Acute kidney injury (AKI) is not uncommon in patients with coronavirus disease 2019 (COVID-19), but thrombotic microangiopathy (TMA) linked to COVID-19 has been exceptionally reported in another patient.1 However, COVID-19–infected patients can develop proteinuria (44%), hematuria (27%), or AKI (5.1%).2 We report a case of atypical hemolytic uremic syndrome (aHUS) associated with COVID-19. This case highlights the importance of a rapid diagnosis, a good medical history, and a genetic analysis to optimize the management of this rare disease. We discuss the role of complement pathways in the pathophysiology of IgA nephropathy and COVID-19 infection. The treatment of aHUS usually requires the use of therapeutic plasma exchange (TPE) and eculizumab, a monoclonal antibody C5 inhibitor that drastically changed the prognosis of TMA. We also wanted to know if TPE impedes natural humoral immunity against COVID-19.
Case Presentation
A 39-year-old fireman was admitted to our nephrology unit for weariness, acute renal failure, diarrhea, and fever (37.5 °C–38 °C) for 6 days. His medical history included hypertension, low-grade esophagitis, and moderate chronic kidney disease (last estimated glomerular filtration rate = 45 ml/min per 1.73 m2, serum creatinine = 2.1 mg %, and residual proteinuria = 1.0 g/d) secondary to biopsy-proven IgA nephropathy. The last biopsy performed 6 months previously was free of TMA lesions, and the kidney disease was progressing very slowly for 10 years. His treatment included perindopril 4 mg/d, irbesartan 300 mg/d, and darbepoetin alpha 10 μg for 2 weeks. He immediately stopped the renin-angiotensin system blockers when diarrhea started. On admission, his blood pressure reached 160/70 mm Hg, his temperature was 37.2 °C, and his physical examination was unremarkable; he had no cough, shortness of breath, purpuric lesions, or edema.
The initial laboratory tests (Table 1) demonstrated AKI, pancytopenia, and hemolysis. C-reactive protein, creatinine kinase, and coagulation tests including fibrinogen were in the normal range. We observed low serum albumin (24.6 g/l), nephrotic-range proteinuria (4.2 g/l–800 ml/d), and an acellular urine sediment with significant hemoglobinuria. Additional analyses confirmed hemolytic anemia with a schistocyte count of 36/1000 red blood cells (<10), a negative Coombs test, normal bilirubin, and unmeasurable haptoglobin < 0.1 g/l (range, 0.3–2.0). A clinical diagnosis of aHUS was made. ADAMTS13 activity was normal (79%, N > 69). The patient had no Shiga toxin in his feces as determined by polymerase chain reaction. We observed a moderate activation of the complement system (C3 = 66 mg/dl [range, 72–156], C3d = 1.7 mg/dl [<1.2], SC5b-9 = 300 ng/ml [<314]), specifically in the alternate pathway (C4 = 0.33 g/l [range, 0.1–0.4], B factor = 11.8 mg/dl [range, 11–22], Bb = 0.9 mg/dl [<0.15]). The main complement system regulators showed no deficiency (i.e., normal dosages of complement factor H [CFH] and CFI genes by nephelometry, normal CFH activity, normal CD46 expression on leukocytes, and absence of detectable CFH autoantibodies). The genetic complement profile was assessed. Other tests for antinuclear antibodies, double-stranded DNA, antineutrophil cytoplasmic antibodies, anti–glomerular basement membrane, and antiphospholipid antibodies (lupus anticoagulant, anticardiolipin IgG/M, anti-β2 glycoprotein IgG/M) were negative.
Table 1.
