Depositions of amyloid‐beta (Aβ) peptides in form of extracellular plaques and cerebral amyloid angiopathy (CAA) are common features of human Alzheimer disease (AD) brains 12. However, Aβ deposits can also occur in individuals without clinical symptoms of cognitive impairment, defining a pathological preclinical phase of AD. Interestingly, the clinical manifestation of AD symptoms correlates with the occurrence of modified Aβ variants phosphorylated at serine residue 8 (pSer8Aβ) 11. Phosphorylated Aβ species have an increased propensity to aggregate 6, show higher resistance against proteolytic degradation 7 and exert increased neurotoxicity 9. Deposits with phosphorylated Aβ variants have been observed in brains of transgenic mouse, human sporadic and familial AD cases 1, 8, 11.
Age‐associated sporadic cerebral amyloidosis has also been shown in mammalian species with the identical amino acid sequence within the Aβ domain as humans 2, 3, 5. Primates and canines not only develop Aβ pathology resembling that of humans, but also show cognitive decline during the natural ageing process. Aβ accumulates in different regions of the brain in a manner that parallels the distribution pattern in the human AD brain 2, 3, 5, 13. Nonhuman primates also represent a valuable model to study AD pathogenesis owing to their close evolutionary relationship to humans. The age‐associated neurodegeneration reported in nonhuman primates is associated with brain atrophy, abundant amyloid plaques and a loss of cholinergic neurons similar to human AD 2, 13. However, nonhuman primates and canines show little if any neurofibrillary tangle pathology, suggesting that these species develop neuropathological features resembling early phases of AD pathogenesis 13. Here, we characterized the deposition of pSer8Aβ and nonphosphorylated Aβ in the brains of Caribbean vervets and canines. Both species revealed abundant deposition of pSer8Aβ in the 5.
We used phosphorylation‐state specific monoclonal antibodies to characterize the deposition of phosphorylated (pSer8Aβ) and nonphosphorylated (npAβ) variants of Aβ in the brains of 15 Caribbean vervets ranging from 7.4 to 32 years of age (Table 1). Archived fixed brain tissues or sections of the Caribbean vervets were obtained from the Behavioral Science Foundation (Basseterre, St. Kitts). Immunohistochemistry was performed as described previously 4, using three different primary antibodies. The mouse monoclonal antibody (mAb) 82E1 (dilution 1:500; Immuno‐Biological Laboratories, Japan) recognizes Aβ and APP C‐terminal fragments starting as Asp 1. mAb 1E4E11 (dilution 1:500) is reactive to Aβ peptides phosphorylated at Ser8 8 and rat mAb 7H3D6 (dilution 1:500) specifically recognizes Aβ peptide not phosphorylated Ser8 position 8. The specificity of the phosphorylation‐state specific antibodies 1E4E11 and 7H3D6 was demonstrated previously by preadsorption with synthetic Aβ peptides with Ser8 in phosphorylated or nonphosphorylated state by western immunoblotting and immunohistochemistry, and by stainings with secondary antibodies alone 8.
Table 1.
Examination summary for Caribbean vervets and Beagle canines.
| Animal | Age (years) | Gender | Brain region examined | 1E4E11 (pSer8Aβ) | 7H3D6 (npAβ) | 82E1 (Aβ1‐x) |
|---|---|---|---|---|---|---|
| Caribbean vervets | ||||||
| Vervet 1 | 7.4 | Female | F | – | – | – |
| T/HC | – | – | – | |||
| Vervet 2 | 11.1 | Female | F | ++ | + | ++ |
| T/HC | – | – | – | |||
| Vervet 3 | 12.2 | Female | F | – | – | – |
| T/HC | – | – | – | |||
| Vervet 4 | 12.4 | Male | F | + | + | + |
| T/HC | – | – | – | |||
| Vervet 5 | 12.8 | Male | F | – | – | + |
| T/HC | – | – | – | |||
| Vervet 6 | 12.9 | Male | F | + | + | + |
| T/HC | + | + | ++ | |||
| Vervet 7 | >15 | Female | F | + | + | +++ |
| T/HC | – | – | + | |||
