Abstract
In this paper, a stochastic SIRV epidemic model with general nonlinear incidence and vaccination is investigated. The value of our study lies in two aspects. Mathematically, with the help of Lyapunov function method and stochastic analysis theory, we obtain a stochastic threshold of the model that completely determines the extinction and persistence of the epidemic. Epidemiologically, we find that random fluctuations can suppress disease outbreak, which can provide us some useful control strategies to regulate disease dynamics. In other words, neglecting random perturbations overestimates the ability of the disease to spread. The numerical simulations are given to illustrate the main theoretical results.
Keywords: Stochastic epidemic model, Threshold value, Extinction, Permanence in the mean
Introduction
Recent global infectious diseases (such as the outbreak of H7N9 influenza in 2013, Ebola disease in 2014, and COVID-19 in 2019) resulted in a lot of biological deaths and substantial financial ruins. Infectious diseases are a major concern of the public. The modeling of infection diseases is extremely important to research the mechanisms of diseases. A mathematical model is considered as an effective way to forecast the outbreak of a disease [1–17].
In fact, our real life is full of randomness and stochasticity. For human disease related epidemics, the nature of epidemic growth and spread is random due to the unpredictability in person to person contacts. Because of environmental noises, the deterministic approach has some limitations in the mathematical modeling transmission of an infectious disease, and several authors began to consider the effect of white noise on the epidemic systems. In order to improve the understanding of the difference of random environmental fluctuations, many scholars have introduced white noise in deterministic models [18–39].
There are different approaches used in the literature to introduce random perturbations into population models, both from a mathematical and biological perspective. One is to perturb the positive endemic equilibria in order to make the equilibria of deterministic models robust. In this situation, the essence of the investigation using the approach is to check if the asymptotic stability of the positive equilibria of deterministic models can be preserved. The other important approach is with parameter perturbation. Many literature works on this approach can be found, for example, [25–29]. In epidemic models, the natural death rate d and the disease transmission parameter β are two of the key parameters to disease transmission. May in [37] pointed out that all the parameters involved in the population model exhibit random fluctuation as the factors controlling them are not constant. And in the real situation, the natural death rate d and the disease transmission parameter β always fluctuate around some average value due to continuous fluctuation in the environment. In this sense, d can seem as a random variable d̃, β changes to a random variable β̃. More precisely, each infected individual makes , potentially infectious contacts with each other individual in .
In recent years, the stochastic SIV and SIRV type epidemic models have been extensively studied, and many important results have been established, see, for example, articles [18–24, 40–43] and the references cited therein. We easily see that most of these research works aim at the models with bilinear incidence, and there exists some research on the models with special nonlinear incidences (see [19–21]). Particularly in [20], the authors studied a class of stochastic SIVS epidemic models with nonlinear saturated incidence:
A threshold value is identified. It is shown that if , then the disease in the stochastic model is extinct, and if , then any solution with positive initial value in is permanent in the mean. In [21], the authors studied a class of stochastic SIVR epidemic models where vaccination is included and such that the immunity is permanent, respectively:
The sufficient conditions on the exponential stability in mean square of disease-free equilibrium are obtained.
Motivated by the above work, in this paper, we consider the following deterministic SIRV epidemic model with nonlinear incidence rate and disease-induced mortality:
| 1.1 |
In model (1.1), the basic reproduction number is a threshold which completely determines the persistence or extinction of the disease. It is shown that, if , then the disease-free equilibrium is globally asymptotically stable, and if , then model (1.1) has a unique endemic equilibrium which is globally asymptotically stable.
Now, we assume that the random effects of the environment make the transmission coefficient β of the disease in deterministic model (1.1) generate random disturbance. That is, , where is a one-dimensional standard Brownian motion defined on some probability space. Thus, model (1.1) will become the following stochastic SIRV epidemic model with nonlinear incidence rate:
| 1.2 |
The biological meaning of all parameters in (1.2) is the same as that in system (1) in [18]. All parameter values are assumed to be nonnegative and λ, . The portion ϵΛ () is vaccinated, whereas the rest remains in the susceptible class.