Relevant laboratory results at admission
| Laboratory tests | Patient result | Reference range |
|---|---|---|
| Hemoglobin | 7.6 g/dl | 13.5–17.5 |
| Platelet count | 80 x 10³/μl | 150–400 |
| WBC count | 2,6 x 109/μl | 3.9–9.5 |
| Schistocyte count | 36/1000 RBC | <10 |
| LDH | 533 IU/l | <255 |
| Haptoglobin | < 0.1 g/l | 0.3–2.0 |
| Creatinine | 4.7 mg/dl | |
| BUN | 218 mg/dl | |
| Albumin | 24.6 g/l | 35.0–52.0 |
| Proteinuria | 4.2 g/d | <0.15 |
BUN, blood urea nitrogen; LDH, lactate dehydrogenase; WBC, white blood cell.
COVID-19 infection was confirmed on a positive reverse transcription polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a nasopharyngeal swab performed in the emergency room. Four days later, this was consolidated by a positive serologic test for anti–SARS-CoV-2 by electrochemiluminescent immunoassay (Elecsys–Cobas 8000; Roche Diagnostics GmbH, Mannheim, Germany). His blood cultures remained negative. A chest computed tomographic scan was normal. A renal ultrasound revealed normal-sized, echogenic kidneys. We performed a kidney biopsy on day 14 to determine the patient’s renal disease.
Kidney Biopsy Findings
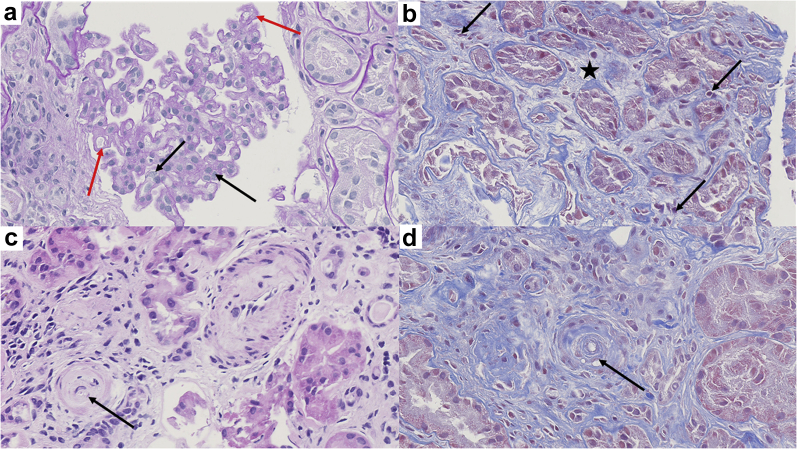
The renal biopsy (Figure 1) contained 7 glomeruli, 3 of which were completely sclerotic and 2 showed synechia with Bowman’s capsule. In the glomerulus, the capillary lumen was narrowed and demonstrated an endocapillary proliferation or endotheliosis. The glomerular basement membrane appeared duplicated. No microthrombi or necrotizing lesions were observed in the glomeruli or in small arteries. However, mucoid thickening and obliteration of the lumen of a small artery were noted, confirming a specific TMA lesion. Acute interstitial nephritis was associated with moderate preexisting tubule interstitial chronic changes, which were noted on the previous kidney biopsy performed 6 months earlier. Immunofluorescence microscopy showed segmental IgA granular deposits in the glomeruli. We found no evidence of SARS-CoV-2 particles using electron microscopy.
Figure 1.
Renal biopsy findings (original magnification ×40). The glomerular capillary lumina were narrowed. Diffuse endocapillary proliferative changes were observed with endothelial swelling (black arrows) and duplication of glomerular basement membrane (red arrows) (a, periodic acid–Schiff staining). The foci of acute interstitial nephritis were characterized by edema (black star) and few mononuclear inflammatory cells (black arrows) (b, Masson trichrome staining). Some small arteries have obliterated lumina (c, hematoxylin eosin staining, black arrows) and mucoid thickening of the intima (d, Masson trichrome staining).
Treatment
Daily 3.0 L TPE and low-molecular-weight heparin were promptly initiated. Intermittent hemodiafiltration therapy was started 3 days after admission because of worsening renal function and fluid overload. A persistent low hemoglobin level (6.7 g %) required 1 red blood cell pack transfusion. A calcium channel blocker, moxonidine, and bumetanide were used to control elevated blood pressure. Methylprednisolone oral therapy (0.8 mg/kg/d) was initiated after the renal biopsy because of the presence of moderate acute interstitial nephritis and the hospitalized COVID-19 patient.