| Vervet 8 | >15 | Male | F | ++ | – | +++ |
| T/HC | + | + | ++ | |||
| Vervet 9 | 16.4 | Female | F | ++ | – | +++ |
| T/HC | ++ | + | +++ | |||
| Vervet 10 | 16.9 | Female | F | + | + | ++ |
| T/HC | + | + | + | |||
| Vervet 11 * | 19 | Female | F | – | – | – |
| T/HC | – | – | – | |||
| Vervet 12 | 24.5 | Female | F | ++ | + | +++ |
| T/HC | ++ | + | ++ | |||
| Vervet 13 | 27.7 | Female | F | +++ | ++ | +++ |
| T/HC | – | + | ++ | |||
| Vervet 14 | 30.0 | Male | F | + | + | +++ |
| T/HC | + | + | ++ | |||
| Vervet 15 | 32.0 | Female | F | + | + | + |
| T/HC | ++ | + | ++ | |||
| Beagle canines | ||||||
| Canine‐1 | 10 | NA | PF | + | + | + |
| Canine‐2 | 12 | NA | PF | ++ | + | ++ |
| Canine‐3 | 14 | NA | PF | +++ | ++ | + |
Semi‐quantitative scoring of general Asp‐1 (82E1)‐ and phosphorylation‐state specific (7H3D6 and 1E4E11)‐ antibody reactivity against immunoreactive Asp1 Aβ plaques (82E1) and non‐phospho and pSer8Aβ‐positive plaques in the brains of 15 Caribbean vervets and 3 canines. Degree of plaques and blood vessel immunoreactivity: ‐, no positive plaques and blood vessels; + low number of positive plaques and blood vessels; ++, moderate number of positive plaques and blood vessels; +++ numerous positive plaques and blood vessels. NA; not available.
*General Aβ plaque immunoreactivity was observed in pre‐frontal cortex in another study, suggesting this animal was in very early stages of plaque deposition.
Of six animals younger than 15 years only three showed Aβ deposits. Aβ plaques in these animals were predominantly found in the frontal cortex and contained both phosphorylated and nonphosphorylated Aβ variants (Table 1, Figure 1). Eight out of nine animals older than 15 years (15–32 years) had abundant deposits of Aβ also containing pSer8Aβ and npAβ peptides. In most of the older animals extracellular plaques were found in the frontal cortex as well as in the temporal cortex and the hippocampal region. Semi‐quantitative analysis revealed an age‐dependent increase in Aβ deposition (Table 1, Figure 1). In addition to diffuse and dense‐core plaques, immunohistochemical analysis also revealed the presence of pSer8Aβ in meningeal and parenchymal blood vessels (Figure 1A,D). Overall, there was a strong overlap in the immunoreactivity for pSer8Aβ and Aβ variants starting with Asp1 of the Aβ sequence (Asp1 Aβ) (Figure 1F). Immunostaining with antibody 7H3D6 detected fewer plaques in both young and older animals (Figure 1B,E; Table 1). This antibody does neither detect Aβ variants that are phosphorylated at Ser8 nor other N‐terminally modified species, including N‐terminally truncated (Aβ3‐42), pyroglutamate Aβ (pyroGluAβ3‐42) and nitrated Aβ (3NTyr10‐ Aβ) 8. Together, these data suggest that Aβ deposits might contain substantial amounts of N‐terminally modified species.
Figure 1.
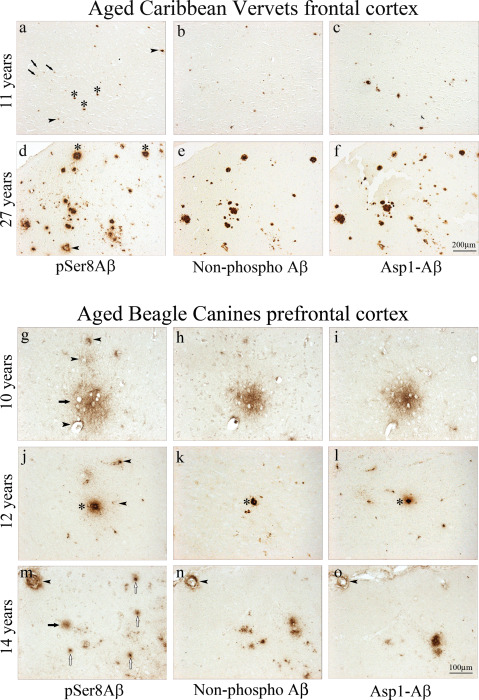
Immunohistochemical analysis of aged Caribbean vervet and canine brains. Immunohistochemistry of formalin‐fixed, paraffin‐embedded frontal cortex sections comparing pSer8Aβ (A,D), non‐phospho Aβ (B,E) and Asp1 Aβ immunoreactivity (C,F) on adjacent serial sections. Both pSer8Aβ (A) and Asp1 Aβ (C) deposition begins in the parenchymal vasculature, as shown in a 11‐year‐old monkey. With aging, cerebral pSer8Aβ immunoreactivity is found in diffuse deposits and also in compacted plaques and CAA (A,D); however, there, pSer8Aβ is only detected in a subset of Asp1 Aβ‐positive plaques (D,F). pSer8Aβ is detected in diffuse plaques (arrows), blood vessels (arrowheads), dense‐cored plaques (asterisks). Immunohistochemistry of paraformaldehyde‐fixed and paraffin‐embedded prefrontal cortex from aged beagle canines with a pSer8Aβ (G,J,M), non‐phospho Aβ (H,K,N) and Asp1 Aβ immunoreactivity (I,L,O) on adjacent serial sections reveals diffuse pSer8Aβ deposits at 10 years of age (g, arrows) that are also positive for non‐phospho Aβ (H) and Asp1‐Aβ IR (I). Staining in a 12‐year‐old canine shows pSer8Aβ‐positive blood vessels (J, arrowheads) that are concomitantly immunolabeled by npAβ and Asp1 Aβ mAb on adjacent serial sections (K,L). Compacted plaques (asterisk) were immunolabeled by all 3 antibodies. In the oldest canine examined in this study (14 years), focal pSer8Aβ‐positive deposits are observed (M); however, only subsets are non‐phospho Aβ and Asp1 Aβ positive (N,O). Superficial vessels are indicated by arrowheads and parenchymal vessels by open arrows.
To also test the deposition of pSer8Aβ in another species with identical amino acid sequence of the Aβ domain like humans and vervets 3, 4, 5, we analyzed brains of beagles of ages between 10 and 14 years with Aβ deposits (brains were obtained from the University of California, Irvine, USA). In all three brains, abundant deposition of pSer8Aβ was detected in extracellular plaques (Table 1 and Figure 1G–O). Here, the 10‐year‐old animal showed predominantly diffuse pSer8Aβ‐positive deposition (Figure 1G). In the two older animals, pSer8Aβ was also detected in compact plaques (Figure 1J,M). Nonphosphorylated Aβ was also detected in these structures (Figure 1H,K,N), indicating co‐deposition of phosphorylated and nonphosphorylated Aβ variants. These deposits were also decorated by antibody 82E1 (Figure 1I,L,O) that selectively recognize the free N‐terminus at Asp1 of Aβ. All 3 canines also displayed abundant CAA (Figure 1G–O). The strongest vascular Aβ deposition was observed in the brain of the oldest animal (Figure 1M). There was a large overlap in the deposition of phosphorylated and nonphosphorylated Aβ in vessels. The immunoreactivity for npAβ and pSer8Aβ overlapped with the 82E1 antibody reactivity, suggesting that CAA contains Aβ species starting with Asp1 in phosphorylated and nonphosphorylated state.
Accumulation of Aβ aggregates in the form of extracellular plaques is a neuropathological hallmark of AD. However, neuropathological assessment and PET imaging revealed that extracellular plaques are also found in cognitively normal individuals. The presence of extracellular Aβ deposits in the absence of cognitive symptoms could represent an early phase of AD pathogenesis, also referred to as pathologically preclinical AD. Interestingly, the presence of pSer8Aβ in extracellular plaques in human brain is strongly associated with the manifestation of clinical symptoms of AD, while unmodified or pyroglutamate‐modified Aβ is also abundant in extracellular plaques of nondemented, pathologically preclinical AD cases, suggesting that pSer8Aβ is critically involved in or reflects the manifestation of clinical symptoms in the pathogenesis of AD 11. These findings also indicate the importance of analyzing the molecular complexity of Aβ aggregates to understand the role of individual Aβ species and the differential composition of Aβ deposits in AD.
In this study, we demonstrate the deposition of pSer8Aβ in brains of both vervets and canines. pSer8Aβ was found in different types of extracellular plaques and in blood vessels, thereby closely resembling the neuropathological features of this Aβ species in the human brain 1, 6, 11. We previously showed the occurrence of post‐translationally modified pyroGlu‐3Aβ in these two animal species 4. Thus, both types of post‐translationally modified Aβ species well characterized in human brains are also found in extracellular deposits in brains of canines and nonhuman primates. The sample size of this study and limited information about the cognitive state of these animals precluded an analysis of the quantitative accumulation during aging, and the potential association of phosphorylated Aβ in the different neuropathological lesions with cognitive performance.
