Abstract
The finding that meningeal solitary fibrous tumors (SFTs) and meningeal hemangiopericytomas (HPCs) are both characterized by NAB2‐STAT6 gene fusion has pushed their inclusion in the WHO 2016 Classification of tumors of the central nervous system (CNS) as different manifestations of the same entity. Given that the clinical behavior of the CNS SFT/HPC spectrum ranges from benign to malignant, it is presently unclear whether the grading criteria are still adequate. Here, we present the results of a study that analyzed the prognostic value of an updated version of the Marseille Grading System (MGS) in a retrospectively assembled cohort of 132 primary meningeal SFTs/HPCs with nuclear overexpression of STAT6. The median patient follow‐up was 64 months (range 4–274 months); 73 cases (55%) were MGS I, 50 cases (38%) MGS II and 9 cases (7%) were MGS III. Progression‐free survival (PFS) and disease‐specific survival (DSS) were investigated using univariate analysis: the prognostic factors for PFS included MGS, extent of surgery, radiotherapy, chemotherapy and mitotic activity ≥5/10 high‐power field (HPF). Moreover, MGS, radiotherapy, mitotic activity ≥5/10 HPF, and necrosis were the prognostic factors measured for DSS. In multivariate analysis, extent of surgery, mitotic activity ≥5/10 HPF, MGS I and MGS III were the independent prognostic factors measured for PFS while necrosis, MGS III and radiotherapy were the independent prognostic factors for DSS. In conclusion, our results show that assessing the malignancy risk of SFT/HPC should not rely on one single criterion like mitotic activity. Therefore, MGS is useful as it combines the value of different criteria. In particular, the combination of a high mitotic activity and necrosis (MGS III) indicates a particularly poor prognosis.
Keywords: central nervous system, grading, hemangiopericytoma, Marseille Grading System, MGS, prognostic factors, solitary fibrous tumor
Introduction
Given the discovery of the NAB2‐STAT6 gene fusion in both solitary fibrous tumors (SFTs) and hemangiopericytomas (HPCs) of the soft tissue and central nervous system (CNS), these entities are no longer regarded as separate neoplasms. They belong to the same spectrum 10. The excellent diagnostic performances of STAT6 immunohistochemistry favored its rapid and wide adoption by pathologists and STAT6 nuclear overexpression is considered mandatory for the diagnosis 9, 10, 12, 15, 20, 31, 34, 37, 39, 43, 49, 50, 51. SFTs/HPCs can manifest anywhere in the body including the meninges, and result in a spectrum of histological features and marked differences in clinical behavior. Assessing the behavior and prognosis of CNS SFT/HPC affects therapeutic management because indolent SFTs/HPCs are typically treated with surgery alone, while adjuvant radiotherapy is believed to be beneficial for patients harboring more aggressive tumors 21, 22, 24, 29, 45, 46.
A spectrum of clinical features renders the grading of SFTs/HPCs problematic, and there are currently three different histological approaches. The first is a grading system used by the World Health Organization (WHO) classification of tumors of the central nervous system (CNS) 29. The second includes histological criteria favoring malignancy by the WHO classification of Tumors of Soft Tissue and Bone 19, and the third is the Marseille Grading System (MGS) 7.
An update of the SFT/HPC WHO grading system has been proposed in the latest revision of the WHO classification of CNS tumors 29. This system is still based on the historical separation of meningeal SFTs and HPCs. It does not reflect the current molecular reunification of these entities, thus an important grading dichotomization is based on the histological phenotype. Therefore, WHO grade I SFTs/HPCs are characterized by the classic SFT phenotype and considered benign, while grade II and III SFTs/HPCs are characterized by the HPC phenotype and are considered malignant and are generally treated by surgery and adjuvant radiotherapy 21, 22, 29, 45. Despite many reports of local recurrences, malignant progression and metastasis for neoplasms harboring the classical SFT phenotype 5, 6, 18, 26, these tumors are still considered benign (i.e., WHO grade I). These cases question the benignity of classical SFT in the current WHO classification. Finally, the criteria used for grading have evolved: necrosis is no longer part of the WHO grading of CNS SFTs/HPCs while the phenotype and mitotic activity are used in the current version 42.
In soft tissues, aggressive behavior of neoplasms that display the classic fibrous phenotype is also well described and the whole spectrum is considered intermediate malignancy. Efforts have been made to define grading criteria suitable for the whole spectrum of SFTs/HPCs 13, 14, 19. For example, high mitotic activity [i.e., >4 mitoses per 10 high‐power field (HPF)] 13, 14, 42, cytonuclear atypia and tumor necrosis are histological indicators of poor outcome 7. Based on these criteria and on clinical parameters, different systems for risk assessment have been proposed 47, but the cohorts on which they are based excluded meningeal cases and are therefore not applicable for meningeal SFT/HPC.
For CNS SFTs/HPCs, we previously proposed a 4‐tiered MGS that is applicable to the whole spectrum 7. This grading system is based on the combination of different histological features: hypercellularity, high mitotic count (>5 mitoses/10 HPF) and necrosis (Table 3). In practice, MGS has limitations prompting for the refinement of specific grading criteria. Specifically, the significance of necrosis in MGS I tumors was not clearly defined, the threshold used for mitotic activity was different compared with the other grading systems, the microscopic surface for the mitotic count and the exact definition of hypercellularity were not clearly stated.
Table 3.
Definitions of WHO grading criteria and the MGS.
| WHO | MGS 2012 23 | Updated MGS |
|---|---|---|
| Grade I | MGS I | MGS I |
| "SFT phenotype” |
Mitotic activity ≤ 5 /10 HPF * No necrosis |
Mitotic activity <5 /10 HPF * (independent of necrosis) |
|
Alternation of hypo‐ and hypercellular areas Abundant collagen Mitotic activity < 5 /10 HPF * |
No hypercellularity | |
| Grade II | MGS IIa | MGS II |
| "HPC phenotype” |
Mitotic activity ≤ 5 /10 HPF * No necrosis |
Mitotic activity ≥5 /10 HPF * No necrosis |
|
Hypercellularity Mitotic activity < 5 /10 HPF * |
Hypercellularity | |
|
MGS IIb Mitotic activity > 5 /10 HPF * No necrosis |
||
| Grade III | MGS III | MGS III |
| Mitotic activity ≥5 /10 HPF * |
Mitotic activity > 5 /10 HPF * and Necrosis and Hypercellularity |
Mitotic activity ≥5 /10 HPF * and Necrosis |
*10 HPF (MGS): counting of 10 adjacent fields with total magnification of 400× (total surface 2.2 mm2) in the most proliferative areas as assessed in a H&E stained slide or guided by Ki67 immunohistochemical staining if available.