As well as we know, in modeling the dynamics of epidemic systems the incidence rate is an important substance. In practice the nonlinear incidence is frequently used for achieving more exact results. We see that some deterministic and stochastic epidemic models with nonlinear incidence have been extensively studied (see, for example, [30–35, 38–44]). However, we see that stochastic epidemic models with nonlinear incidence and vaccination are barely studied. Therefore, our first question is: Can we also establish a series of similar results on the extinction (i.e., disease-free) or persistence (i.e., endemic) of the disease for the stochastic SIRV epidemic model with nonlinear incidence and vaccination?
The main focus of this article is to investigate how environment fluctuations affect disease dynamics through studying the global dynamics of an SIRV model with nonlinear incidence in both the deterministic and the corresponding stochastic version. The organization of this paper is as follows. In Sect. 2, we give some useful lemmas and fundamental assumption for general nonlinear incidence functions. In Sect. 3, the results on the extinction of the disease with probability one are stated and proved. In Sect. 4, we prove that the disease is persistent under one condition. In Sect. 5, the numerical simulations are presented. Finally, in Sect. 6, a conclusion is given.
Preliminaries
Denote . For any integrable function defined for , we denote . The initial condition for model (1.2) is given by
| 2.1 |
where . Moreover, for a nonlinear function in model (1.2), we always introduce the following assumptions (see [34]).
(H) The function is two-order continuously differentiable for any with , strictly monotone increasing for for any fixed , and monotone increasing for for any fixed . Moreover, the function is bounded and monotone decreasing for for any fixed , and for all .
Remark 2.1
Define , from assumption , is continuous and monotone increasing for . By simple calculation, we can obtain for any and .
Lemma 2.2
For any initial value , model (1.2) has a unique solution with initial condition (2.1) defined for all , and the solution remains in with probability one for any . Furthermore,
where .
Proof
Since the coefficients of model (1.2) are locally Lipschitz continuous, by the fundamental theory of stochastic differential equations, for any initial value , model (1.2) has a unique local solution defined for and satisfies a.s. for all , where is the explosion time (see [36]). Let , then we have
| 2.2 |
Consequently,
| 2.3 |
Therefore, we further have, for any ,
| 2.4 |
This shows that , a.s. for all . It follows from assumption that there is a constant such that a.s..
To show that the solution is global, we only need to prove that a.s. Let be large enough such that all lie within the interval . For each integer , define the following stopping time:
Throughout this paper, we set , where Ø denotes the empty set. It is clear that is increasing as . Set , then a.s. Namely, we need to show that a.s. Assume that there exist a pair of constants and such that . Then there is an integer such that, for all ,
| 2.5 |
Define a -function as follows:
The nonnegativity of can be seen from for . Using Itô’s formula (see [45]), we obtain
where
Clearly, we further have
where . Therefore, we have
| 2.6 |
Integrating (2.6) from 0 to and then taking expectations, we can obtain
| 2.7 |
Set for , then by (2.5). Noticing that, for every , there is at least one of , , , that equals to . Hence,
| 2.8 |
In view of (2.7) and (2.8), we have
where is the indicator function of . Let lead to the contradiction
Therefore, we must have a.s..
Furthermore, since (2.3) and (2.4) hold for all , we can obtain
and when we also have a.s. for all . This competes the proof. □
Remark 2.3
Denote the region
The proof of Lemma 2.2 shows that Γ is globally attractive and positive invariant with respect to model (1.2) with probability one. Therefore, in the following discussions we can assume that the initial value for any solution of model (1.2).
Lemma 2.4
Let be the solution of model (1.2) with initial value . Then
| 2.9 |
where
, , and
Furthermore,
| 2.10 |
where
| 2.11 |
Proof
From (2.2) and model (1.2), we can obtain
| 2.12 |
| 2.13 |
and
| 2.14 |
Combining with (2.12), (2.13), and (2.14), we can obtain
| 2.15 |
where
and .
By (2.15), we further have
| 2.16 |
Integrating (2.16) from 0 to t and multiplying by , we can obtain
| 2.17 |
By substituting (2.17) into (2.15), we further obtain (2.9).
Next, we prove (2.10), from (2.2) and model (1.2) we further have
and
Consequently,
Therefore,
Thus, we finally obtain (2.10). This completes the proof. □
Extinction of the disease
Define the parameter
where we can easily see that is the basic reproduction number of deterministic model (1.1). On the extinction of the disease in probability for model (1.2) we have the following result.
