Graphical abstract
Keywords: SARS-CoV-2, Endocytosis, Endosomal acidification, Lipid rafts, Cholesterol
Abstract
Coronavirus disease 2019 is a kind of viral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, the mechanism whereby SARS-CoV-2 invades host cells remains poorly understood. Here we used SARS-CoV-2 pseudoviruses to infect human angiotensin-converting enzyme 2 (ACE2) expressing HEK293T cells and evaluated virus infection. We confirmed that SARS-CoV-2 entry was dependent on ACE2 and sensitive to pH of endosome/lysosome in HEK293T cells. The infection of SARS-CoV-2 pseudoviruses is independent of dynamin, clathrin, caveolin and endophilin A2, as well as macropinocytosis. Instead, we found that the infection of SARS-CoV-2 pseudoviruses was cholesterol-rich lipid raft dependent. Cholesterol depletion of cell membranes with methyl-β-cyclodextrin resulted in reduction of pseudovirus infection. The infection of SARS-CoV-2 pseudoviruses resumed with cholesterol supplementation. Together, cholesterol-rich lipid rafts, and endosomal acidification, are key steps of SARS-CoV-2 required for infection of host cells. Therefore, our finding expands the understanding of SARS-CoV-2 entry mechanism and provides a new anti-SARS-CoV-2 strategy.
1. Introduction
Coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly increased in epidemic scale of the world since its initial outbreak during the winter of 2019 [1], [2]. Up to march 2021 the number of confirmed cases worldwide exceeds 100 million, with more than 2.15 million deaths. This pandemic is of international concern and subject to intense investigations and many pathological features of COVID-19 have yet to be characterized.
To enter the host cells, viruses specifically interact with a membrane component and subsequently integrate in the cell membrane to release the genome into the cytosol, or alternatively take advantage of the endocytic pathway [3], [4]. The cellular entry of SARS-CoV-2 has been shown to be facilitated by the serine protease transmembrane protease serine 2 (TMPRSS2) for spike (S) protein priming [5]. Moreover, Hoffmann et al. [5] and Zhu et al. [6] found that SARS-CoV-2 preferentially enters cells through the plasma membrane fusion pathway in Calu-3 cells expressing the TMPRSS2, whereas SARS-CoV-2 enters via endosomal pathway in HEK293T and Vero cells that express no or minimal TMPRSS2. In the endocytic pathway, the virus activation is driven by successful internalization, proteolysis and processing in endosomes/lysosomes, a process typically enhanced by an acidic endosomal pH [7]. Angiotensin-converting enzyme 2 (ACE2) has been reported as the cellular receptor for both SARS-CoV-1 and SARS-CoV-2 [8], [9], [10]. However, we still lack the detailed understanding of the cell endocytic process of how SARS‐CoV‐2 enters the host cells after binding to the ACE2 receptor.
Viruses typically make use of various endocytic mechanisms physiologically directed to promote the absorption of fluids, solutes, and small particles. Briefly, the endocytosis pathways could be divided into dynamin-dependent pathways such as clathrin-mediated endocytosis (CME), caveolin-mediated endocytosis (CaME), fast endophilin-mediated endocytosis (FEME), and dynamin-independent pathways including macropinocytosis and lipid rafts, all of which have been studied in the context of virus entry [3], [11]. A number of viruses including vesicular stomatitis viruses (VSV), Influenza A and Semliki forest take advantage of CME pathway to enter host cells [12], [13]. A number of adaptors, scission factors, cytoskeleton and regulatory factors participate in this process, such as dynamin 2, AP-2, Eps15, epsin1, actin, Rab5, Rab7, PI(3,4)P and PI(4,5)P2 [3], [14]. CaME is another important pathway for viruses to enter host cells and caveolin1 is necessary (and perhaps sufficient) for caveolar biogenesis. Simian virus 40 (SV40) has been widely studied and proposed to enter cells by CaME [15], [16], [17]. Recently, it was reported that SV40 particles enter the cell by binding to the carbohydrate moiety of the GM1 ganglioside in the plasma membrane of the host cell [18]. CaME is dynamin 2-dependent and is closely related to actin, Rab5, tyrosine kinases, phosphatases and protein kinase C (PKC) [3], [19]. In 2015, Boucrot et al. found a new endocytic route called FEME, which is mediated by endophilin and independent of clathrin and AP-2. FEME can be blocked by inhibitors of dynamin, Rac, phosphatidylinositol-3-OH kinase, PAK1 and actin polymerization, and be activated upon CDC42 inhibition [20]. Endophilin A2 has also been found to be associated with enterovirus 71 (EV71) entry [21], exocytosis of neurosecretory vesicles [22], and early uptake structures that are induced by the bacterial Shiga and cholera toxins [23].