The 2016 WHO classification does not provide a definition for hypercellularity and “10 HPF.”
The goal of this study was to improve the grading of CNS SFTs/HPCs by re‐evaluating different histological criteria on an expanded international cohort of 132 primary meningeal SFTs/HPCs, and to analyze their respective prognostic value to ultimately refine our definitions of the MGS.
Materials and Methods
Sample selection and patient clinical information
Patients with a diagnosis of SFTs/HPCs of the CNS were selected from the (referral) files of the authors and from the archives of colleagues who participated in this study. Clinical patient information including age at first histological diagnosis, sex, treatment details and follow‐up data were extracted from the (electronic) patient records. The neurosurgeon defined the extent of surgery as complete or incomplete. For each case, a representative tumor tissue block of the primary resection specimen was selected and included for histological and immunohistochemical assessment. The SFT/HPC diagnosis was confirmed by STAT6 nuclear staining using an already published protocol 49. In short, STAT6 immunohistochemistry (IHC) was performed by applying the STAT6 monoclonal antibody (clone YE361; dilution 1:250; Abcam Cambridge, The United Kingdom) on 4 µm histological sections and an automated immunostainer (Ventana benchmark XT, Ventana Medical Systems Inc, Tucson, AZ) with an indirect biotin‐free system based on polymer (Ultraview universal DAB kit, Ventana medical Systems Inc.).
Follow‐up data included information about recurrence(s), regional or distant metastases, progression‐free survival (PFS) and disease‐specific survival (DSS). Recurrent and metastatic disease were detected by imaging techniques (in patients without any other known malignancies), and in a subset of cases corroborated by histological assessment. PFS was counted in months from the date of the first histological diagnosis to the date of diagnosis of the (first) local recurrence, detection of progressive disease for which treatment was necessary, or detection of metastases by imaging techniques. A distinction was made between patients who died because of disease‐specific factors and patients who died because of other causes. DSS was counted accordingly until the date of death because of disease causation. Patients who died directly because of (complications of) surgery were excluded from further survival analysis.
The French samples used in this study were stored and retrieved from the Assistance Publique Hopitaux de Marseille Biobank (APHM‐Biobank). The APHM‐Biobank (authorization number: AC2013‐1786‐BIOBANQUES BB‐0033‐00097) respects the ethical charter of the French National Cancer Institute to store and deliver samples for scientific research according to the French Public Health Code (articles L. 1243‐4 and R. 1243‐61). All samples were collected after obtaining informed consent from the patients. The study of the Dutch samples was approved by the local scientific review board of the Radboud University Medical Center and was performed in accordance with the Code of Conduct of the Federation of Medical Scientific Societies in the Netherlands. Because this research was not interventional, it did not require approval by an ethics committee. The French committee for the treatment of biomedical research information approved the data management of this study (C.C.T.I.R.S.−09.084Ter).
Histological features
Histological sections (4‐µm) were stained with hematoxylin and eosin, and all cases were independently reviewed and scored by two pathologists (CB, NM). The following histological features were also assessed based on our previous study 7:
Presence/absence of hypercellularity (defined as presence of at least a HPF of densely packed cells without intervening collagenous stroma between the cells)
Presence/absence of necrosis
Mitotic activity: mitoses were counted in 10 adjacent HPFs in the most proliferative areas as assessed in a H&E stained slide (10 HPF with total magnification of 400× corresponded to 2.2 mm2)
Absence of hypercellularity as defined above with abundant intervening collagenous stroma and a generally lower cellular density corresponds with the SFT phenotype. For discrepant cases, a consensus was reached, and with regard to mitotic count, dichotomization was achieved with ≥5 mitoses/2.2 mm2 as a cut‐off point.
Statistical analysis
Clinico‐pathological variables based on grading were compared using the chi‐square test (or Fisher's exact test when at least one subgroup was n < 5) for categorical variables, and the Mann–Whitney U‐test for continuous variables. All statistical tests were two‐sided, and the threshold for statistical significance was P = 0.05. Survival curves were calculated according to the Kaplan–Meier method and compared using the log‐rank test. Variables with a significant P‐value ≤ 0.10 were used to build the multivariate Cox proportional hazard models, and limitation of the number of variables was performed with respect to the number of events. The results are reported as two‐sided P‐values with 95% confidence intervals (95% CI). Analyses were conducted with SPSS statistics version 23.0.0.0 (IBM Corp.) and Prism 7.0a (Graphpad Software, Inc.).
Results
Clinical and follow‐up data (Supporting Information Table SA)
The primary tumors of 132 patients with a median age at diagnosis of 53 years (range 22–86 years) and an almost equal male–female ratio were analyzed. All patients were treated with surgical resection. According to the surgical reports, the intraoperative assessment of the extent of surgery by the neurosurgeon [gross total resection (GTR)] was achieved in 47 patients and was incomplete in 47 patients. It was not possible to retrieve data from 38 patients. Data revealed that 67 patients (51%) received adjuvant radiotherapy, but this information was not available for eight patients. Of the 60 patients for which information about chemotherapy was available, 10 (16%) received adjuvant chemotherapy when extracranial metastases were detected.
PFS and DSS data were available for 131 and 132 patients, respectively. The median follow‐up period was 64 months with a range of 4–274 months. Recurrent disease occurred in 52 patients (39.4%) after a median period of 36 months (38 patients recurred before 10 years of follow‐up, 11 patients between 10 and 20 years 3 patients after 20 years of follow‐up). About 16 of the 71 patients (22.5%) had extracranial metastatic disease (missing data regarding metastases for 61 patients). Eight patients had metastases in the lungs, liver and/or bones. One patient had metastases in the abdominal cavity, spleen and liver. However, specific data regarding exact site(s) of metastases were not available for seven patients.
