Abstract
The main objective of this study was to analyse neurological symptoms during a Covid-19 infection and determine the pattern of symptoms by comparing outpatients with inpatients. A further goal was to identify possible predictors, such as pre-existing conditions and neurological symptoms.
We recorded the clinical data of 40 inpatients and 42 outpatients in this retrospective, cross sectional study. Of them, 68 patients (83%), evenly distributed between the two groups, suffered from neurological symptoms. We identified the onset of neurological symptoms and the related time ranges in 41 patients (36 outpatients and 5 inpatients). Of these, 63.4% reported neurological symptoms on the first or second day of illness. 49 patients (72%) showed combinations of at least two to a maximum of seven different neurological symptoms. A more severe course of disease was correlated with age and male sex, but age was not identified as a predictor for the occurrence of neurological symptoms. Women suffered from central and neuromuscular symptoms more often than men (p = 0,004). The most common symptoms were fatigue (54%), headache (31%), loss of taste (31%), and loss of smell (27%).
Pre-existing dementia was associated with increased lethality; similarly, pre-existing stroke was associated with a more severe course of Covid-19 infection. Hallucinations and confusion were related to an increased likelihood of death.
The present data demonstrate the importance of comprehensive neurological support of inpatients and outpatients affected by Covid-19.
Keywords: SARS-CoV-2, Covid-19, Neurological symptom, Outpatient, Inpatient, Predictor of severity, Pre-existing neurological condition
1. Introduction
The different characteristics and clinical presentations of novel Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2 Viruses, Covid-19) have garnered huge interest in the scientific community.
In the six previously known human-infecting Coronaviruses (CoV) [1], neurological signs and symptoms were reported only in a small number of patients infected by the respiratory coronaviruses SARS-CoV-1 (Severe Acute Respiratory Syndrome-Coronavirus-1) and MERS-CoV (Middle East Respiratory Syndrome-Coronavirus). Nonetheless, only a few neuropathological studies have shown involvement of the neurological system in human infection by SARS-CoV-1 [2].
Patients affected by Covid-19 infected with SARS-CoV-2 suffer from a variety of disorders of the central nervous (CNS) and neuromuscular systems [3]. To date, many clinical studies have described large variations in the occurrence of neurological symptoms during Covid-19 infection [4]. Case reports have shown rare complications, as haemorrhagic encephalitis [5], Guillain-Barre syndrome [6], and others [7,8].
Most studies have investigated hospitalised patients. As a result, current data is mainly related to severely affected patients. To gather more realistic data on CNS and neuromuscular system involvement in Covid-19, we examined two groups. One included outpatients exhibiting a mild or moderate course of disease. The other was formed by hospitalised inpatients with a severe or critical course.
To date, none of the published clinical Covid-19 studies has explored possible relationships between pre-existing diseases, neurological diseases and neurological Covid-19 symptoms.
2. Material and methods
This retrospective study was conducted in the Austrian districts of Melk and Neunkirchen with populations of 77,585 and 12,251 inhabitants, respectively. We collected patient data according to the Covid-19 infection severity scale division of the Robert Koch Institution [9] and divided them into two groups. One group included outpatients with a mild or moderate course of disease; the other was formed by critically or severely affected, hospitalised patients. Inclusion criteria were a positive SARS-CoV-2 polymerase chain reaction (PCR) test using a deep nasopharyngeal or oropharyngeal swab, and age over 18 years.
The hospitalised, severe and critical patients were treated at a designated Covid-19 disease hospital in Melk. Patients from other hospitals with a severe course of disease were also transferred to the Melk facility. Data were collected from 13.03.2020 to 14.04.2020.
During the same time period, data were gathered on outpatients through general practitioners (GP) and during antibody testing at the Reichenau an der Rax (2.515 citizens) municipality in the Neunkirchen district. Patients fulfilling the inclusion criteria and who agreed to participate in this study were contacted via phone three weeks to one month after the last negative SARS-CoV-2 PCR test. We collected patients' neurological symptoms and all other below-mentioned variables through a standardised, dichotomised interview. In cases of persistent neurological symptoms at the time of the telephone interview, further clinical neurological examinations were performed and carried out by a neurologist. Nerve conduction studies were performed in patients with neuromuscular symptoms.