Theorem 3.1
Let be any solution of model (1.2) with initial value . Assume that one of the following conditions holds:
and is decreasing for ; .
Then we have
Proof
Using Itô’s formula to , and then integrating from 0 to t for any , we can obtain
| 3.1 |
By the Cauchy–Schwarz inequality, we further have
| 3.2 |
If condition holds, we further have
| 3.3 |
where
By the mean value theorem, we have
| 3.4 |
where ξ is situated between and S. Since is decreasing for , if , then , we obtain
| 3.5 |
and if , then , we also have
| 3.6 |
Substituting (3.5) and (3.6) into (3.4) yields that
| 3.7 |
Substituting (2.10) into (3.7), we further get
Since by assumption , we hence have
| 3.8 |
We obtain immediately from substituting (3.8) into (3.3) that
where
From Lemma 2.2 and expression (2.11) of , we can obtain a.s., which implies that and a.s.. Therefore, by the large number theorem for martingales, we finally have
| 3.9 |
If condition holds, then from (3.2) we have
With the large number theorem for martingales, we obtain
| 3.10 |
This completes the proof. □
Theorem 3.2
Assume that the conditions of Theorem 3.1hold. Then, for any solution of model (1.2) with initial value , we have
where .
Proof
From (3.9) and (3.10) we easily obtain a.s. Now, let us prove the assertion a.s., a.s., and a.s. as . According to (2.10) we get
Clearly, , and
| 3.11 |
Using L’Hospital’s rule, we compute to obtain
| 3.12 |
| 3.13 |
and
| 3.14 |
From (3.11), (3.12), (3.13), and (3.14), it follows that a.s.. Therefore, a.s..
From (2.12), we easily get a.s. Then, by using (2.12), we further obtain
where . This completes the proof. □
Permanence in the mean
On the permanence in the mean with probability one for model (1.2) we have the following result.
Theorem 4.1
Assume . Then any solution of model (1.2) with initial value is permanent in the mean. Namely, we have
where the constants , , , M̃, , and T will be defined below.
Proof
We consider formula (3.1). From (2.9), by the mean value theorem, for any , we have
| 4.1 |
and
| 4.2 |
where is situated between and and . According to Lemma 3 given in [34], we obtain
and
From (3.1) and combining with (4.1) and (4.2), we can obtain
| 4.3 |
We then compute to obtain
| 4.4 |
| 4.5 |
and
| 4.6 |
where
From (4.4), (4.5), and (4.6), we can rewrite (4.3) as
| 4.7 |
where
According to L’Hospital’s rule, we get
| 4.8 |
and
| 4.9 |
From equations (4.8) and (4.9), by the strong law of large numbers, we obtain that a.s.. Therefore, we finally have
| 4.10 |
Next, we prove the permanence in the mean of . The proof is similar to [34]. According to Lemma 3 given in [34], we have . Integrating the first equation of model (1.2) from 0 to t, we can obtain
Taking and the strong law of large numbers, we further have
Secondly, from (4.10) and integrating the third equation of model (1.2) from 0 to t, we can obtain
Lastly, integrating the last equation of model (1.2), we obtain
Therefore, taking , we finally have
This completes the proof. □
Remark 4.2
Comparing with , we can easily see that for any and if , then . Namely, the environmental noise can greatly change the properties of an epidemic model.
Numerical simulation
In this section we analyze the stochastic behavior of model (1.2) by means of the numerical simulations in order to make readers understand our results better. The numerical simulation method can be found in [32]. The corresponding discretization system of model (1.2) is given as follows:
where () are the Gaussian random variables which follow the standard normal distribution .
Example 1
In model (1.2), we take (standard incidence).