Information about disease status at the end of follow‐up period was available for all patients: 16 patients died because of disease‐related factors (DOD) after a median period of 70 months (22–268 months), 10 patients died because of other or unknown causes (DOC), 98 patients presented with no evidence of disease (NED) during follow‐up and 8 patients were alive with disease (AWD). Of the AWD patients, one patient had only residual disease for which a “wait‐and‐see” policy was arranged, two patients had recurrent disease and five patients had metastatic disease at the end of follow‐up. Four patients died during surgery or because of post‐operative complications (within the first month of follow‐up) and were excluded from survival analysis.
Association of the clinico‐pathological features and grading system with survival (Tables 1 and 2)
Table 1.
Association of clinical and histological parameters with survival (median follow‐up 64 months, range 4–274 months).
| Variables | No. of patients | PFS (months) | No. of patients | DSS (months) | ||||
|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Log‐rank | Median | 95% CI | Log‐rank | |||
| Age | 0.511 | 0.422 | ||||||
| ≤ 53 years | 63 | 72 | 35.9–108.0 | 63 | 249 | 51.4–446.5 | ||
|
> 53 years Missing values |
68 1 |
85 | 77.5–92.4 |
69 – |
268 | / | ||
| Sex | 0.885 | 0.641 | ||||||
| Female | 63 | 83 | 76.9–89.9 | 64 | 268 | / | ||
|
Male Missing values |
67 2 |
101 | 18.8–183.1 |
67 1 |
NR | / | ||
| Surgical resection | <0.0001 | 0.182 | ||||||
| Incomplete | 47 | 36 | 23.8–48.1 | 47 | 268 | / | ||
|
Complete Missing values |
46 39 |
217 | 54.1–379.8 |
47 38 |
249 | / | ||
| Adjuvant treatment | 0.034 | 0.001 | ||||||
| RT (−) | 57 | 101 | 78.4–123.5 | 57 | 268 | / | ||
|
RT (+) Missing values |
66 9 |
48 | 20.0–75.9 |
67 8 |
170 | 52.5–287.4 | ||
| CT (−) | 49 | 102 | 61.2–142.7 | 0.045 | 50 | NR | / | 0.196 |
|
CT (+) Missing values |
10 73 |
48 | 33.5–62.4 |
10 72 |
NR | / | ||
| Mitoses | 0,0004 | 0.007 | ||||||
| <5/10 HPF | 73 | 128 | 61.7–194.2 | 73 | 268 | 239.4–296.5 | ||
|
≥5/10 HPF Missing values |
58 1 |
47 | 37.7–58.2 |
59 – |
170 | 56.1–283.8 | ||
| Necrosis | 0.739 | 0.001 | ||||||
| Absent | 117 | 85 | 70.7–99.2 | 118 | 268 | 239.4–296.5 | ||
|
Present Missing values |
14 1 |
145 | / | 14 | 116 | 72.9–159.0 | ||
| Hypercellularity | 0.090 | 0.101 | ||||||
| Absent | 36 | 128 | 76.4–179.5 | 36 | 249 | / | ||
|
Present Missing values |
95 1 |
72 | 41.3–102.6 | 96 | NR | / | ||
PFS, progression‐free survival; DSS, disease specific survival; RT, radiotherapy; CT, chemotherapy; HPF, high power field.
Table 2.
Association of the updated MGS with survival.
| Variables | No. of patients | PFS (months) | No. of patients | DSS (months) | ||||
|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Log‐rank | Median | 95% CI | Log‐rank | |||
| MGS I | 73 | 128 | 61.7–194.2 | 0.0018 | 73 | 268 | 239.4–296.5 | 0.0001 |
| MGS II | 49 | 47 | 36.8–57.1 | 50 | NR | / | ||
| MGS III | 9 | 85 | 64.5–105.4 | 9 | 116 | 65.474–166.5 | ||
PFS, progression‐free survival; DSS, disease specific survival.
In univariate analysis, the age at diagnosis and patient sex was not significantly correlated with prognosis. Incomplete surgical resection was significantly correlated with shorter PFS (P < 0.0001), but not with DSS. Adjuvant radiotherapy and chemotherapy were associated with shorter PFS (P = 0.034 and P = 0.045 respectively) and—for radiotherapy only—shorter DSS (P = 0.001).
Regarding histological features, high mitotic activity (≥5/10 HPF) was significantly correlated with shorter PFS (P = 0.0004) and DSS (P = 0.007). The presence of necrosis was also strongly correlated with shorter DSS (P < 0.0001), but not with PFS. Hypercellularity was not statistically correlated with prognosis (Table 1).
Refinement of clinico‐pathological features and grading
We refined the criteria used for MGS to address the issue that some MGS cases could be incorporated into more than one MGS category [e.g., cases with low mitotic count and presence of necrosis (n = 6)]. Mitotic activity was correlated with PFS and DSS, and therefore was the main discriminating factor between all MGS groups. Of note, other studies also revealed a relationship between mitotic activity and prognosis 13, 14, 16, 17, 25, 27, 42. Hypercellularity was not correlated with prognosis, therefore it has not been taken into account in the updated MGS. Necrosis remained an important discriminating factor defining MGS III neoplasms when combined with high mitotic activity.
MGS I tumors were defined by mitotic count <5/10 HPF with or without necrosis, MGS II tumors were defined by mitotic count ≥5/10 HPF without necrosis and MGS III tumors were defined by mitotic count ≥5/10 HPF with necrosis. These adaptations resulted in an updated MGS, as shown in Table 3. According to this updated system, there were 73 MGS I tumors (55%), 50 MGS II tumors (38%) and 9 MGS III tumors in our series (7%) (Supporting Information Table SA; Figure 1). Recurrent disease was noted in 21 of 73 patients with an MGS I tumor (28.7%; 5 patients with GTR), in 25 patients with a MGS II tumor (50%; 3 patients with GTR) and in 6 patients with a MGS III tumor (66%; 1 patient with GTR). DOD was noted for six patients with a MGS I tumor (8.2%), five patients with a MGS II tumor (10%) and five patients with a MGS III tumor (55%). No statistical differences were observed between the different MGS groups with respect to patient age, sex or extent of surgery (Supporting Information Table SB). Concerning adjuvant therapy, radiotherapy was given significantly more often for higher‐grade tumors (P < 0.0001).
