Dear Editor,
We are writing this letter in response to the article published by Ravula et al. [1] where it was proposed that whole blood derived covid convalescent plasma (WB−CCP) might be a feasible cost-effective alternative to treat Covid-19 disease in developing countries. Herein we would like to share our experiences from a resource limited province in India where the covid convalescent plasma (CCP) was actually made available to the ailing people by collecting whole blood from Covid-19 recovered individuals.
1. Background
On 11th March 2020 the outbreak of severe acute respiratory syndrome due to SARS-CoV-2 infection was declared as pandemic by WHO [2]. India has become one of the worst affected countries due to this ongoing pandemic. After a yearlong battle against the Covid-19, today, on 11th March 2021 the number of total Covid-19 cases in India stands at 11.3 million with more than 150,000 casualties [3]. Till date no treatment directed against the Covid-19 has been shown to reduce mortality in hospitalized patients. In September-2020, during the peak of the pandemic CCP was considered one of only a few available options to contend with COVID-19 disease in India, although, adequate data on CCP from well designed controlled clinical trials were unavailable at that time. The problem has increased by several folds in some of the resource limited regions due to the limited access to the apheresis facilities, inadequate infrastructures and lack of technical expertise to collect CCP by plasmapheresis.
2. Establishing whole blood derived covid convalescent plasma collection facility
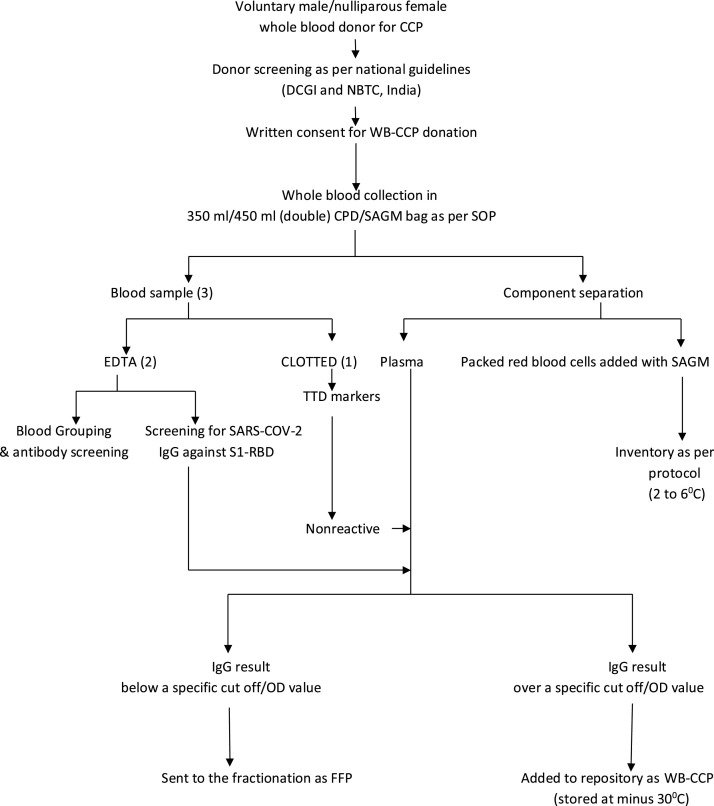
On 2nd September 2020 a standard operating procedure (SOP) was issued by the State Blood Transfusion Council (SBTC) of West Bengal, India to facilitate the off-label use of WB−CCP across the state due to the limited availability of apheresis facilities in this region [4]. A proposal to establish 20 plasma collection facilities from whole blood across the state was approved considering the rapid spread of COVID-19 infection in local population which was reflected by reporting of more than 2500 positive cases per day consecutively for two weeks [5].The consultants/medical officers, technical supervisors/ technologists, and nurses posted at different blood centers across the state were given the responsibility to select the eligible donor, collect the plasma, and to store, process and label the plasma as WB−CCP for use in indicated hospitalized Covid-19 patients. The detail work-flow is described in Fig. 1 . These personnel were also given responsibility to document the details of the product in an integrated computer based inventory system approved by SBTC to ensure better visibility of the available product to the nearby hospitals.
Fig. 1.
WORK FLOW OF WB−CCP.
2.1. Selection of donor
The selection of whole blood donors was according to the guidelines stated in the Drug and Cosmetic Act 1940 and interim guidance for blood transfusion services in view of Covid-19 in India [6]. All recovered Covid-19 individuals who once tested positive for COVID-19 and/or had symptoms of Covid-19 were eligible to donate when they were free of symptoms for at least 28 days. As the capacity for SARS-CoV-2 testing was limited in the peripheral hospitals negative molecular testing to confirm the viral clearance was not made mandatory as donor eligibility criteria.