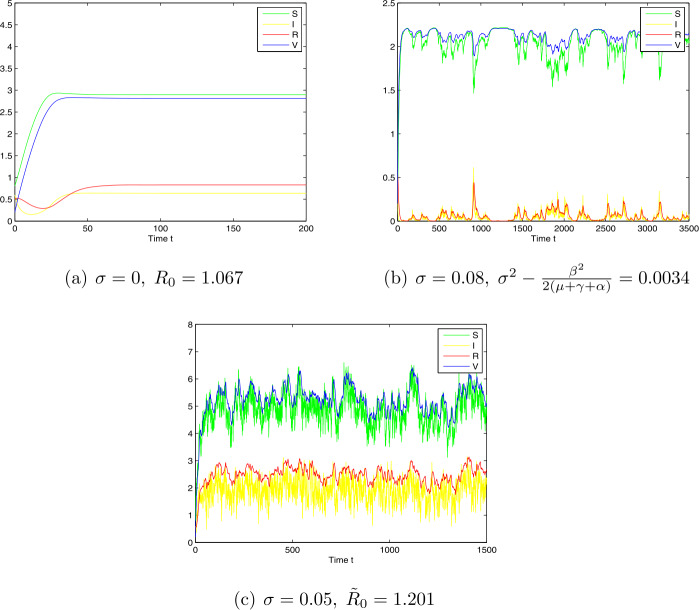
Case 1. (i) Choose , , , , , , , , and . For deterministic model (1.1), . From the numerical simulations (see Fig. 1.a), it is clear that the endemic equilibrium is also globally asymptotically stable if only the basic reproduction number is greater than one. For the corresponding stochastic model (1.2), we have , which is the case of Theorem 3.1. From the numerical simulations, we see that the disease will die out (see Fig. 1.b).
Figure 1.
Simulation for paths , , , and for the stochastic system and the corresponding deterministic system with initial value and step size
(ii) Choose , , , , , , , , and . By computing, we have , which is the case of Theorem 3.1. From the numerical simulations, we see that the disease will die out (see Fig. 1.c).
Case 2. Choose , , , , , , , , and . By computing, we have , . The numerical simulations are given in Figs. 2.a and 2.b, which show that model (1.2) is permanent in the mean with probability one.
Figure 2.
Simulation for paths , , , and for the stochastic system with initial value for different noise intensities (a) and (b)
Example 2
In model (1.2), take , where ω is a positive constant.
Case 1. Choose , , , , , , , , , and . By computing, we have , which is the case of Theorem 3.1. From the numerical simulations given in Fig. 3.b, we see that disease will die out.
Figure 3.
Simulation for paths , , , and for the stochastic system and the corresponding deterministic system with initial value and step size
Case 2. Choose , , , , , , , , , and . We have , . The numerical simulations found in Fig. 3.c show that model (1.2) is permanent in the mean with probability one.
Example 3
In model (1.2), take (Beddington–DeAngelis incidence), where and are nonnegative constants.
Case 1. Choose , , , , , , , , , , and . By computing we have , , which is the case of Theorem 3.1. From the numerical simulations given in Fig. 4.b, we see that the disease will die out.
Figure 4.
Simulation for paths , , , and for the stochastic system and the corresponding deterministic system with initial value and step size
Case 2. Choose , , , , , , , , , , and . By computing, we have , . The numerical simulations given in Fig. 4.c show that model (1.2) is permanent in the mean with probability one.
Conclusion
Environmental noises have a critical influence on the development of an epidemic. In this paper, we study the dynamics of a stochastic SIRV model with general nonlinear incidence rate. We assume that the stochastic perturbation is a white noise type which perturbs the disease transmission coefficient β. This is a well-established way of introducing stochastic environmental noise into biologically realistic population dynamic models that were used in [44, 46].
The value of our study lies in two aspects: Mathematically, we show that the global dynamics of deterministic model (1.1) can be governed by its reproduction number , while the dynamics of its stochastic version (1.2) is seen to be governed by . In addition, we have provided the analytic results on the existence of the global positive solution, the extinction (i.e.,disease-free) or persistence (i.e.,endemic) of the disease for stochastic model (1.2).
Epidemiologically, we summarize our main findings as follows:
1. Noise can suppress the disease outbreak: Theorem 3.1 (a) indicates that the extinction of the disease in stochastic model (1.2) occurs if the basic reproduction number . We show that deterministic model (1.1) admits a unique endemic equilibrium which is globally asymptotically stable if its basic reproduction number . Notice that , and it is possible that . This is the case when deterministic model (1.1) has an endemic (see Fig. 1a) while stochastic model (1.2) has disease extinction with probability one (see Fig. 1b). This implies that large noise intensities can inhibit the spread of a disease, which means the random perturbations can change the disease dynamics.