Importantly, SARS-CoV entry into host cells has been shown to occur through a novel clathrin- and caveolin-independent endocytic pathway, suggesting an involvement of dynamin-independent pathways [7]. Macropinocytosis, also known as cell drinking, is characterized by the formation of large (greater than200 nm in diameter) and ruffled protrusions of the cell membrane, driven by actin rearrangements, which is primarily mediating non-selective internalization of fluid and membrane [24]. Vaccina virus mature virion (VV MV), adenovirus 3 (Ad3), coxsackievirus B (CVB), herpes simplex virus 1 (HSV1), echovirus 1 (EV1) and Zaire ebolavirus (ZEBOV) were found to be depended on macropinocytosis for its internalization and infection [24], [25], [26], [27], [28]. Macropinocytosis is characterized by the involvement of cell signaling factors PAK1, PI(3)K, PKC, Akt, PKC, PLCc, Rab34 and PLC-A2 that act to promote membrane ruffling by stimulating actin remodeling through CDC42 and Rac [24]. Lipid rafts are liquid-ordered membranes, enriched in cholesterol and sphingolipids, involved in biosynthetic transport, endocytosis and signal transduction. Viruses are also believed to take advantage of lipid rafts for entry into their cell host, assembly, and budding [29]. For several viruses, membrane rafts have been suggested to be involved in the mediation of the entry of enveloped viruses, including influenza virus, Sindbis virus (SIN), human immunodeficiency virus (HIV), murine leukemia virus (MLV), measles virus, Ebola virus and SARS-CoV [29], [30], [31], [32], [33], [34]. In addition, other endocytic pathways such as IL-2, ADP-ribosylation factor 6 (Arf6) and flotillin-dependent pathways have also been studied regarding their importance for viral entry [3], [35], [36]. However, the exact pathway for the entry to the host cells of SARS-CoV-2 is still unclear.
As SARS-CoV-2 is highly contagious, studies on this virus must be handled in biosafety level 3 or 4 containment laboratories, which often hinders pathological research of the SARS-CoV-2 and development of vaccine. Pseudoviruses are deprived of critical gene sequences of importance for virulence, while envelopes of these viral particles could have similar conformational structures as wild-type viruses, making it feasible to be handled in biosafety level 2 laboratories [37]. Particularly, the artificially inserting reporter sequences of luciferase and green fluorescence protein (GFP) makes the pseudoviruses to be a powerful tool for studying early infection events in the life cycle of SARS-CoV-2. Here, we used SARS-CoV-2 spike-bearing pseudoviruses to infect HEK293T-ACE2hi cells, and characterized the contribution of each endocytic pathway to the entry and infection of pseudoviruses by quantitative analysis of luminescence and fluorescence intensity. The cholesterol-rich lipid rafts and endosomal acidification dependence of SARS-CoV-2 infection was finally clarified. This study provides novel information regarding the entry of SARS-CoV-2, which is likely to be useful in understanding the pathological characteristics of this virus, and also provide attractive therapeutic targets to block SARS-CoV-2 infections.