Figure 1.
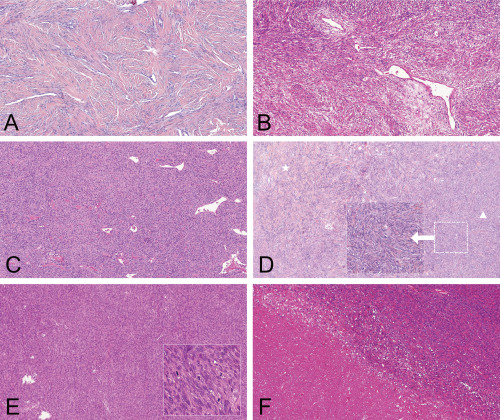
Histological features and grading of meningeal SFT/HPC. A. MGS grade I: low cellularity and plenty of intervening collagen (“classic fibrous phenotype”), mitotic activity < 5/HPF. (H&E, 100×). B. MGS grade I: variable cellularity (intervening collagen is still present between most of the cells: “classic fibrous phenotype”), mitotic activity < 5/HPF. (H&E, 100×). C. MGS grade I: hypercellularity (“HPC phenotype”), rare intervening collagen and mitotic activity < 5/HPF (H&E, 100×). D. MGS grade I: The cellularity level of this tumor is hard to define precisely: the left area still has collagen between tumor cells and corresponds to a classic “SFT phenotype” (star), whereas more densely packed cells are present in the right side of the microphotograph: possible “HPC phenotype” (arrowhead)—using the updated version of MGS, which is independent of hypercellularity, this tumor is classified MGS I as it displays a mitotic activity < 5/HPF (H&E, 100×. Inset: focus of possible hypercellularity 200×). E. MGS grade II: mitotic activity ≥ 5/HPF without necrosis (H&E, 100×). Inset: high mitotic activity; 400×). F. MGS grade III: necrosis and mitotic activity ≥ 5/HPF (H&E, 100×).
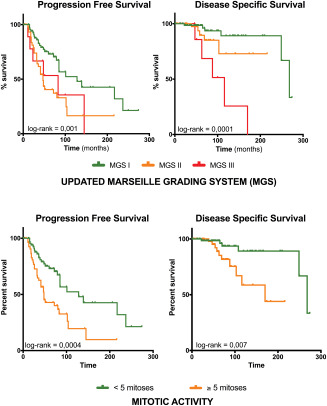
The updated MGS was correlated with PFS (P = 0.001) and DSS (P = 0.0001) in univariate analysis (Table 2; Figure 2).
Figure 2.
Progression free survival (PFS) and disease specific survival (DSS) curves for MGS and mitotic activity.
In multivariate analysis, the Cox model for PFS involved mitotic activity ≥5/10 HPF, each of the different MGS subgroups vs. incomplete surgical resection, hypercellularity and radiotherapy. Incomplete surgical resection (for MGS III tumors), mitotic activity ≥5/10 HPF and MGS III were all independent adverse prognostic factors for PFS. Conversely, MGS I was correlated with a better PFS. For DSS, the model involved mitotic activity ≥5/10 HPF and necrosis for each of the different MGS subgroups vs. radiotherapy: MGS III, radiotherapy and necrosis were independent adverse prognostic factors. Mitotic activity ≥5/10 HPF was not an independent prognostic factor for DSS, although a trend was noted (Supporting Information Table SC).
Discussion
Like the 2013 WHO Classification of Tumors of Soft Tissue and Bone 19, the revised WHO Classification of Tumors of the Central Nervous System regrouped SFT and HPC into a single entity: SFT/HPC. SFTs/HPCs encompass a spectrum consisting of two main phenotypes 29, but it is important to note that tumors with intermediate morphology or evolution from one phenotype to another are also encountered, often making discrimination between these phenotypes arbitrary 2. The NAB2‐STAT6 gene fusion and the subsequent nuclear relocation of STAT6, which are both present in the whole spectrum, proved to be highly sensitive and specific for the diagnosis. STAT6 immunohistochemistry has been rapidly and widely adopted by pathologists for an accurate diagnosis of these neoplasms 9, 10, 12, 15, 20, 31, 34, 37, 39, 43, 49, 50, 51.
Grading of meningeal SFTs/HPCs is important to predict prognosis and optimize the therapeutic management of individual patients. Of note, grading criteria as included in the WHO 2016 classification have not been specifically designed for meningeal SFTs/HPCs as one tumor entity, and are still based on the dichotomy of histological phenotypes. The MGS, which was specifically designed for and could be applied to the whole spectrum of SFTs/HPCs, was found to be of prognostic value for PFS and OS in a series of 89 meningeal SFTs/HPCs 7. Results were comparable in an independent cohort of 58 meningeal SFTs/HPCs by another research group 52. In this expanded cohort of 132 primary meningeal SFTs/HPCs, we re‐evaluated the prognostic value of several clinical and histological variables to refine our definition of the MGS. Moreover, we hoped to harmonize some of the criteria used with the WHO classifications, and analyze the prognostic value of this updated MGS for PFS and DSS. Table 3 shows an overview of the histological criteria used.
Application of the updated MGS revealed prognostic value for PFS and DSS in uni‐ and multivariate analysis. Multivariate analysis showed that a combination of necrosis and high mitotic activity (i.e., MGS III) was linked with the worst prognosis with a median DSS of 88 months. The separation of tumors into MGS II and III had important prognostic value, as the 5‐ and 10‐year survival rates for MGS III tumors were worse than for MGS II tumors: 5‐year DSS of 68% vs. 89% and 10‐year DSS of 25% vs. 72%, respectively.
The strength of the MGS is in the combination of different criteria. For example, when mitotic activity ≥5/10 HPF was used as a single criterion (thus without adding necrosis), patients with the poorest prognosis were merged with those with a better prognosis, resulting in an overall 10‐year DSS rate of 58%. High mitotic activity (i.e., ≥5/10 HPF) was associated with a worse prognosis in univariate analysis but not in multivariate analysis (although a trend was noted). This is consistent with other reports showing that mitotic activity carries a prognostic value 13, 14, 16, 17, 25, 27, 42.