We documented age, Body Mass Index (BMI), sex, pre-existing conditions, and all neurological symptoms, including their chronological sequence, either by reviewing medical histories (inpatients) or by standardised telephone consultation (outpatients). Besides recording all neurological symptoms, we also included fatigue syndrome because of its importance in Covid-19 infections, its occurrence in various neurological disorders, and its multifactorial aetiology.
The statistical analysis was carried out using SPSS 24. Metric variables, where normally distributed, were compared with the t-test, otherwise with the Mann-Whitney U test. A chi-square test was performed for categorial variables. In the case of metric predictors and dichotomous dependent variables, a binary logistic regression was calculated. In the statistical analyses, a level of significance of p < 0.05 was assumed. The calculations were not adjusted for multiple comparisons. Given the possibility of a type-1 error, the results should be interpreted in an exploratory and descriptive way.
3. Results
82 patients, 44 men and 38 women, with a mean age of 56 ± 15.5 years, were included in the study.
Table 1 presents the data on 40 patients with a severe or critical course of disease, leading to hospitalisation, and 42 outpatients with a mild or moderate course.
Table 1.
Demographic data and pre-existing neurological and non-neurological conditions.
| Total (n = 82) | Hospitalised (n = 40) | Outpatient (n = 42) | |
|---|---|---|---|
| Gender (m/f) | 44/38 | 29/11 | 15/27 |
| Age (years) | 56 ± 15.5 | 61.1 ± 15.9 | 51 ± 13.5 |
| Pre-existing general conditions | |||
| Hypertension | 24 | 18 | 6 |
| Diabetes type 2 | 12 | 9 | 3 |
| Hypothyroidism | 9 | 5 | 4 |
| Hyperlipidaemia | 8 | 7 | 1 |
| Coronary heart disease, cardiomyopathy | 7 | 5 | 2 |
| Pulmonary disease | 6 | 5 | 1 |
| Depression | 3 | 3 | 0 |
| Neurological pre-existing conditions | |||
| Dementia | 4 | 4 | 0 |
| Stroke | 4 | 4 | 0 |
| Polyneuropathy | 3 | 1 | 2 |
| Parkinson's disease | 1 | 1 | 0 |
| Multiple sclerosis | 0 | 0 | 0 |
| Other pre-existing neurological conditionsa | 6 | 4 | 2 |
Including schizoaffective disorder, minor stroke, cerebral small vessel disease, Wernicke encephalopathy, metastasis in the optical nerve and fibromyalgia.
The mean age of the inpatient cohort was 61.1 ± 15.9, indicating a significantly higher age compared to the outpatient group (51 ± 13.5 years p < 0.004). Age was a significant predictor not only for the need for hospitalisation (x2(1) =13.985, p = 0.002), but also for a lethal course of disease (x2(1) =13.985, p < 0.001). For each year of age, the probability of hospitalisation due to SARS-CoV2 infection increased by 4.9% (p = 0.005) and the likelihood of the course of disease having a lethal outcome rose by 15.6% (p = 0.008).
The need for hospitalisation was significantly higher in men than in women (p = 0.001). However, the rate of lethality showed no significant gender differences (p = 1.000).
The average BMI for the patients overall was 25.2 (± 4.4). The inpatients had an average BMI of 26.8 (± 2.9) compared to the outpatients' BMI of 24.9 (± 4.6). There was no significant correlation between BMI and the duration of disease (p = 0.211). In addition, BMI was not a predictor for an inpatient or outpatient course of disease (x2(1) = 0.89; p = 0.346).
In total, neurological symptoms during Covid-19 disease were observed in 68 out of 82 patients (83%). There was no significant difference between inpatients (86%) and outpatients (80%). Women suffered more frequently than men from neurological symptoms (in total p = 0.004; inpatient p = 0.033; outpatient p = 0.085). Age was not a significant predictor for the occurrence of neurological symptoms (p = 0.338). Neurological symptoms during Covid-19 disease showed an early onset with an average of 1.6 ± 2.7 days.