Genetic Analysis
On month later, we received the results of genetic screening of a panel of genes involved in TMAs (ADAMTS13, C3, C5, CD46, CFB, CFH, CFHR5, CFI, DGKE, MMACHC, PLG, and THBD genes). We sequenced the targeted exons (KAPA HypercapV3; Roche, Basel, Switzerland) of these genes (including −14 to +6 bases of adjacent intronic regions) using Illumina (San Diego, CA) technology with a coverage of at least 100× per exon and 50× per base. Sequenced data were filtered and classified according to American College of Medical Genetics and Genomics criteria. The variants found were confirmed by Sanger sequencing. Genetic testing demonstrated a heterozygous missense variant of the C3 gene (c.481C>T encoding for a R161W substitution [p.Arg161Trp], corresponding to a R139W substitution of the mature C3 protein). In addition, the patient was homozygous for the at-risk CD46 haplotype (GGAAC), homozygous for a frequent polymorphism of the CFH gene (c.184G>A (p.Val62Ile; V62 variant), and heterozygous for a polymorphism in the thrombomodulin (THBD) gene (c.1418C>T [p.Ala473Val]).
Follow-up
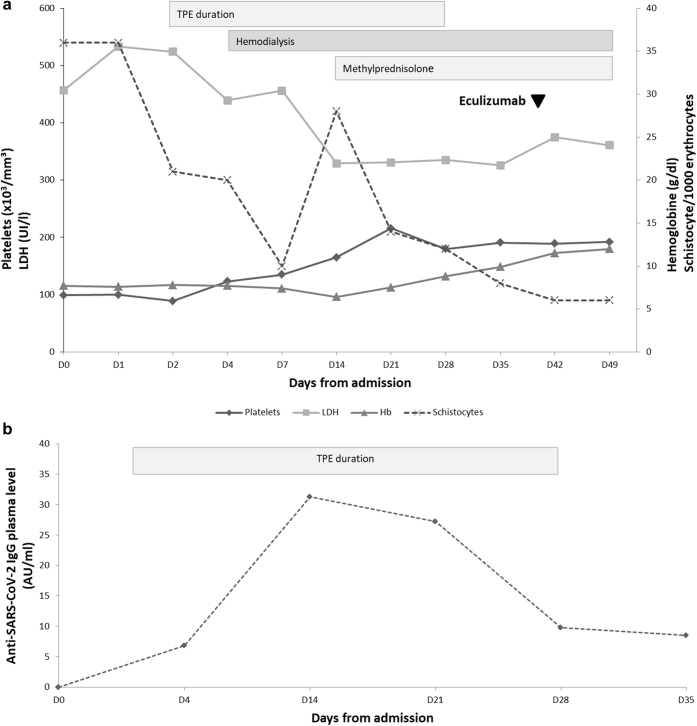
After 2 weeks of plasma exchanges, platelets recovered to 190,000/μl, the schistocyte count decreased to 15/1000 red blood cells, and the lactate dehydrogenase level returned to 230 U/l (Figure 2a) despite a persistent low haptoglobin level < 0.1 g/l. The patient was in good condition and was able to return home after 3 weeks of hospitalization. TPEs were continued for 4 weeks. The demonstration of a C3 gene mutation (with homozygous CD46 haplotype and polymorphism of factor H) encouraged us to start the first dose of C5 inhibitor eculizumab on day 40. After vaccination against meningococcus strains and pneumococcus, he received the initial dose of 900 mg together with daily azithromycin. Unfortunately, we could not continue eculizumab because it was not granted due to administrative delays. Alexion Pharmaceutical (Cheshire, CT) offered the treatment on compassionate grounds. The patient still pursues hemodialysis and decreasing corticotherapy, but his schistocyte count has normalized (6/1000 red blood cells). Despite treatment with TPE, we observed a significant rise in the level of SARS-CoV-2 IgG antibodies (Figure 2b).