2.2. Collection and separation
Collection was done either inside the premises of a licensed blood center with component separation facilities or in a blood mobile vehicle (BMV) or in an outdoor blood donation camp maintaining strict medical precautions and social distancing norms. Separation was performed according to the departmental SOP for component preparation.Volume of plasma per unit to be used as WB−CCP was not less than 200 mL. No pooling of plasma from different donors was allowed. Each WB−CCP was given a unique identification number before entering the unit into an integrated inventory system.
2.3. Testing
Blood grouping and irregular red cell antibody screening was performed by conventional tube test (CTT) after donation. Transfusion transmitted disease markers were screened by ELISA (HIV I&II, HBV, HCV) and immunochromatography (malaria, syphilis). Enzyme immunoassays (i.e. ELISAs) were performed to detect SARS-CoV-2 IgG antibodies to spike protein, receptor-binding domain (S1-RBD of Covid-19). While detecting Covid-19 anti-S1-RBD IgG by ELISA, plasma was labeled as WB−CCP based on the test result obtained over a specific test value (signal/Cut-off or OD) which was in accordance with the concerned manufacturer’s instruction and also with the departmental SOP in such a manner that the established value would be corroborative with the value of IgG titre, already established to be effective in the treatment of Covid-19 patients as per available literatures [7,8]. Conversely, the plasma yielding result below the cut off value was not considered as WB−CCP, but as FFP and sent to the fractionation. All donor samples were stored in aliquots at −80 °C for future neutralization assays.
2.4. Labelling and storage
All units of WB−CCP were labelled specifically with a unique identification number. The base label was same as for regular plasma. However, following cautionary statement -“Caution: New Drug - Limited by National law to investigational use” was mentioned over the label. The WB−CCP was stored at −30 °C within 24 h of the end of the collection. It was recommended to store the WB−CCP at constant temperature below −30 °C until administration up to 12 months. A separate rack with proper signage facility was dedicated in the deep freezer for storage of WB−CCP.
2.5. Transportation
All WB−CCP after thawing at 37 °C were transported in a temperature-controlled condition to the nearest Covid hospital. It was advised that under certain circumstances when freezer was not available, thawed plasma stored between 2 °C and 6 °C could be used up to 5 days prior to transfusion [9]. In emergency cases bulk transfer or single unit transfer of WB−CCP between facilities across the state was permitted.
2.6. Distribution and issue
WB−CCP units were issued to the covid-19 patients only after receiving a valid requisition along with a blood sample from the hospitals. A patient’s request form along with an informed consent was designed and distributed among treating hospitals for off-label use of WB−CCP. All WB−CCP issued for transfusion were ABO compatible. Patient’s blood group was confirmed twice at respective blood centres by CTT. Blood product appropriateness, labeling, blood group, volume, and expiry date were checked twice by two different technologists before releasing any WB−CCP unit. All records were maintained in the blood component issue register. An information system was developed in such a manner that each blood centre should receive an update once a released product was transfused to a patient.
2.7. Selection of patients
WB−CCP was transfused in COVID-19 patients with moderate to severe disease to limit further disease associated complications. It was not transfused in pregnant and breastfeeding women or patients who were already in septic shock or under mechanical ventilation for more than 5 days. Patients who had allergy to blood components were also not considered eligible for transfusion.
2.8. Dose
All patients had received an initial dose of 200−250 mL followed by one additional dose of 200−250 mL at 24 h interval according to the disease severity and tolerance observe during first transfusion. The second plasma from a different ABO compatible donor was preferred based on the availability.
2.9. Adverse reaction
No serious adverse event was reported due to the transfusion of WB−CCP except few cases of minor allergic reactions which were successfully managed with antihistaminic.
2.10. Follow-up of patients receiving WB−CCP
It was not possible to follow-up each patient for further analysis because of heterogeneity of the infrastructures and inadequate resources at the peripheral sites.
3. Results
A state wise audit on collection and utilization of WB−CCP was conducted after one month to assess the functionality of WB−CCP program across 20 centres. It was found that from October-20 to November-20 total 672 units of WB−CCP were collected (B > O>A > AB), among them 502 units were found suitable for transfusion according to the test results for SARS-CoV-2 IgG antibodies and 145 units were transfused to the patients. Total 357 units of WB−CCP were kept ready for transfusion at the end of November-20.