Of the 40 patients requiring hospitalisation, 6 patients had a lethal outcome (15%). In all cases, the cause of death was respiratory insufficiency.
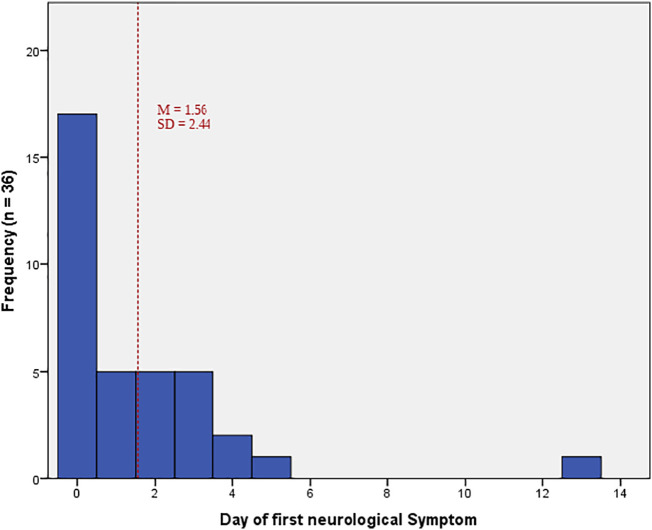
Table 2 reports the occurrence of neurological symptoms, the time range of first occurrence and symptom duration, as far as could be defined. Of the 68 patients with neurological symptoms, only 41 (36 outpatients and 5 inpatients) were able to define symptom onset and time sequence. Of these, 95.1% reported onset to be in week 1 (63.4% on day 1 and 2, and 31.7% between days 3 and 7). Only 2.8%, and after the second week, 2.1% of these patients reported the first onset of neurological symptoms to have been during week 2. Fig. 1 illustrates the time sequence for the first neurological symptoms in outpatients in relation to disease onset.
Table 2.
Demographic data and pre-existing neurological and non-neurological conditions.
| Total (n = 82) | Hospitalised (n = 40) | Outpatient (n = 42) |
|||
|---|---|---|---|---|---|
| Onset (day) | Duration (days) | ||||
| Neurological symptoms | 68 (83%) | 32 (80%) | 36 (86%) | 1.6 ± 2.4 | 28.5 ± 29.2 |
| Neurological symptoms | |||||
| Fatigue | 44 (54%) | 21 (53%) | 23 (55%) | 2.5 ± 3.0 | 11.0 ± 16.7 |
| Headache | 25 (31%) | 13 (33%) | 12 (29%) | 1.0 ± 2.2 | 3.2 ± 1.3 |
| Loss of taste | 25 (31%) | 4 (10%) | 21 (50%) | 4.5 ± 3.2 | 21.6 ± 28.5 |
| Loss of smell | 22 (27%) | 3 (8%) | 19 (45%) | 5.5 ± 4.4 | 28.5 ± 30.7 |
| Vertigo | 13 (16%) | 6 (15%) | 7 (17%) | 2.6 ± 2.4 | 15.9 ± 33.2 |
| Mood disturbance | 13 (16%) | 8 (20%) | 5 (12%) | 24.1 ± 25.9 | 16.2 ± 11.4 |
| Hallucinations/confusion | 9 (11%) | 9 (23%) | 0 (0%) | ||
| Back pain | 8 (10%) | 4 (10%) | 4 (10%) | 0.8 ± 1.5 | 8.5 ± 3.8 |
| Sensory symptoms | 5 (6%) | 1 (3%) | 4 (10%) | 26.4 ± 16.3 | 22.2 ± 20.9 |
| Myalgia | 5 (6%) | 2 (5%) | 3 (7%) | 2.7 ± 3.8 | 17.0 ± 6.1 |
| Mononeuropathy | 3 (4%) | 0 (0%) | 3 (7%) | 26.7 ± 16.5 | 33.7 ± 7.4 |
| Reduced alertness | 3 (4%) | 3 (8%) | 0 (0%) | ||
| Unsteadiness | 2 (2%) | 2 (5%) | 0 (0%) | ||
| Epileptic seizure | 1 (1%) | 1 (3%) | 0 (0%) | ||
| Dysphagia | 1 (1%) | 1 (3%) | 0 (0%) | ||
Fig. 1.