2. Materials and methods
2.1. Cells and culture
In order to examine the infection of SARS-CoV-2 pseudoviruses, we obtained an HEK293T cell line which stably and highly expressed human ACE2. HEK293T cells were from ATCC and HEK293T-ACE2hi cells were constructed by Genomeditech (Shanghai, China) [38]. Briefly, HEK293T-ACE2hi cells were produced by constructing hACE2 overexpression vector (pGMLV-PA6) and using lentivirus (pGMLV-CMV-H-ACE2-PGK-Puro) to infect the target cells to construct a stable cell line. HEK293T cells were cultured in DMEM high glucose medium (Hyclone, USA) containing 10% FBS (HyClone, USA) and 1% penicillin–streptomycin at 37 °C in a humidified atmosphere of 5% CO2. HEK293T-ACE2hi cells were maintained in DMEM with 10% FBS, 1% penicillin–streptomycin and 4 μg/ml puromycin at 37 °C in a humidified atmosphere of 5% CO2.
2.2. Inhibitors, antibodies, and reagents
Chloroquine, the purity of 98%, was from Macklin (Shanghai, China). Hydroxychloroquine, the purity of 98%, was provided by Energy Chemical, (Shanghai, China). Ammonium chloride (NH4Cl) and bafilomycin A1 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Chemical inhibitors used in the drug inhibition assays including dynasore (Cat. No. HY-15304), chlorpromazine hydrochloride (CPZ) (Cat. No. HY-B0407A), 5-(N-ethyl-N-isopropyl) amiloride (EIPA) (Cat. No. HY-101840), IPA-3 (Cat. No. HY-15663), NSC 23,766 trihydrochloride (Cat. No. HY-15723A), methyl-β-cyclodextrin (MβCD) (Cat. No. HY-101461), ML141 (Cat. No. HY-12755) were purchased from Med Chem Express (MCE, USA). Mouse anti-endophilin A2 monoclonal antibody (sc-365704) was purchased from Santa Cruz Biotechnology (CA, USA). Rabbit anti-Caveolin-1 polyclonal antibody (abs122936) was purchased from Absin Bioscience Inc (Shanghai, USA). Rabbit anti-GAPDH polyclonal antibody (bs-2188R) was purchased from BIOSS (Beijing, China). Lipo8000 Transfection Reagent was purchased from Beyotime Biotechnology (Shanghai China). Luciferase Assay System kit (Cat. No. E1500) was provided by Promega (USA). The SARS-CoV-2 spike-bearing pseudovirus were purchased from Sino Biological (Cat. No. PSV001) (Beijing, China) and Biodragon (Cat. No. BDAA0026) (Beijing, China). The pseudoviruses were produced in HEK293T cells with a retrovirus backbone, which membrane surface contains the S protein of SARS-CoV-2, and the RNA sequence of green fluorescent protein (zsGreen) and luciferase is packaged in its genome.
2.3. Cytotoxicity assay
Cell viability was evaluated using a cell counting kit 8 (CCK-8) (Bimake, USA) and following the manufacturer's instructions. Briefly, HEK293T-ACE2hi cells were seeded into 96‐well plates at a density of five thousand cells per well and then treated with different concentrations of chemical inhibitors for 24 h, and then 10 μl of CCK-8 solution was added to each well followed by an additional 2 h incubation at 37 °C. Relative cell viability was assessed by monitoring the absorption at 450 nm using a multiskan spectrum (Thermo, USA). The percentage of viable cells was calculated using the formula: [(ODTreated − ODBlank) / (ODControl − ODBlank)] × 100%. Each sample was assayed with four replicates per measurement, and each experiment was repeated three times.
2.4. Pseudovirus infection assay
The SARS-CoV-2 spike-bearing pseudovirus infection assays were conducted as described previously [38]. In brief, 2 × 104 of HEK293T-ACE2hi cells were seeded into white 96‐well plates. SARS-CoV-2 spike-bearing pseudoviruses (multiplicity of infection (MOI) = 10) were added into medium and incubated in a 37 °C incubator containing 5% CO2 for 4 h. Subsequently, cells were washed once with PBS and replaced by 200 μl of fresh DMEM. To test the pseudovirus infection, the luciferase luminescence was detected by a microplate reader (Thermo Electron Corporation) and the GFP in HEK293T-ACE2hi cells was observed with a fluorescence microscope (Nikon, Japan).