2. The effects of the intensity of noise level: From part (b) of Theorem 3.2, under large noise intensity case, i.e., the condition holds, the disease will become extinct exponentially. In other words, in the case of sufficiently large noise, we should use the stochastic model rather than the deterministic model to describe the population dynamics (see Fig. 1.c). One can know that if , model(1.1) admits a globally stable endemic equilibrium . In this case, when the noise intensity σ is small enough to imply that from Theorem 4.1, one can know that the stochastic model preserves the property of the global stability, and the noise can force the solutions of model (1.2) to oscillate strongly around the endemic point (see Figs. 2.a and 2.b). In addition, from Figs. 2.a and 2.b, one can observe the effects of increasing noise intensity σ on the increased level of non-equilibrium fluctuation in the stochastic dynamics of model (1.2).
Furthermore, from Theorems 3.1, 4.1 and numerical simulation results (e.g., Figs. 1–4), we can conclude that, when the intensity of noise is small, the stochastic model preserves the property of the global stability. In this case, we can ignore noise and use the deterministic model to approximate the population dynamics. However, the large intensity of noise can force the solution of model (1.2) to oscillate strongly around the disease-free or endemic points, or the extinction. In these cases, we cannot ignore the effect of noise and, therefore, we cannot use the deterministic model but the stochastic model to describe the disease dynamics.
Some interesting topics deserve further investigations. SDEs are being increasingly used in a wide range of areas, for example, finance and biology. There has recently been a large explosion in the number of papers using SDEs to model how diseases spread. However, these papers introduce stochasticity in a different way by parameter perturbation, which is appropriate if one of the parameters is a random variable. Another way to introduce stochasticity into deterministic models is telegraph noise where the parameters switch from one set to another according to a Markov switching process. Therefore, we may study a stochastic version of model (1.2) including Markovian switching into all parameters. These studies are in progress.
Acknowledgements
The authors would like to thank the editor and the anonymous reviewers for their valuable comments and constructive suggestions.
Authors’ contributions
All authors contributed equally and significantly in writing this paper. All authors read and approved the final manuscript.
Funding
This research is supported by the Natural Science Foundation of Xinjiang (Grant No. 2020D01C178).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Capasso V., Serio G. A generalization of the Kermack-McKendrick deterministic epidemic model. Math. Biosci. 1978;42:43–61. doi: 10.1016/0025-5564(78)90006-8. [DOI] [Google Scholar]
- 2.Xiao D., Ruan S. Global analysis of an epidemic model with nonmonotone incidence rate. Math. Biosci. 2007;208:419–429. doi: 10.1016/j.mbs.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kermack W.O., Mckendrick A.G. Contributions to the mathematical theory of epidemics. Philos. Trans. R. Soc. A, Math. Phys. Eng. Sci. 1927;115:700–721. [Google Scholar]
- 4.Kermack W.O., Mckendrick A.G. Contributions to the mathematical theory of epidemics. II. The problem of endemicity. Proc. R. Soc. Lond. Ser. A. 1932;138:55–83. doi: 10.1098/rspa.1932.0171. [DOI] [Google Scholar]
- 5.Sene N. SIR epidemic model with Mittag-Leffler fractional derivative. Chaos Solitons Fractals. 2020;137:109833. doi: 10.1016/j.chaos.2020.109833. [DOI] [Google Scholar]
- 6.Sene N. Analysis of the stochastic model for predicting the novel coronavirus disease. Adv. Differ. Equ. 2020;2020:568. doi: 10.1186/s13662-020-03025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M.A., Atangana A. Modeling the dynamics of novel coronavirus (2019-nCov) with fractional derivative. Alex. Eng. J. 2020;59(4):2379–2389. doi: 10.1016/j.aej.2020.02.033. [DOI] [Google Scholar]