The MGS and the WHO grading system are both based on mitotic activity. However, the cut‐offs used by each system are slightly different: >5 mitoses/10 HPF for the initial MGS 7 and ≥5 mitoses/10 HPF for the WHO classifications (CNS and the soft tissue) 19, 29. We statistically tested both thresholds and found that >5 and ≥5 mitoses/10 HPF carried a prognostic value for PFS and DSS in univariate analysis, and both tended to be as significant as an independent prognostic factor for PFS and DSS. The association with necrosis (defining MGS III tumors) was correlated significantly with poor prognosis in uni‐ and multivariate analysis for PFS and DSS, regardless of the mitotic cut‐off used. Therefore, we integrated this ≥5 HPF threshold in the updated MGS, to allow a better harmonization of the MGS criteria with others 19, 29, 42.
The distinction between the classical SFT and the HPC phenotype in the WHO classification is based in part on the evaluation of cellularity, a criterion which was also used in the previous version of the MGS. In the current study, hypercellularity displayed a trend toward worse PFS and, to a lesser degree, DSS, but these results were not statistically significant. For the classical SFT phenotype, some authors have suggested that hypercellularity does not carry a prognostic value, but might be an important criterion when combined with other histological findings such as nuclear atypia, necrosis or mitosis 6. However, conflicting results have been observed regarding the prognostic value of hypercellularity 14, 25. Hypercellularity is probably prone to high inter‐observer variability, as some cases may display alternating collagenous and more hypercellular areas that are prone to sampling bias. To address these limitations, hypercellularity is no longer a criterion used in the MGS. Therefore, former MGS I and IIa groups have been merged into MGS I, which is defined only by a low mitotic activity, regardless of the WHO phenotype or cellularity.
In our previous study, we did not find necrosis to be an independent predictor of prognosis 7. Nevertheless, necrosis proved to be a prognostic indicator in this expanded cohort, particularly when combined with high mitotic activity. This is consistent with the former 2007 classification of the CNS 30, which separated grade II and III HPCs based on the criteria defined by Mena et al, including necrosis and high mitotic activity as prognostic indicators 33. Also, a recent report regarding soft tissue SFT/HPC, has integrated necrosis into its grading scheme 14. We also analyzed the value of necrosis for non‐mitogenic tumors and found that 5 SFTs/HPCs displayed necrosis with a low mitotic activity, and all patients had a favorable outcome without recurrence or death (follow‐up range 34–85 months).
Hypercellularity was present in samples of three patients without evidence of recurrence (median PFS = 64 months) or death related to disease (median DSS = 64 months) during follow‐up. Conversely, among the six MGS I tumors that proved to be lethal during follow‐up, no cases displayed necrosis and two cases (44%) showed a fibrous and hypocellular phenotype.
Clinical variables such as age and sex had no effect on the prognosis in this study. In previous studies, older age has been reported to be associated with worse overall survival for CNS SFT/HPC 48 and for soft tissue SFT 42. Other clinical features like exact tumor location and tumor volume also reportedly provide prognostic information 6, 26, 32. Unfortunately, data regarding these features were only available for a limited subset of cases in our cohort and therefore could not be statistically analyzed. Studies regarding the predictive value of these parameters should take into account the clinical, radiological and pathological criteria to clarify this relationship further. Treatment of CNS SFT/HPC generally consists of surgical resection, with a better prognosis when tumors are completely excised 52. In our study, gross total resection was associated with better PFS without an effect on DSS, similar to recent findings 8. Nevertheless, despite apparent complete surgical resection, long‐term recurrences or metastatic spread have been reported for the whole spectrum of SFT/HPC of the CNS 6. In our cohort, 9 of the 47 patients (19%) with complete surgical resection of their SFT/HPC experienced recurrent disease. In the CNS, the quality of the surgical resection is assessed by the surgeon during the procedure, and therefore is prone to potential evaluation bias. Data regarding prognostic value of surgical margins in soft tissue are sometimes conflicting 12, 14, 25, 41.
It has been postulated that HPCs can benefit from adjuvant radiotherapy, but this is still a matter of debate 21, 22, 38, 40, 44, 45, 46, 52. Thereby, studies regarding its prognostic value do not adequately correlate different tumor grades (either WHO or MGS) with outcome. In our study, radiotherapy was an adverse prognostic factor for PFS and DSS. Selection bias toward higher‐grade tumors in the group of patients who received radiotherapy is the most likely explanation for this finding. Furthermore, the exact time period in which radiotherapy was applied in these patients could not be retrieved. Prospective studies with adequate grading of meningeal SFTs/HPCs are required to define the role of radiotherapy in the therapeutic regimen.
Long‐term follow‐up is advised for all patients with a meningeal SFT/HPC regardless of grade, because recurrence(s) and/or metastasis can occur several years after initial diagnosis, even following complete surgical resection 11, 26, 28. It is important to note that such adverse events can also happen for meningeal SFT/HPC with the classic fibrous phenotype 2, 35. In our cohort, recurrence was observed in eight cases (6%) harboring a classic fibrous phenotype with low mitotic activity (all of which were MGS I) and one of these cases recurred after complete surgical resection.
WHO grade I tumors of the CNS are traditionally considered benign. In CNS SFT/HPC, this view might not accurately reflect the particular behavior and the prognosis uncertainty of some of the “benign‐looking” classic fibrous end of the SFT/HPC spectrum. Such tumors may display recurrences and even metastasis during follow‐up 2, 35. In our cohort, six MGS I tumors (4.5%) recurred after a median follow‐up of 60 months (despite 2 cases with complete surgical resection). Notably, two of these cases harbored the classic fibrous phenotype (with abundant collagen, without hypercellularity and with a low mitotic activity) and would be classified as WHO grade I. All six patients died of their disease including two cases harboring metastasis. Other examples of tumors with the classical SFT phenotype exhibiting aggressive behavior during long‐term follow‐up have been documented 2. Recent data suggest late metastatic capacity even for apparently “benign” soft‐tissue SFTs 23.