2.5. siRNA assay
siRNA against endophilin A2 (si-EA2) (5′-GCU GGA UGA UGA CUU CAA A-3′, 5′-UUU GAA GUC AUC AUC CAG C-3′) clathrin (si-clathrin) (5′-GAG GUC AAU UCU CUA CUG A-3′, 5′-UCA GUA GAG AAU UGA CCU C-3′), dynamin (si-dynamin) (5′-CCG CAG CCA GAA GGA UAU U-3′, 5′-UUU GAA GUC AUC AUC CAG C-3′) and caveolin-1 (si-CAV1) (5′-CCA GAA GGG ACA CAC AGU U-3′, 5′-AACUGUGUGUCCCUUCUGG-3′) were designed and produced by GenePharma (Shanghai, China), and nonspecific siRNA sequences were used as a control (si-NC). The siRNA was transfected into HEK293T-ACE2hi cells using Lipo8000 Transfection Reagent according to the manufacturer’s instructions. The protein levels of EA2 and caveolin 1 were tested by Western blotting to evaluate the silence efficiency before the subsequent assays were performed. For the infection assay, HEK293T-ACE2hi cells transfected with siRNA were incubated with SARS-CoV-2 spike-bearing pseudoviruses for 4 h, and then the unbound pseudovirus were removed by washing with PBS, and cells were incubated in fresh DMEM medium. The GFP fluorescence intensity and luciferase activity were detected at indicated time points.
2.6. Virus infection in the presence of inhibitors
HEK293T-ACE2hi cells were pre-treated with different concentrations of inhibitors (chloroquine, hydroxychloroquine, NH4Cl, bafilomycin A1, dynasore, CPZ, EIPA, NSC 23,766 trihydrochloride, IPA-3, MβCD or ML141) for 1 h and then incubated with SARS-CoV-2 spike-bearing pseudoviruses at 37 °C in a humidified atmosphere of 5% CO2 for 4 h. In these total 5 h, the inhibitors were present in the medium. Subsequently, the unbound pseudoviruses were removed by washing with PBS, and cells were incubated in fresh DMEM medium. At different time points, the GFP fluorescence intensity and luciferase activity were detected.
2.7. Cholesterol supplement
For cholesterol supplement experiments, cells, firstly pre-treated with MβCD (2.5 mM) for 1 h, were added with different concentration of cholesterol for 1 h, followed by the incubation of SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) for 4 h. In addition, cells were directly treated with different concentration of cholesterol for 1 h and incubated with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) for 4 h.
2.8. Protein extraction and Western blotting
Total protein lysates from cells were extracted by using the RIPA solution (Beyotime, China) with a cocktail of protease and phosphatase inhibitors (Roche), followed with incubation on ice for 30 min and centrifugation at 12,000 × g for 15 min at 4 °C. The final protein concentration of each sample was determined by a BCA kit (Thermo Scientific).
The protein expression level was detected by Western blotting analysis. The supernatants (20 μg total protein) from protein lysates were subjected to SDS-PAGE gel according to standard procedures in Bio-Rad system. Mouse anti-endophilin A2 antibody, rabbit anti-Caveolin-1and rabbit anti-GAPDH were used as the primary antibody. The signal was further detected by using the secondary antibody of goat anti-rabbit IgG or goat anti-mouse IgG. The intensity of specific binding bands was calculated against the endogenous control (GAPDH), and data were showed as fold change against the control.
2.9. Statistical analysis
Quantitative data were expressed as mean ± SEM. The statistical analysis of differences between experimental groups was performed using One-way ANOVA or Student's t-test. P value < 0.05 was considered significant.