- 8.Jahanshahi H., Shanazari K., et al. Numerical analysis of Galerkin meshless method for parabolic equations of tumor angiogenesis problem. Eur. Phys. J. Plus. 2020;135(11):866. doi: 10.1140/epjp/s13360-020-00716-x. [DOI] [Google Scholar]
- 9.Khan A., Gómez-Aguilar J.F., et al. Stability analysis and numerical solutions of fractional order HIV/AIDS model. Chaos Solitons Fractals. 2019;122:119–128. doi: 10.1016/j.chaos.2019.03.022. [DOI] [Google Scholar]
- 10.Gómez-Aguilar J.F., Abro K.A., et al. Chaos in a calcium oscillation model via Atangana-Baleanu operator with strong memory. Eur. Phys. J. Plus. 2019;134(4):140. doi: 10.1140/epjp/i2019-12550-1. [DOI] [Google Scholar]
- 11.Abuasad S., Yildirim A., et al. Fractional multi-step differential transformed method for approximating a fractional stochastic SIS epidemic model with imperfect vaccination. Int. J. Environ. Res. Public Health. 2019;16(6):973. doi: 10.3390/ijerph16060973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Aguilar J., López-López M., et al. Chaos in a cancer model via fractional derivatives with exponential decay and Mittag-Leffler law. Entropy. 2017;19(12):681. doi: 10.3390/e19120681. [DOI] [Google Scholar]
- 13.Khan M.A., Atangana A., et al. The dynamics of COVID-19 with quarantined and isolation. Adv. Differ. Equ. 2020;2020:425. doi: 10.1186/s13662-020-02882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atangana E., Atangana A. Facemasks simple but powerful weapons to protect against COVID-19 spread: can they have sides effects? Results Phys. 2020;19:103425. doi: 10.1016/j.rinp.2020.103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atangana A. Modelling the spread of COVID-19 with new fractal-fractional operators: can the lockdown save mankind before vaccination? Chaos Solitons Fractals. 2020;136:109860. doi: 10.1016/j.chaos.2020.109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borah M.J., Hazarika B., et al. Examining the correlation between the weather conditions and COVID-19 pandemic in India: a mathematical evidence. Results Phys. 2020;19:103587. doi: 10.1016/j.rinp.2020.103587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panda S.K. Applying fixed point methods and fractional operators in the modelling of novel coronavirus 2019-nCoV/SARS-CoV-2. Results Phys. 2020;19:103433. doi: 10.1016/j.rinp.2020.103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Jiang D., O’Regan D. The extinction and persistence of the stochastic SIS epidemic model with vaccination. Physica A. 2013;392:4916–4927. doi: 10.1016/j.physa.2013.06.009. [DOI] [Google Scholar]
- 19.Lin Y., Jiang D., Wang S. Stationary distribution of a stochastic SIS epidemic model with vaccination. Physica A. 2014;394:187–197. doi: 10.1016/j.physa.2013.10.006. [DOI] [Google Scholar]
- 20.Zhao D., Zhang T., Yuan S. The threshold of a stochastic SIVS epidemic model with nonlinear saturated incidence. Physica A. 2016;443:372–379. doi: 10.1016/j.physa.2015.09.092. [DOI] [Google Scholar]
- 21.Tornatore E., Vetro P., Buccellato S.M. SIVR epidemic model with stochastic perturbation. Neural Comput. Appl. 2014;24:309–315. doi: 10.1007/s00521-012-1225-6. [DOI] [Google Scholar]
- 22.Zhao D., Yuan S. Persistence and stability of the disease-free equilibrium in a stochastic epidemic model with imperfect vaccine. Adv. Differ. Equ. 2016;2016:280. doi: 10.1186/s13662-016-1010-4. [DOI] [Google Scholar]
- 23.Driss K., Lahcen B. Stationary distribution and dynamic behaviour of a stochastic SIVR epidemic model with imperfect vaccine. J. Appl. Math. 2018;2018:1291402. [Google Scholar]
- 24.Ma Y., Yu X. Threshold dynamics of a stochastic SIVS model with saturated incidence and Lévy jumps. Adv. Differ. Equ. 2020;2020:273. doi: 10.1186/s13662-020-02723-9. [DOI] [Google Scholar]