Figure 2.
(a) Evolution of the biochemical parameters (i.e., platelets, lactate dehydrogenase [LDH], hemoglobin [Hb], and schistocytes) after admission and during therapies (i.e., therapeutic plasma exchange [TPE], hemodialysis, methylprednisolone, and initiation of eculizumab). (b) The trend of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG antibodies during TPE.
Discussion
Differential Diagnosis
The presence of AKI, hemolytic anemia associated with schistocytes, thrombocytopenia, and C3 hypocomplementemia strongly suggested the presence of complement-mediated TMA. A thrombotic thrombocytopenic purpura was ruled out by normal ADAMTS13 activity. Shiga toxin–mediated hemolytic uremic syndrome was excluded by the absence of Shiga toxin in the stool. There were no specific drug exposures (quinine or sulfamethoxazole-trimethoprim) that suggested drug-induced TMA. No autoimmune disease or malignancy was identified. The male condition excluded HELLP syndrome. Other rare TMA syndromes refer to transplantation, abnormal metabolism of cobalamin, or invasive pneumococcal infections. Finally, we think that the differential diagnosis that needs to be discussed is an IgA-associated TMA,3 but the presence of some anatomopathologic elements contradict this hypothesis. The arguments against this hypothesis are the presence of glomerular TMAs (which are absent in IgA-associated TMAs) and the presence of the pathogenic mutation of the alternative complement pathway. Moreover, in the present case, IgA nephropathy was stable until the patient developed COVID-19; this makes it more likely that the virus acted as a trigger for complement activation.4, 5, 6 Therefore, we should consider that IgA nephropathy and aHUS due to COVID-19 are 2 concomitant diseases.
Kidney Biopsy
Kidney biopsy findings were characterized by active TMA lesions without necrosis or thrombi. Acute tubulointerstitial nephritis was associated with moderate preexisting tubulointerstitial chronic changes. Immunofluorescence examination showed glomerular IgA deposits in a patient known to have IgA nephropathy. In the present case, the presence of glomerular TMA excluded IgA-associated TMA. Indeed, in IgA-associated TMA, the TMA lesions are only arterial and arteriolar. We consider IgA nephropathy as a concomitant condition. No viral particles were noted on electron microscopy.
COVID-19 and aHUS
COVID-infected patients can develop proteinuria (44%), hematuria (27%), or AKI (5.1%).2 Pathologic findings include acute tubular nephritis, minimal change disease, membranous glomerulopathy, anti–glomerular basement membrane nephritis, exacerbation of preexisting autoimmune glomerulonephritis, collapsing glomerulopathy, or allograft rejection.7,8 Exceptionally, a few cases of renal microangiopathies were reported1,9,S1 on the ground of multiple organ failure. In case of COVID-19–associated renal TMA, recent evidence suggests that SARS-CoV-2 would lead to cytokine storm–mediated kidney injury through the direct activation of the alternative pathway of the complement rather than direct viral infection of the kidney.S2 Nevertheless, a direct kidney infection through angiotensin-converting enzyme 2 receptors present in tubules and podocytes is not yet elucidated.6, 7, 8 The thrombotic events can occur despite the patients receiving thromboprophylaxis. There are also preliminary reports of lupus anticoagulant, anti–β2-glycoprotein I, and anticardiolipin or other antiphospholipid antibodies implicated in COVID-19 coagulopathy.4, 5, 6 A case of TMA associated with COVID-19 reported by Jhaveri et al.9 was characterized with diffuse cortical necrosis and widespread microthrombi in the kidney biopsy. In the present case, the lesions of TMA were moderate, probably because of the fast diagnosis and treatment.