3. Results
3.1. High expression of human ACE2 facilitates the infection of SARS-CoV-2 spike-bearing pseudoviruses
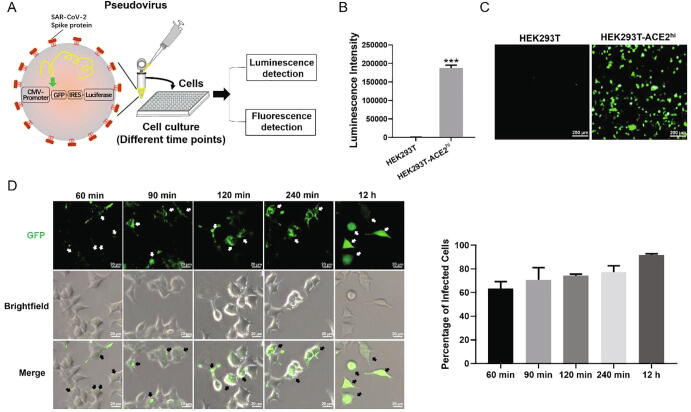
In this study, SARS-CoV-2 spike-bearing pseudoviruses were used to investigate the virus infection. They contain the sequences of GFP and luciferase (Fig. 1A), so that successful infection can be easily quantified by detecting the fluorescence intensity of GFP or luminescent intensity of luciferase, even with minute amounts of pseudoviruses. It has been shown that SARS-CoV-2 uses its spike protein binding ACE2 receptors to invade host cells [39]. We used SARS-CoV-2 pseudoviruses to infect HEK293T cells and HEK293T-ACE2hi cells. The luminescence intensity showing the luciferase activity was detected after 48 h incubation. It was found that the SARS-CoV-2 pseudoviruses only infected HEK293T-ACE2hi cells and rarely entered to HEK293T cells (Fig. 1B and C). After incubation with pseudoviruses for 60 min, GFP expression could be observed in HEK293T-ACE2hi cells and fluorescence intensity gradually increases with time. GFP expression was observed in the whole cell at 12 h post-infection with the pseudoviruses (Fig. 1D). The percentage of infected cells (with GFP expression) were also analyzed, and the result showed that it increased from 63% in 60 min to 91% in 12 h (Fig. 1D).
Fig. 1.
SARS-CoV-2 spike-bearing pseudovirus entering cells is ACE2-dependent. (A) Schematic of SARS-CoV-2 spike-bearing pseudovirus infection and detection. GFP: green fluorescent protein; IRES: internal ribosome entry site. (B) HEK293T (n = 3) and HEK293T-ACE2hi cells (n = 3) were infected with SARS-CoV-2 spike-bearing pseudoviruses at MOI of 10. The unbound pseudoviruses were washed out at 4 h post-infection and the chemiluminescence was detected by a microplate reader at 48 h post-infection. (C) Fluorescence images are shown of HEK293T and HEK293T-ACE2hi cells at 48 h post-infection (MOI = 10). Scale bar indicates 200 μm. (D) HEK293T-ACE2hi cells were continuously infected with SARS-CoV-2 spike-bearing pseudovirus (MOI = 10) for 1 h, 1.5 h, 2 h, 4 h and 12 h, and live cells were visualized by fluorescent microscopy to locate the GFP protein. Scale bar indicates 20 μm. Statistical data is presented as the mean ± SD of three independent experiments with repeated three times. *** P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. SARS-CoV-2 spike-bearing pseudoviruses are internalized through endocytosis sensitive to pH
The established view on the endocytic pathway of viruses is that it is pH-dependent and involve an activation step of the endocytosed virions in the acidic endosome. The SARS-CoV enter cells via endocytosis based on observations using ACE2-GFP and chemical inhibitors for endosomal acidification [7]. To analyze the pathway of SARS-CoV-2 spike-bearing pseudovirus infection, lysosomotropic weak bases (such as chloroquine, hydroxychloroquine and ammonium chloride (NH4Cl)) and a v-ATPase inhibitor (bafilomycin A1) were used to confirm that the viruses require low pH for infection [3]. All inhibitors used were tested on HEK293T-ACE2hi cells by cytotoxicity assays to obtain the proper working concentration range (Fig. S1A-D). As shown in Fig. 2A-D, pretreatment with different concentrations of inhibitors for endosomal acidification all significantly decreased viral infectivity in a dose-dependent manner. After incubation of HEK293T-ACE2hi cells with pseudoviruses for 48 h, chloroquine (20 μM), hydroxychloroquine (20 μM), NH4Cl (50 mM) and bafilomycin A1 (100 nM) respectively caused 77% (Fig. 2A), 73% (Fig. 2B), 78% (Fig. 2C) and 99% (Fig. 2D) reduction in pseudovirus infection by detecting the luciferase luminescence value. We found that green fluorescence was seen in whole cells infected with SARS-CoV-2 spike-bearing pseudoviruses, while the green fluorescence intensity in cells pretreated with different endosomal acidification inhibitors was significantly reduced at 12 h post-infection with the pseudoviruses (Fig. 2E). These results demonstrate that the infection of SARS-CoV-2 spike-bearing pseudoviruses is via endocytosis and pH-dependent.