- 25.Mao X., Marion G., Renshaw E. Environmental Brownian noise suppresses explosions in population dynamics. Stoch. Process. Appl. 2002;97(1):95–110. doi: 10.1016/S0304-4149(01)00126-0. [DOI] [Google Scholar]
- 26.Allen L.J.S., Allen E.J. A comparison of three different stochastic population models with regard to persistence time. Theor. Popul. Biol. 2003;64(4):439–449. doi: 10.1016/S0040-5809(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 27.Allen L.J.S., Kirupaharan N. Asymptotic dynamics of deterministic and stochastic epidemic models with multiple pathogens. Int. J. Numer. Anal. Model. 2005;2(3):329–344. [Google Scholar]
- 28.Allen L.J.S., Driessche P. Stochastic epidemic models with a backward bifurcation. Math. Biosci. Eng. 2006;3(3):445–458. doi: 10.3934/mbe.2006.3.445. [DOI] [PubMed] [Google Scholar]
- 29.Allen L.J.S. Math. Epidemiology. 2008. An introduction to stochastic epidemic models; pp. 81–130. [Google Scholar]
- 30.Zhao Y., Jiang D. The threshold of a stochastic SIRS epidemic model with saturated incidence. Appl. Math. Lett. 2014;34:90–93. doi: 10.1016/j.aml.2013.11.002. [DOI] [Google Scholar]
- 31.Rifhat R., Muhammadhaji A., Teng Z. Asymptotic properties of a stochastic SIRS epidemic model with nonlinear incidence and varying population sizes. Dyn. Syst. 2020;35:56–80. doi: 10.1080/14689367.2019.1620689. [DOI] [Google Scholar]
- 32.Zhou Y., Zhang W., Yuan S. Survival and stationary distribution of a SIR epidemic model with stochastic perturbations. Appl. Math. Comput. 2014;244:118–131. [Google Scholar]
- 33.Yang Q., Mao X. Stochastic dynamics of SIRS epidemic models with random perturbation. Math. Biosci. Eng. 2014;11:1003–1025. doi: 10.3934/mbe.2014.11.1003. [DOI] [Google Scholar]
- 34.Rifhat R., Ge Q., Teng Z. The dynamical behaviors in a stochastic SIS epidemic model with nonlinear incidence. Comput. Math. Methods Med. 2016;2016:5218163. doi: 10.1155/2016/5218163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y., Jiang D. The threshold of a stochastic SIS epidemic model with vaccination. Appl. Math. Comput. 2014;243:718–727. [Google Scholar]
- 36.Mao X. Stochastic Differential Equations and Applications. Chichester: Horwood; 1997. [Google Scholar]
- 37.May R.M. Stability and Complexity in Model Ecosystems. Princeton: Princeton University Press; 1973. [Google Scholar]
- 38.Lahrouz A., Settati A. Necessary and sufficient condition for extinction and persistence of SIRS system with random perturbation. Appl. Math. Comput. 2014;233:10–19. [Google Scholar]
- 39.Gray A., Greenhalgh D., Hu L., Mao X., Pan J. A stochastic differential equation SIS epidemic model. SIAM J. Appl. Math. 2011;71:876–902. doi: 10.1137/10081856X. [DOI] [Google Scholar]
- 40.Lu R., Wei F. Persistence and extinction for an age-structured stochastic SVIR epidemic model with generalized nonlinear incidence rate. Physica A. 2019;513:572–587. doi: 10.1016/j.physa.2018.09.016. [DOI] [Google Scholar]
- 41.Yang J., Martchev M., Wang L. Global threshold dynamics of an SIVS model with waning vaccine-induced immunity and nonlinear incidence. Math. Biosci. 2015;268:1–8. doi: 10.1016/j.mbs.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Wen B., Teng Z., Li Z. The threshold of a periodic stochastic SIVS epidemic model with nonlinear incidence. Physica A. 2018;508:532–549. doi: 10.1016/j.physa.2018.05.056. [DOI] [Google Scholar]
- 43.Cai Y., Kang Y., Wang W. A stochastic SIRS epidemic model with nonlinear incidence rate. Appl. Math. Comput. 2017;305:221–240. [Google Scholar]
- 44.Cai Y., Kang Y., Malay B., Wang W. A stochastic SIRS epidemic model with infectious force under intervention strategies. J. Differ. Equ. 2015;259:7463–7502. doi: 10.1016/j.jde.2015.08.024. [DOI] [Google Scholar]
- 45.Lei Q., Yang Z. Dynamical behaviors of a stochastic SIRI epidemic model. Appl. Anal. 2016;96:1–13. [Google Scholar]
- 46.Liptser R. A strong law of large numbers for local martingales. Stochastics. 1980;3:217–228. doi: 10.1080/17442508008833146. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.