Recently, risk assessment approaches have been proposed to address this prognosis uncertainty for soft tissue SFTs, with a risk stratification model by Demicco et al and a risk calculator by Salas et al 14, 42. Both systems integrate clinical and histological criteria, and share common age and mitotic activity factors. The SFT risk calculator 42 also includes tumor location (other than limb), and the SFT risk stratification model 14 is partly based on tumor necrosis and size. Neither system includes meningeal SFTs/HPCs, nor they have not been validated for CNS SFT/HPC. In the present cohort, age was not correlated with PFS or DSS in uni‐ and multivariate analysis. Therefore, we did not include this parameter in our system. Unfortunately, detailed information regarding tumor size was only available for a subset of cases and could not be analyzed in this report. Since Demicco et al included tumor size as a criterion in their risk stratification model 13, 14, it might be good to investigate the prognostic value of tumor size in CNS SFT/HPC in future, multi‐center studies in order to aim to an unified grading system for these neoplasms.
In summary, this study showed that the combination of histological variables, mitotic activity ≥5/10 HPF and necrosis is valuable in grading meningeal SFTs/HPCs. In particular, the updated MGS allowed the distinction of a subgroup of patients with a poor outcome (i.e., patients with MGS III tumors). Furthermore, we conclude that prediction of behavior should not be assessed solely on mitotic count, but by integration of different histological criteria. Meanwhile, patients with CNS SFT/HPC at a histologically favorable end of the spectrum might still develop recurrence and/or metastasis, as reported by us and others. Therefore, long‐term follow‐up is advised for the entire spectrum of SFT/HPC.
Further advances in grading SFT/HPC might be possible by integrating molecular information into specific grading subgroups. For example, different NAB2‐STAT6 gene fusion variants in soft tissue SFTs are reportedly correlated to different clinical outcomes, and TERT promoter mutations have also been associated with adverse prognosis 1, 3, 4, 36, 47. The prognostic relevance of these molecular aberrations in CNS SFTs/HPCs needs to be further investigated in a larger number of tumors 20.
Supporting information
Table SA. Clinico‐pathological characteristics of the 132 patients with meningeal SFT/HPC included in this study.
Table SB. Association of the updated MGS grade with clinical features.
Table SC. Correlation of different clinico‐pathological variables and outcome—results of multivariate analysis.
Acknowledgments
This study was performed with the aid of a supporting research grant from Stichting STOP Hersentumoren, the Netherlands.
6 French CNS SFT/HPC Consortium—Corinne Bouvier: Department of Pathology and Neuropathology, Timone Hospital, Marseille, France, INSERM, UMR 911, Marseille, France, and Aix‐Marseille Université, Faculté de Médecine, Marseille, France; Philippe Cornu: Department of Neurosurgery, Pitié‐Salpétrière Hospital, Paris, France; Henry Dufour: Department of Neurosurgery, Timone Hospital, Marseille, France; Dominique Figarella‐Branger: Department of Pathology and Neuropathology, Timone Hospital, Marseille, France, INSERM, UMR 911, Marseille, France, and Aix‐Marseille Université, Faculté de Médecine, Marseille, France; Jacques Guyotat: Department of Neurosurgery, CHU Lyon, Lyon, France; Anne Jouvet: Department of Neuropathology, CHU Lyon, Lyon, France; Nicolas Macagno: Department of Pathology and Neuropathology, Timone Hospital, Marseille, France, INSERM, UMR 911, Marseille, France, and Aix‐Marseille Université, Faculté de Médecine, Marseille, France; Philippe Métellus: Department of Neurosurgery, Pitié‐Salpétrière Hospital, Paris, France, INSERM, UMR 911, Marseille, France, and Aix‐Marseille Université, Faculté de Médecine, Marseille, France; Karima Mokhtari: Department of Neuropathology, Pitié‐Salpétrière Hospital, Paris, France; Alexandre Vasiljevic: Department of Neurosurgery, CHU Lyon, Lyon, France; Pascale Varlet: Department of Neuropathology, Sainte‐Anne Hospital, Paris, France.
7 Dutch CNS SFT/HPC Consortium—R Vogels: Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands, Department of Pathology, Stichting PAMM, Eindhoven, The Netherlands; U Flucke: Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands, Department of Pathology, Princess Máxima Center for Pediatric Oncology, Utrecht, and University Medical Center Utrecht, The Netherlands; B Küsters: Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands, Department of Pathology, Maastricht University Medical Center+, Maastricht, The Netherlands; P Groenen: Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands; P Wesseling: Department of Pathology, Princess Máxima Center for Pediatric Oncology, Utrecht, and University Medical Center Utrecht, The Netherlands, Department of Pathology, VU University Medical Center, Amsterdam, The Netherlands; E Bekers: Pathologie‐DNA, Location Jeroen Bosch Hospital, Den Bosch, The Netherlands; M Verdijk: Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands; M Djafarihamedani: Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands; E Kurt: Department of Neurosurgery, Radboud University Medical Center, Nijmegen, The Netherlands, Department of Neurosurgery, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands; H Küsters‐Vandevelde: Department of Pathology, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands; R Fleischeuer: Department of Pathology, St. Elisabeth Hospital, Tilburg, The Netherlands; S Leenstra; Department of Neurosurgery, St. Elisabeth Hospital, Tilburg, The Netherlands, Department of Neurosurgery, Erasmus Medical Center, Rotterdam, The Netherlands; P Robe: Department of Neurosurgery, University Medical Center Utrecht, The Netherlands; W Spliet: Department of Pathology, University Medical Center Utrecht, The Netherlands; D Troost: Department of Pathology, Amsterdam Medical Center, Amsterdam, The Netherlands; W van Furth: Department of Neurosurgery, Amsterdam Medical Center, Amsterdam, The Netherlands.