Fig. 2.
Inhibition of endosomal acidification prevents SARS-CoV-2 spike-bearing pseudovirus infection. (A–D) HEK293T-ACE2hi cells were pre-treated with different doses of chloroquine (n = 3) (A), hydroxychloroquine (n = 3) (B), NH4Cl (n = 3) (C) and bafilomycin A1 (n = 3) (D) then infected with SARS-CoV-2 spike-bearing pseudoviruses at MOI of 10. The pseudoviruses infectivity was analyzed by luciferase activity at 48 h post-infection. (E) Fluorescence pictures and brightfield are shown of control, chloroquine (20 μM), hydroxychloroquine (20 μM), NH4Cl (50 mM) and bafilomycin A1 (100 nM) at 12 h post-infection. Scale bar indicates 20 μm. Statistical data is presented as the mean ± SD of three independent experiments with repeated three times. ** P < 0.01, *** P < 0.001.
3.3. Dynamin related endocytic pathways are not involved in SARS-CoV-2 spike-bearing pseudovirus infection
CME, CaME, as well as FEME, are the most studied pathways to be involved in virus internalization [3], [20]. To identify the specific endocytic pathway of SARS-CoV-2, siRNA and biochemical inhibitors targeting each endocytic pathway were used to evaluate the effect on SARS-CoV-2 spike-bearing pseudovirus infection. Our results indicated that the clathrin-, caveolar-, and endophilin A2-dependent pathway are not likely to be involved in the SARS-CoV-2 spike-bearing pseudovirus infection. Chlorpromazine (CPZ) is a drug commonly used to inhibit clathrin-mediated endocytosis and prevent the assembly of clathrin coating pits on the cell surface [40], [41]. We obtained the proper working concentration range of CPZ by cytotoxicity assays (Fig. S1E). Our result showed that CPZ pretreatment did not affect the luciferase and fluorescence signal compared with vehicle control treated cells at 24 h and 48 h post-infection with the SARS-CoV-2 spike-bearing pseudoviruses, while infection of VSV-G pseudoviruses as a positive control was inhibited by CPZ pretreatment (Fig. 3A and B, Fig. S2A). We also used RNAi method to explore the role of clathrin in SARS-CoV-2 spike-bearing pseudovirus infection. Silencing of clathrin showed similar results in luciferase luminescence, compared with the results using clathrin inhibitors (Fig. 3C). Next we used a siRNA approach to explore the role of CaME in SARS-CoV-2 spike-bearing pseudovirus infection. The silencing efficiency of cavelin-1 (CAV1) siRNA in HEK293T-ACE2hi cells was detected by Western blotting (Fig. 3D). Silencing of CAV1 failed to show any significant effect on infection of spike-bearing pseudoviruses by monitoring the luminescence intensity of luciferase (Fig. 3D). We next investigated whether FEME was related to SARS-CoV-2 spike-bearing pseudovirus infection by knocking down EA2 using its specific siRNA, and the silencing efficiency of EA2 siRNA was also detected by Western blotting (Fig. 3E). In line with silencing of CAV1, silencing of EA2 displayed no effect on spike-bearing pseudovirus infection in HEK293T-ACE2hi cells compared to the siNC control (Fig. 3E). This result probably suggested that EA2 might not contribute to the pseudovirus infection, however, we remain not to completely rule out the FEME pathway due to the compensation effect from the other endophilin isoforms.