Day of first reported neurological symptom in outpatients.
The most common symptom was fatigue (54%), equally distributed in both groups. Fatigue frequently occurred together with gastrointestinal symptoms (p = 0.046), and limb pain (p = 0.026). The duration of fatigue ranged from 1 to 76 (mean 11) days.
Another common symptom was headache (25 patients per group, 31%). It was the earliest neurological symptom to occur (day 1 ± 2.2) and was reported in 76% of cases on day 1. Headache was characterised as a pressure-like headache (Visual Pain Scale (VPS) 7 ± 1.86, range 3–10). The average duration of headache was 3.2 ± 1.3 days. It was not associated with any other symptom. Furthermore, this symptom was not associated with a severe course or increased lethality.
One patient suffered from an intense pressure-like headache (VPS 9) without any other symptom. On day 3 of headache, a SARS-CoV-2 PCR test was conducted after the patient's husband had tested positive. On day 4, the headache was greatly reduced and on day 5 the patient recovered without suffering from any other kind of Covid-19 symptom.
In total, loss of taste occurred as frequently as headache (25 patients) and was more frequently reported in outpatients (50%) than inpatients (10%). The reduction of taste was correlated with anosmia (p < 0.001), dysesthesia (p = 0.028), hoarseness, and swallowing difficulties (p = 0.018).
In the outpatient group this symptom was correlated with gastrointestinal symptoms (p = 0.006), loss of appetite (p = 0.040), and skin alterations (p = 0.048).
Anosmia was observed in 22 patients (27%) and occurred in outpatients (19%) with gastrointestinal symptoms (p = 0.014) and skin alterations (p = 0.014). Both, reduced taste and smell, occurred at the end of week 1 and lasted longer than 3 weeks (Table 2).
Hallucinations are the only symptom which occurred solely in hospitalised patients (23%) and indicated a higher risk of lethality (p = 0.001). Furthermore, a significant association was found with pre-existing stroke (p = 0.004) and dementia (p = 0.000). The origin of the hallucinations could not be analysed in more detail owing to the situation in the general medicine intensive care unit.
Diffuse back pain occurred in 8 patients (10%), equally distributed in both groups and with an early onset (day 0.8 ± 1.5). There was an association between back pain and the occurrence of myalgia (p = 0.006).
Sensory symptoms (6%) and mononeuropathies (4%) occurred more frequently in the outpatient group. Both symptoms showed late onset and long duration (Table 2). Sensory symptoms presented in a stocking distribution. Mononeuropathies affected the upper extremities, twice the ulnar nerve and once the median nerve, involving only sensory fibres in both cases. In two out of three patients nerve conduction velocity studies were conducted. The results were within the normal range.
In total, more than one neurological symptom was recorded in 76.8% of patients (Fig. 1). Of them, 8.5% showed 5 or more neurological symptoms. There was a correlation between number of neurological symptoms and duration of illness (r = 0.43; p = 0.005).
The association between pre-existing diseases and the further course of disease are described in Table 3 . High blood pressure (p = 0.002), diabetes mellitus II (p = 0.049) and hyperlipidaemia (p = 0.027) were significantly correlated with severe Covid-19 infection. However, no correlation could be found between pre-existing diseases and lethal outcome.
Table 3.