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/TpQRwd
References
- 1. Akaike K, Kurisaki‐Arakawa A, Hara K, Suehara Y, Takagi T, Mitani K et al (2015) Distinct clinicopathological features of NAB2‐STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Hum Pathol 46:347–356. [DOI] [PubMed] [Google Scholar]
- 2. Apra C, Mokhtari K, Cornu P, Peyre M, Kalamarides M (2017) Intracranial solitary fibrous tumors/hemangiopericytomas: first report of malignant progression. J Neurosurg 23:1–6. [DOI] [PubMed] [Google Scholar]
- 3. Bahrami A, Lee S, Schaefer I‐M, Boland JM, Patton KT, Pounds S, Fletcher CD (2016) TERT promoter mutations and prognosis in solitary fibrous tumor. Mod Pathol 29:1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barthelmeß S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H et al (2014) Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2‐STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol 184:1209–1218. [DOI] [PubMed] [Google Scholar]
- 5. Bisceglia M, Dimitri L, Giannatempo G, Carotenuto V, Bianco M, Monte V et al (2011) Solitary fibrous tumor of the central nervous system: report of an additional 5 cases with comprehensive literature review. Int J Surg Pathol 19:476–486. [DOI] [PubMed] [Google Scholar]
- 6. Bisceglia M, Galliani C, Giannatempo G, Lauriola W, Bianco M, D'Angelo V et al (2011) Solitary fibrous tumor of the central nervous system: a 15‐year literature survey of 220 cases (August 1996‐July 2011). Adv Anat Pathol 18:356–392. [DOI] [PubMed] [Google Scholar]
- 7. Bouvier C, Métellus P, de Paula AM, Vasiljevic A, Jouvet A, Guyotat J et al (2012) Solitary fibrous tumors and hemangiopericytomas of the meninges: overlapping pathological features and common prognostic factors suggest the same spectrum of tumors. Brain Pathol 22:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Champeaux C, Khan AA, Wilson E, Thorne L, Dunn L (2017) Meningeal haemangiopericytoma and solitary fibrous tumour: a retrospective bi centre study for outcome and prognostic factor assessment. J Neurooncol 125:651. [DOI] [PubMed] [Google Scholar]
- 9. Cheah AL, Billings SD, Goldblum JR, Carver P, Tanas MZ, Rubin BP (2014) STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology 46:389–395. [DOI] [PubMed] [Google Scholar]
- 10. Chmielecki J, Crago AM, Rosenberg M, O'Connor R, Walker SR, Ambrogio L et al (2013) Whole‐exome sequencing identifies a recurrent NAB2‐STAT6 fusion in solitary fibrous tumors. Nat Genet 45:131–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi J, Park S‐H, Khang SK, Suh Y‐L, Kim SP, Lee YS et al (2016) Hemangiopericytomas in the central nervous system: a multicenter study of korean cases with validation of the usage of STAT6 immunohistochemistry for diagnosis of disease. Ann Surg Oncol 23:954–961. [DOI] [PubMed] [Google Scholar]
- 12. Demicco EG, Harms PW, Patel RM, Smith SC, Ingram D, Torres K et al (2015) Extensive survey of STAT6 expression in a large series of mesenchymal tumors. Am J Clin Pathol 143:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE et al (2012) Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol 25:1298–1306. [DOI] [PubMed] [Google Scholar]
- 14. Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang W‐L (2017) Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol 3:461. [DOI] [PubMed] [Google Scholar]
- 15. Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL (2014) Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 27:390–395. [DOI] [PubMed] [Google Scholar]
- 16. England DM, Hochholzer L, McCarthy MJ (1989) Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 13:640–658. [DOI] [PubMed] [Google Scholar]
- 17. Enzinger FM, Smith BH (1976) Hemangiopericytoma. An analysis of 106 cases. Hum Pathol 7:61–82. [DOI] [PubMed] [Google Scholar]
- 18. Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR (2011) The central nervous system solitary fibrous tumor: a review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg 113:703–710. [DOI] [PubMed] [Google Scholar]
- 19. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (2013) World Health Organization Classification of Tumours of Soft Tissue and Bone, 4th edn. International Agency for Research on Cancer (IARC): Lyon, France. [Google Scholar]
- 20. Fritchie KJ, Jin L, Rubin BP, Burger PC, Jenkins SM, Barthelmeß S et al (2016) NAB2‐STAT6 gene fusion in meningeal hemangiopericytoma and solitary fibrous tumor. J Neuropathol Exp Neurol 75:263–271. [DOI] [PubMed] [Google Scholar]
- 21. Ghia AJ, Allen PK, Mahajan A, Penas‐Prado M, McCutcheon IE, Brown PD (2013) Intracranial hemangiopericytoma and the role of radiation therapy: a population based analysis. Neurosurgery 72:203–209. [DOI] [PubMed] [Google Scholar]
- 22. Ghia AJ, Chang EL, Allen PK, Mahajan A, Penas‐Prado M, McCutcheon IE, Brown PD (2013) Intracranial hemangiopericytoma: patterns of failure and the role of radiation therapy. Neurosurgery 73:624–630. [DOI] [PubMed] [Google Scholar]
- 23. Gholami S, Cassidy MR, Kirane A, Kuk D, Zanchelli B, Antonescu CR et al (2017) Size and location are the most important risk factors for malignant behavior in resected solitary fibrous tumors. Ann Surg Oncol 24:3865–3871. [DOI] [PubMed] [Google Scholar]
- 24. Ghose A, Guha G, Kundu R, Tew J, Chaudhary R (2017) CNS hemangiopericytoma: a systematic review of 523 patients. Am J Clin Oncol 40:223–227. [DOI] [PubMed] [Google Scholar]
- 25. Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ et al (2002) Clinicopathologic correlates of solitary fibrous tumors. Cancer 94:1057–1068. [PubMed] [Google Scholar]
- 26. Han N, Kim H, Min SK, Paek S‐H, Park C‐K, Choi S‐H et al (2016) Meningeal solitary fibrous tumors with delayed extracranial metastasis. J Pathol Transl Med 50:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Insabato L, Siano M, Somma A, Gentile R, Santangelo M, Pettinato G (2009) Extrapleural solitary fibrous tumor: a clinicopathologic study of 19 cases. Int J Surg Pathol 17:250–254. [DOI] [PubMed] [Google Scholar]