4. Discussion
The objective of this letter was to propose a practical approach, by utilizing limited available resources, for the use of WB−CCP in Covid-19 patients in resource constraint healthcare facilities. It is a known fact that the plasma collection using apheresis facility is ideal because it optimizes efficiency and frequency of collections. But the whole blood donation has certain advantage as to the donor may easily be convinced for donation due to the shorter collection time and collection could be done outside blood centre premises with minimal infrastructure and least expenditure. The major limitation of WB−CCP is such donor can only donate again after 3 months interval. Furthermore, WB−CCP collected from a single donor can only be utilized for a single patient for a single transfusion. Some donors were also interested to donate because they wanted to store their Covid IgG rich plasma for their own future use in case requirement arises during the second wave of pandemic. The requirement of CCP was reduced after December-20 in our region due to the gradual improvement of pandemic situation and rolling out of vaccination against Covid-19. As combining results of available clinical trials together on CCP [[10], [11], [12], [13], [14]] including the data from RECOVERY trial [15] did not show significant benefit of using CCP in Covid-19 patients that could be another reason for recent decrease in interest regarding CCP usage.
The effect of this pandemic on blood transfusion services across the world is staggering, but considering all the limitations such as limited access to apheresis facilities, inadequate number of molecular testing facilities, unavailability of pathogen reduction technique etc. the developing countries are fighting hard to contain this pandemic by scientific improvisations, and establishment of WB−CCP collection facility could be considered as one of such efforts to reduce the loss of precious human lives.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.transci.2021.103140.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Ravula U., Chunchu S.R., Gente V.K. Whole blood derived covid convalescent plasma: an economical option among developing countries. Transfus Apher Sci. 2021;2(January) doi: 10.1016/j.transci.2020.103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization: Coronavirus disease (COVID-19) outbreak. https://www.who.int/emergencies/diseases/novelcoronavirus-2019. [Last accessed 27 April 2020].
- 3.Covid-19 India. https://www.mohfw.gov.in/index1.php. [Last accessed 11 March 2021].
- 4.Standard operating procedure (SOP) for whole blood derived convalescent covid-19 plasma (WB-CCP) for off-label use. Govt. of West Bengal, India. Order number: HFW-28013(99)/6/2020-SBTC SEC-Dept. of H &FW, dated on 02.09.2020.
- 5.WEST BENGAL COVID-19 HEALTH BULLETIN –. 30th NOVEMBER 2020. http://www.manupatrafast.in/covid_19/West%20Bengal/Govt/2020/Dec/01/Health%20Bulletin_30.pdf. [Last accessed 10 December 2020].
- 6.NBTC Interim guidance for blood transfusion services in view of COVID-19 dated on 25.03.20. http://www.naco.gov.in/nbtc-interim-guidance-blood-transfusion-services-view-covid-19. [Last accessed 10 December 2020].
- 7.US FDA authorisation letter in response to the request that the Food and Drug Administration (FDA) issue an Emergency Use Authorization (EUA) for emergency use of COVID-19 convalescent plasma for the treatment of hospitalized patients with Coronavirus Disease 2019 (COVID-19), as described in the Scope of Authorization (Section II) of this letter, pursuant to Section 564 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. 360bbb-3) dated on 28.03.20.
- 8.Nayak K., Gottimukkala K., Kumar S., et al. Characterization of neutralizing versus binding antibodies and memory B cells in COVID-19 recovered individuals from India. Virology. 2021;558:13–21. doi: 10.1016/j.virol.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadlbauer D., Baine I., Amanat F., et al. Anti-SARS-CoV-2 spike antibodies are stable in convalescent plasma when stored at 4° Celsius for at least 6 weeks. Transfusion. 2020;60:2457–2459. doi: 10.1111/trf.16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A., Mukherjee A., Kumar G., et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371(October) doi: 10.1136/bmj.m3939. m3939 Erratum in: BMJ. 2020 Nov 3;371:m4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonovich V.A., Burgos Pratx L.D., Scibona P., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajpai M., Kumar S., Maheshwari A., et al. Efficacy of convalescent plasma 575 therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot 576 randomized controlled trial. medRxiv. 2020 2020.10.25.20219337. [Google Scholar]
- 13.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., et al. Convalescent Plasma 573 for COVID-19. A randomized clinical trial. medRxiv. 2020 2020.07.01.20139857. [Google Scholar]
- 14.Ray Y., Paul S.R., Bandopadhyay P., et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. 2020 2020.11.25.20237883. [Google Scholar]
- 15.Horby P.W., Estcourt L., Peto L., et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial The RECOVERY Collaborative Group. medRxiv. 2021 doi: 10.1016/S0140-6736(21)00897-7. 03.09.21252736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.