaci F, Bargagli E, Bravi F, Rottoli P. Concentrations of major elements and mercury in unstimulated human saliva. Biol Trace Elem Res. 2002;89:193–203. doi: 10.1385/BTER:89:3:193. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Hu B, Liu Y, Ding G, Zhang C, Wang S. The effects of fixed orthodontic appliances on saliva flow rate and saliva electrolyte concentrations. J Oral Rehabil. 2009;36:781–785. doi: 10.1111/j.1365-2842.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahn S-J, Kho H-S, Lee S-W, Nahm D-S. Roles of salivary proteins in the adherence of oral streptococci to various orthodontic brackets. J Dent Res. 2002;81:411–415. doi: 10.1177/154405910208100611. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg D, Eyal S. Initial biofilm formation of Streptococcus sobrinus on various orthodontics appliances. J Oral Rehabil. 2004;31:1041–1045. doi: 10.1111/j.1365-2842.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 22.Campos MJ da S, Raposo NRB, Ferreira AP, Vitral RWF. Salivary alpha-amylase activity: a possible indicator of pain-induced stress in orthodontic patients. Pain Med. 2011;12:1162–1166. doi: 10.1111/j.1526-4637.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 23.Adhitya S, Sudiono J, Kusnoto J, Roeslan BO. Effect of orthodontic tooth movement on salivary aspartate aminotransferase activity. J Dent Indones. 2013;20:15–19. [Google Scholar]
- 24.Husin E, Tjandrawinata R, Juliani M, Roeslan BO. Orthodontic force application in correlation with salivary lactate dehydrogenase activity. J Dent Indones. 2013;19:10–13. [Google Scholar]
- 25.Totan A, Greabu M, Totan C, Spinu T. Salivary aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase: possible markers in periodontal diseases? Clin Chem Lab Med. 2006;44:612–615. doi: 10.1515/CCLM.2006.096. [DOI] [PubMed] [Google Scholar]
- 26.Banu S, Jabir NR, Mohan R, et al. Correlation of toll-like receptor 4, interleukin-18, transaminases and uric acid in the patients with chronic periodontitis and healthy adults. J Periodontal. 2015;86:431–419. doi: 10.1902/jop.2014.140414. [DOI] [PubMed] [Google Scholar]
- 27.Pietraforte D, Castelli M, Metere A, et al. Salivary uric acid at the acidic pH of the stomach is the principal defense against nitrite-derived reactive species: sparing effects of chlorogenic acid and serum albumin. Free Radic Biol Med. 2006;41:1753–1763. doi: 10.1016/j.freeradbiomed.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Tothova L, Celecova V, Celec P. Salivary markers of oxidative stress and their relation to periodontal and dental status in children. Dis Markers. 2013;34:9–15. doi: 10.3233/DMA-2012-00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chenin DA, Trosien AH, Fong PF, Miller RA, Lee RS. Orthodontic treatment with a series of removable appliances. J Am Dent Assoc. 2003;134:1232–1239. doi: 10.14219/jada.archive.2003.0358. [DOI] [PubMed] [Google Scholar]
- 30.Atug Özcan SS, Ceylan I, Özcan E, Kurt N, Dagsuyu IM, Çanakçi CF. Evaluation of oxidative stress biomarkers in patients with fixed orthodontic appliances. Dis Markers. 2014;2014:1–7. doi: 10.1155/2014/597892. [DOI] [PMC free article] [PubMed] [Google Scholar]