The accumulation of Aβ in walls of blood vessels is observed in almost all patients with AD, but can also be also detected nondemented people, and patients with familial CAA 10. In this study, we found that pSer8Aβ accumulated in the walls of cerebral blood vessels including leptomeningeal arteries, cortical arteries, capillaries and smaller arterioles in canine brains. Aβ in the wall of a cerebral blood vessel could affect brain circulation, the blood‐brain barrier and cause microhaemorrhages 10. Previous reports indicate that the age‐related neuropathological lesions and cognitive impairment in aged canine and vervets is comparable to that of humans, suggesting that these species could represent natural animal models of sporadic cerebral amyloidosis to study the mechanisms involved in the development and progression of AD 2, 3, 5, 13. Post‐translationally modified Aβ variants are abundant in the human brain and likely contribute to the complex pathways of Aβ aggregation, deposition and neurotoxicity. Thus, future investigations on the spatiotemporal accumulation and deposition of phosphorylated and other post‐translationally modified Aβ species in animal species naturally developing Aβ pathology in relation to the cognitive performance could provide interesting insights into the complexity and functional implication of different Aβ species in human AD pathogenesis.
Acknowledgments
We thank Kevin X. Le, Qiaoqiao Shi and Sandra Theil for technical assistance. This work was funded by Deutsche Forschungsgemeinschaft grant (WA1477/4 (JW)), an Alzheimer Forschung Initiative grant (#12854 (SK)) and an Anonymous Foundation (CAL).
CAL and JW are co‐senior authors
References
- 1. Ashby EL, Miners JS, Kumar S, Walter J, Love S, Kehoe PG (2015) Investigation of Abeta phosphorylated at serine 8 (pAbeta) in Alzheimer's disease, dementia with Lewy bodies and vascular dementia. Neuropathol Appl Neurobiol 41:428–444. [DOI] [PubMed] [Google Scholar]
- 2. Braidy N, Poljak A, Jayasena T, Mansour H, Inestrosa NC, Sachdev PS (2015) Accelerating Alzheimer's research through ‘natural’ animal models. Curr Opin Psychiatry 28:155–164. [DOI] [PubMed] [Google Scholar]
- 3. Cotman CW, Head E, Woodruff‐Pak D (2008) The canine (dog) model of human aging and disease: Dietary, environmental and immunotherapy approaches. J Alzheimers Dis 15:685–707. [DOI] [PubMed] [Google Scholar]
- 4. Frost JL, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmour RM et al (2013) Pyroglutamate‐3 amyloid‐beta deposition in the brains of humans, non‐human primates, canines, and Alzheimer disease‐like transgenic mouse models. Am J Pathol 183:369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Head E (2013) A canine model of human aging and Alzheimer's disease. Biochim Biophys Acta 1832:1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar S, Rezaei‐Ghaleh N, Terwel D, Thal DR, Richard M, Hoch M et al (2011) Extracellular phosphorylation of the amyloid beta‐peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer's disease. EMBO J 30:2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar S, Singh S, Hinze D, Josten M, Sahl H‐G, Siepmann M et al (2012) Phosphorylation of amyloid‐beta peptide at serine 8 attenuates its clearance via insulin‐degrading and angiotensin‐converting enzymes. J Biol Chem 287:8641–8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar S, Wirths O, Theil S, Gerth J, Bayer TA, Walter J (2013) Early intraneuronal accumulation and increased aggregation of phosphorylated Abeta in a mouse model of Alzheimer's disease. Acta Neuropathol 125:699–709. [DOI] [PubMed] [Google Scholar]
- 9. Kumar S, Wirths O, Stüber K, Wunderlich P, Koch P, Theil S et al (2016) Phosphorylation of the amyloid β‐peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity. Acta Neuropathol 131:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Love S, Miners JS (2016) Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol 131:645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rijal Upadhaya A, Kosterin I, Kumar S, von Arnim CAF, Yamaguchi H, Fandrich M et al (2014) Biochemical stages of amyloid‐beta peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer's disease. Brain 137:887–903. [DOI] [PubMed] [Google Scholar]
- 12. Selkoe DJ (2001) Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev 81:741–766. [DOI] [PubMed] [Google Scholar]
- 13. Walker LC, Jucker M (2017) The exceptional vulnerability of humans to Alzheimer's disease. Trends Mol Med 23:534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]