Relationship between pre-existing conditions and course of illness.
| Course of disease (outpatient vs. hospitalised) | Lethal course of disease | |
|---|---|---|
| General pre-existing conditions | ||
| Hypertension | p = 0.002 | n.s. |
| Diabetes type 2 | p = 0.049 | n.s. |
| Hypothyroidism | n.s. | n.s. |
| Hyperlipidaemia | p = 0.027 | n.s. |
| Coronary heart disease, cardiomyopathy | n.s. | n.s. |
| Pulmonary disease | n.s. | n.s. |
| Depression | n.s. | n.s. |
| Pre-existing neurological conditions | ||
| Dementia | n.s. | p < 0.001 |
| Stroke | n.s. | p = 0.008 |
| Polyneuropathy | n.s. | n.s. |
| Parkinson's disease | n.s. | n.s. |
| Other pre-existing neurological conditions | n.s. | n.s. |
Pre-existing dementia (p = 0.000) and stroke (p = 0.004) were associated with a significantly higher risk of death. The association was still present when considering only patients aged 50 years and older (p = 0.001 for dementia, p = 0.025 for stroke).
4. Discussion
Most currently available studies of neurological signs and symptoms during a Covid-19 infection focus on hospitalised patients [3,[10], [11], [12]]. Our study compares the spectrum of neurological signs and symptoms in inpatients and outpatients, with the aim to increase practical knowledge. Results show a high rate of neurological symptoms (more than 80%) in Covid-19 infected patients, which were reported in both groups.
Neurological symptoms were equally distributed in both groups regardless of the severity of infection. Age is known to be a predictor of more severe Covid-19 infection [13] and of a higher risk of death [14], but the occurrence of neurological symptoms was unrelated to age. General symptoms of Covid-19 affect men more often than women [15,16] in contrast to neurological symptoms, which showed a higher frequency in females.
One of the key results of this study is that hallucinations and confusion, which only occurred in hospitalised patients, were associated with a greater likelihood of death. This may be related to pre-existing dementia and stroke, which are also associated with higher mortality. These clinically relevant results could be supportive in therapeutic decision-making. No other neurological symptom was associated with the course of the Covid-19 infection. However, the occurrence of multiple neurological symptoms was associated with a longer course of disease.
For the first time, we present evidence that not only cardiovascular [17] and metabolic disorders [16] are associated with an unfavourable course of disease, but also pre-existing conditions of the CNS (dementia and stroke) could negatively affect the progress of the infection. Accordingly, these pre-existing neurological conditions should become part of medical history-taking in SARS CoV-2 patients. Further studies are required to confirm these data, particularly in the context of other cerebrovascular diseases, age and dementia.
The early onset of a great number of neurological symptoms were detected in our study. This may help in differential diagnosis and will support early performance of specific virus tests. Conversely, late onset of neurological symptoms, as dysesthesia and mononeuropathies, are important in outpatient and inpatient management.
More than one in two patients suffered from fatigue, corroborating the results of other studies [12,[18], [19], [20]]. The relationship between fatigue and the nervous system is ill-defined and is also related to systemic inflammation and/or desaturation due to lung involvement; fatigue is often seen in patients with neurological disorders. In our opinion, the symptom of fatigue should be given due consideration in Covid-19 patients with neurological symptoms.
This study highlights long-lasting fatigue of up to 76 days' duration. Further examinations are required to determine the risk of fatigue leading to a post Covid-19 syndrome, described in approximately 10% of infected persons [21].
The prevalence of headache varies widely [19,22]. A recently published meta-analysis showed a frequency of 10% [23]. In our study, headache frequently occurred very early, presenting as an initial symptom, but also as an isolated symptom.
Both initial headache and very early back pain, both non-specific symptoms, are associated with Covid-19. To date, back pain has been reported as a Covid-19 symptom in only one study, which analysed clinical presentation of pain [11]. Although back pain did not occur as often in our study as in the above-mentioned paper, it is important to point out that it is a possible symptom of Covid-19, which has not been described in many currently published studies. Moreover, in our study, back pain was frequently observed in association with myalgia and dysesthesia. This finding indicates a significant correlation between back pain, abdominal pain and female gender [11], in contrast to the present literature. However, there was no clearly identifiable aetiology for these symptoms. The time sequence of early back pain followed 3 weeks later by dysesthesia, suggests a possible immune-mediated genesis. In acute inflammatory demyelinating polyneuropathy, two-thirds of patients present with low back pain as an early symptom [24]. Further studies, focusing on this specific SARS-CoV-2 effect, are therefore warranted. The rare occurrence of sensory mononeuropathies in this study also calls for further analysis.