- 28. Kumar N, Kumar R, Kapoor R, Ghoshal S, Kumar P, Salunke PS et al (2012) Intracranial meningeal hemangiopericytoma: 10 years experience of a tertiary care Institute. Acta Neurochir (Wien) 154:1647–1651. [DOI] [PubMed] [Google Scholar]
- 29. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella‐Branger D et al. (2016) WHO Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer (IARC): Lyon, France. [Google Scholar]
- 30. Louis DN, Wiestler OD, Burger PC (2007) WHO Classification of Tumours of the Central Nervous System, 4th edn. International Agency for Research on Cancer (IARC): Lyon, France. [Google Scholar]
- 31. Macagno N, Figarella‐Branger D, Mokthari K, Métellus P, Jouvet A, Vasiljevic A et al (2016) Differential diagnosis of meningeal SFT‐HPC and meningioma: which immunohistochemical markers should be used?. Am J Surg Pathol 40:270–278. [DOI] [PubMed] [Google Scholar]
- 32. Melone AG, D'Elia A, Santoro F, Salvati M, Delfini R, Cantore G, Santoro A (2014) Intracranial hemangiopericytoma–our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg 81:556–562. [DOI] [PubMed] [Google Scholar]
- 33. Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE (1991) Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol 22:84–91. [DOI] [PubMed] [Google Scholar]
- 34. Mohajeri A, Tayebwa J, Collin A, Nilsson J, Magnusson L, Steyern von FV et al (2013) Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer 52:873–886. [DOI] [PubMed] [Google Scholar]
- 35. Muñoz E, Prat A, Adamo B, Peralta S, Ramón y Cajal S, Valverde C (2008) A rare case of malignant solitary fibrous tumor of the spinal cord. Spine 33:E397–E399. [DOI] [PubMed] [Google Scholar]
- 36. Nakada S, Minato H, Nojima T (2016) Clinicopathological differences between variants of the NAB2‐STAT6 fusion gene in solitary fibrous tumors of the meninges and extra‐central nervous system. Brain Tumor Pathol 33:169–174. [DOI] [PubMed] [Google Scholar]
- 37. Ouladan S, Trautmann M, Orouji E, Hartmann W, Huss S, Büttner R, Wardelmann E (2015) Differential diagnosis of solitary fibrous tumors: a study of 454 soft tissue tumors indicating the diagnostic value of nuclear STAT6 relocation and ALDH1 expression combined with in situ proximity ligation assay. Int J Oncol 46:2595–2605. [DOI] [PubMed] [Google Scholar]
- 38. Ramakrishna R, Rostomily R, Sekhar L, Rockhill J, Ferreira M (2014) Hemangiopericytoma: radical resection remains the cornerstone of therapy. J Clin Neurosci 21:612–615. [DOI] [PubMed] [Google Scholar]
- 39. Robinson DR, Wu Y‐M, Kalyana‐Sundaram S, Cao X, Lonigro RJ, Sung Y‐S et al (2013) Identification of recurrent NAB2‐STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 45:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T et al (2012) Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118:1628–1636. [DOI] [PubMed] [Google Scholar]
- 41. Salas S, Jiguet‐Jiglaire C, Campion L, Bartoli C, Frassineti F, Deville J‐L et al (2014) Correlation between ERK1 and STAT3 expression and chemoresistance in patients with conventional osteosarcoma. BMC Cancer 14:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salas S, Resseguier N, Blay J‐Y, Le Cesne A, Italiano A, Chevreau C et al (2017) Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Ann Oncol 28:1979–1987. [DOI] [PubMed] [Google Scholar]
- 43. Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE et al (2013) Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2‐STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol 125:651–658. [DOI] [PubMed] [Google Scholar]
- 44. Sonabend AM, Zacharia BE, Goldstein H, Bruce SS, Hershman D, Neugut AI, Bruce JN (2014) The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a Surveillance, Epidemiology, and End Results analysis. J Neurosurg 120:300–308. [DOI] [PubMed] [Google Scholar]
- 45. Spina A, Boari N, Gagliardi F, Donofrio CA, Franzin A, Mortini P (2016) The current role of Gamma Knife radiosurgery in the management of intracranial haemangiopericytoma. Acta Neurochir (Wien) 158:635–642. [DOI] [PubMed] [Google Scholar]
- 46. Stessin AM, Sison C, Nieto J, Raifu M, Li B (2013) The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma‐experience from the SEER database. Int J Radiat Oncol Biol Phys 85:784–790. [DOI] [PubMed] [Google Scholar]
- 47. Tai H‐C, Chuang I‐C, Chen T‐C, Li C‐F, Huang S‐C, Kao Y‐C et al (2016) NAB2‐STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol 5:159–1335. [DOI] [PubMed] [Google Scholar]
- 48. Trifiletti DM, Mehta GU, Grover S, Sheehan JP (2017) Clinical management and survival of patients with central nervous system hemangiopericytoma in the National Cancer Database. J Clin Neurosci 44:169–174. [DOI] [PubMed] [Google Scholar]
- 49. Vogels R, Vlenterie M, Versleijen‐Jonkers Y, Ruijter E, Bekers EM, Verdijk M et al (2014) Solitary fibrous tumor ¿ clinicopathologic, immunohistochemical and molecular analysis of 28 cases. Diagn Pathol 9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A et al (2014) STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 38:552–559. [DOI] [PubMed] [Google Scholar]
- 51. Yuzawa S, Nishihara H, Wang L, Tsuda M, Kimura T, Tanino M, Tanaka S (2016) Analysis of NAB2‐STAT6 gene fusion in 17 cases of meningeal solitary fibrous tumor/hemangiopericytoma: review of the literature. Am J Surg Pathol 40:1031–1040. [DOI] [PubMed] [Google Scholar]
- 52. Zeng L, Wang Y, Wang Y, Han L, Niu H, Zhang M et al (2017) Analyses of prognosis‐related factors of intracranial solitary fibrous tumors and hemangiopericytomas help understand the relationship between the two sorts of tumors. J Neurooncol 131:153–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SA. Clinico‐pathological characteristics of the 132 patients with meningeal SFT/HPC included in this study.
Table SB. Association of the updated MGS grade with clinical features.
Table SC. Correlation of different clinico‐pathological variables and outcome—results of multivariate analysis.