COVID-19 and TPEs
Conventional treatment of aHUS includes TPE, hemodialysis, and increasingly humanized monoclonal antibody C5 inhibitor.4, 5, 6 We were concerned about the benefits to risk ratio of TPE during a severe infection, such as COVID-19. Humoral immunity is of critical importance in clearing SARS-CoV-2, and treatment with convalescent plasma containing viral-specific neutralizing antibodies has even been used to treat critically ill COVID-19 patients.4,5 In this context, we were very cautious before using TPE because the procedure itself might remove critically important neutralizing antibodies against SARS-CoV-2. The rapidly favorable clinical and biological evolutions reassured us. We were comforted by the significant rise in the level of IgG antibodies against SARS-Cov-2 (Figure 2b). To our knowledge, this is an important finding that has not been previously reported.
Genetic Identification
The diagnosis of aHUS may sometimes be confirmed by genetic analysis. This condition accounts for 5% to 10% of all cases of hemolytic uremic syndrome and is characterized by an increased activity of the alternative pathway of the complement caused by a genetic mutation or less often acquired. A mutation is found in 60% of aHUS patients and mainly involves CFH. The C3 mutations only account for 2% to 10%S3 of mutations, and an environmental trigger (infection, pregnancy, or drugs) has been identified in more than 70% of such cases.S4 It has been demonstrated that this genetic variant, leading to a R139W C3 mutant protein, is a direct gain-of-function mutation leading to a hyperactive C3 convertase. Very recently, the first case of an aHUS (due to CD46 mutation) relapse episode triggered by COVID-19 has been published,1 but in contrast to this case, our patient remained on dialysis despite the initiation of eculizumab. Both cases are illustrations that SARS-CoV-2 might be added to the list of the potential triggers of aHUS. It is known that C3 mutations are associated with a high risk of end-stage renal disease, relapse, death, and recurrence after renal transplantation.S5 Our patient is also homozygous for at-risk CD46 haplotype and CFH V62 variant and heterozygous for Ala473Val variant of THBD A473. It has been shown that the concomitant presence of the at-risk CD46 haplotype and a mutation might increase the penetrance of the disease.S6
Conclusions
We report a case of biopsy-proven TMA associated with COVID-19. aHUS was diagnosed. Genetic testing demonstrated a heterozygous missense variant of the C3 gene, a homozygous polymorphism of the CFH gene, a heterozygous polymorphism in the THBD gene, and the at-risk CD46 haplotype. TPE, corticoids, low-molecular-weight heparin, and intermittent hemodiafiltration therapy were promptly initiated. We showed that SARS-CoV-2 could trigger the development of a renal TMA through activation of the complement alternative pathway. We also showed that TPE does not seem to impede the natural course of SARS-CoV-2 seroprotection (Table 2).
Table 2.
Teaching points
|
|
|
|
aHUS, atypical hemolytic uremic syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMA, thrombotic microangiopathy; TPE, therapeutic plasma exchange.
Disclosure
All the authors declared no competing interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Acknowledgments
The authors thank Professor Yves Pirson for his precious advice; Professor Karin Dahan for genetic identification; Patrick Stordeur, PhD, for complement pathway investigation; and Vanessa Arcolia, PhD, for proofreading and technical support.
Footnotes
Supplementary References
Supplementary Material
Supplementary References
References
- 1.Ville S., Le Bot S., Chapelet-Debout A. Atypical HUS relapse triggered by COVID-19. Kidney Int. 2021;99:267–268. doi: 10.1016/j.kint.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Karoui K., Hill G., Karras A. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol. 2012;23:137–148. doi: 10.1681/ASN.2010111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merrill J., Erkan D., Winakur J., James J. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16:581–589. doi: 10.1038/s41584-020-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Sahu K., Cerny J. Coagulopathy, endothelial dysfunction, thrombotic microangiopathy and complement activation: potential role of complement system inhibition in COVID-19. J Thromb Thrombolysis. 2020;15:1–6. doi: 10.1007/s11239-020-02297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavriilaki E., Brodsky R. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189:222–265. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 7.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–237. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhaveri K., Meir L., Chang B. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98:509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.