Our results are based mainly on data, collected through patients' medical histories. Hence, further studies should focus on clinical-neurological and laboratory examinations and further testing, according to the specific symptom [25]. This will pave the way to better understanding of the underlying pathological mechanism of the existing neurological involvement. Furthermore, due to the exploratory nature of our methodology as well as the varying methodologies of the two groups, the small number of patients and the retrospective design, further studies should determine whether the observed associations hold true even after more thorough examination.
GPs and medical health professionals should be aware of the wide spectrum of SARS-CoV-2 symptoms, generally and neurologically, in order to be able to classify the differential diagnosis, especially during the influenza season. Covid-19 and influenza present many similar symptoms, such as fever, cough and myalgia [26]. Nevertheless, neurological symptoms, reported after 1–5 days of systemic symptoms, also occur due to neurovirulent strains of the influenza virus [27]. These conditions can include seizures [28], acute necrotising encephalopathy (ANE) - reported to be the most frequent neurological complication of influenza infection [29] [30] - movement disorder [31], and Guillain-Barre syndrome [32]. Upper respiratory tract infection is seen as a prodromal condition, followed by fever and a rapidly worsening level of consciousness [33].
In relation to neurological symptoms, ongoing prospective studies [34] and future analysis of out- and inpatient clinical-neurological examinations are needed to ensure continuous improvements in the care of patients affected by Covid-19. However, these findings can already help GPs and medical health professionals in a clear, efficient way in the management of in- and outpatients with suspected and confirmed SARS-CoV-2.
5. Conclusion
In our study, the prevalence of neurological symptoms of CoV-19 infection exceeded 80% in hospitalised and outpatients. Certain neurological symptoms characterise Covid-19 infection, such as fatigue, pressure-like headache, loss of taste and/or smell. The occurrence of hallucinations and confusion was associated with a lethal outcome and could be considered when managing intensive care units, especially when faced with the risk of reaching full capacity. Similarly, if proven reliable, the association between dementia and stroke and a severe course of Covid-19, may also be an important factor in infected patient management. Additionally, identifying the wide variety of neurological symptoms in mildly or moderately affected Covid-19 outpatients will allow accurate, efficient symptomatic treatment.
Funding
This research did not receive grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
There is no COI of any other authors.
Acknowledgements
The study was approved by the ethics committee of lower Austria, “GS1-EK-4/651-2020, NÖ Ethikkommission”.
References
- 1.Corman V.M., Lienau J., Witzenrath M. Coronaviruses as the cause of respiratory infections. Internist (Berl) 2019;60(11):1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Román G.C., Spencer P.S., Reis J., Buguet A., Faris M.E.A., Katrak S.M., Láinez M., Medina M.T., Meshram C., Mizusawa H., et al. The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020;414:116884. doi: 10.1016/j.jns.2020.116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krett J.D., Jewett G.A.E., Elton-Lacasse C., Fonseca K., Hahn C., Au S., Koch M.W. Hemorrhagic encephalopathy associated with COVID-19. J. Neuroimmunol. 2020;346:577326. doi: 10.1016/j.jneuroim.2020.577326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahimi K. Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. Neurol. Sci. 2020;41(11):3149–3156. doi: 10.1007/s10072-020-04693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger J.R. COVID-19 and the nervous system. J. Neuro-Oncol. 2020;26(2):143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharifian-Dorche M., Huot P., Osherov M., Wen D., Saveriano A., Giacomini P.S., Antel J.P., Mowla A. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J. Neurol. Sci. 2020;417:117085. doi: 10.1016/j.jns.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ständiger Arbeitskreis der Kompetenz- und Behandlungszentren für Krankheiten durch hochpathogene Erreger am RKI: Hinweise zu Erkennung . Robert Koch-Institut; 2020. Diagnostik und Therapie von Patienten mit COVID-19. [Google Scholar]
- 10.Liotta E.M., Batra A., Clark J.R., Shlobin N.A., Hoffman S.C., Orban Z.S., Koralnik I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann. Clin. Transl. Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murat S., Dogruoz Karatekin B., Icagasioglu A., Ulasoglu C., İçten S., Incealtin O. Clinical presentations of pain in patients with COVID-19 infection. Ir. J. Med. Sci. 2020:1–5. doi: 10.1007/s11845-020-02433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynants L., Van Calster B., Collins G.S., Riley R.D., Heinze G., Schuit E., Bonten M.M.J., Damen J.A.A., Debray T.P.A., De Vos M., et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. Bmj. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., Guo G.Y., Du J., Zheng C.L., Zhu Q., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020:55(5). doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020;20(7):442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., Bonanomi E., Cabrini L., Carlesso E., Castelli G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y., Wang G., Cai X.P., Deng J.W., Zheng L., Zhu H.H., Zheng M., Yang B., Chen Z. An overview of COVID-19. J Zhejiang Univ Sci B. 2020;21(5):343–360. doi: 10.1631/jzus.B2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2020;75(3) doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivan M., Taylor S. NICE guideline on long covid. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 22.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., Huet K., Plzak J., Horoi M., Hans S., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020;288(3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam M.A., Alam S.S., Kundu S., Hossan T., Kamal M.A., Cavestro C. Prevalence of headache in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 14,275 patients. Front. Neurol. 2020:11(1492). doi: 10.3389/fneur.2020.562634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zifko U., Grisold W. Schmerzsyndrome bei neuroimmunologischen Krankheitsbildern. Neuropsychiatrie. 1994;8:102–106. [Google Scholar]
- 25.Ferrarese C., Silani V., Priori A., Galimberti S., Agostoni E., Monaco S., Padovani A., Tedeschi G. An Italian multicenter retrospective-prospective observational study on neurological manifestations of COVID-19 (NEUROCOVID) Neurol. Sci. 2020;41(6):1355–1359. doi: 10.1007/s10072-020-04450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oette M. COVID-19 in der Grippesaison: Sind wir sicher in der klinischen Einschätzun? MMW Fortschr. Med. 2020;162(17):48–53. doi: 10.1007/s15006-020-3450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasai T., Togashi T., Morishima T. Encephalopathy associated with influenza epidemics. Lancet. 2000;355(9214):1558–1559. doi: 10.1016/S0140-6736(05)74614-6. [DOI] [PubMed] [Google Scholar]
- 28.Chiu S.S., Tse C.Y., Lau Y.L., Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001;108(4) doi: 10.1542/peds.108.4.e63. [DOI] [PubMed] [Google Scholar]
- 29.Steininger C., Popow-Kraupp T., Laferl H., Seiser A., Gödl I., Djamshidian S., Puchhammer-Stöckl E. Acute encephalopathy associated with influenza A virus infection. Clin. Infect. Dis. 2003;36(5):567–574. doi: 10.1086/367623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troedson C., Gill D., Dale R.C. Emergence of acute necrotising encephalopathy in Australia. J. Paediatr. Child Health. 2008;44(10):599–601. doi: 10.1111/j.1440-1754.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- 31.Ryan M.M., Procopis P.G., Ouvrier R.A. Influenza A encephalitis with movement disorder. Pediatr. Neurol. 1999;21(3):669–673. doi: 10.1016/s0887-8994(99)00062-4. [DOI] [PubMed] [Google Scholar]
- 32.Flewett T.H., Hoult J.G. Influenzal encephalopathy and postinfluenzal encephalitis. Lancet. 1958;2(7036):11–15. doi: 10.1016/s0140-6736(58)90003-5. [DOI] [PubMed] [Google Scholar]
- 33.Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19(2):81–92. doi: 10.1016/s0387-7604(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 34.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]