Fig. 3.
SARS-CoV-2 spike-bearing pseudovirus infection of HEK293T-ACE2hi cells is independent of dynamin, clathrin, caveolin, and endophilin A2. (A) HEK293T-ACE2hi cells were pre-treated with CPZ (25 μM) (n = 3) and infected with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) or the control pseudoviruses VSV-G (n = 3). The infection was then measured by firefly luciferase activity at 48 h post-infection. (B) Fluorescence images of cells are shown of control and pretreatment with CPZ (25 μM) at 48 h post-infection with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10). Scale bar indicates 200 μm. (C-E) HEK293T-ACE2hi cells were transfected with si-Clathrin (C) si-CAV1 (D) or si-EA2 (E) and then infected with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10), and infectivity of with si-Clathrin (n = 3) (C) si-CAV1 (n = 3) (D) or si-EA2 (n = 3) (E) was measured by firefly luciferase activity at 48 h post-infection. (G, H) HEK293T-ACE2hi cells were pre-treated with dynasore (80 μM) (n = 3), and infected with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) or the control pseudoviruses VSV-G (n = 3). The infection efficiency was evaluated as above mentioned. (H) HEK293T-ACE2hi cells were transfected with si-Dynamin and then infected with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) or VSV-G pseudoviruses (MOI = 10), and infectivity was measured by firefly luciferase activity at 48 h post-infection (n = 3). Scale bar indicates 200 μm. Statistical data is presented as the mean ± SD of three independent experiments with repeated three times. *** P < 0.001.
Dynamin is a cellular GTPase and plays an essential role in some modes of endocytosis, including CME, FEME, CaME as well as some of the non-clathrin/non-caveolin-dependent pathways [42]. Accordingly, viruses may use either dynamin-dependent or -independent modes of endocytosis or a combination of the two [43]. Our above results indicated that dynamin-related pathways are not likely to be involved in the entry of SARS-CoV-2. To double confirm our finding we used dynasore, an effective endocytic pathway inhibitor that could inhibit dynamin activity by rapidly blocking coated vesicle formation within seconds of dynasore addition [42]. The proper working concentration range of dynasore was obtained by cytotoxicity assays in HEK293T-ACE2hi cells (Fig. S1F). Results showed that pretreatment of dynasore at different concentrations did not affect SARS-CoV-2 spike-bearing pseudovirus infectivity by detection of luciferase luminescence at 48 h post-infection with the pseudoviruses (Fig. 3F and Fig. S2B), while infection of VSV-G pseudoviruses as a positive control was inhibited by dynasore pretreatment. The representative fluorescence image also showed that dynasore had no significant effect on SARS-CoV-2 spike-bearing pseudovirus infection (Fig. 3G). Knocking down of dynamin using siRNA showed similar results in luciferase luminescence compared with the results using dynasore (Fig. 3H).
3.4. SARS-CoV-2 spike-bearing pseudoviruses are internalized without involvement of macropinocytosis
Then we further investigated the dynamin-independent pathways in virus infection. Macropinosome formation requires a variety of cellular factors activity, such as Na+/H+ exchange (NHE), PKC, p21-activated kinase 1 (PAK1) and Rho GTPase (CDC42 and RAC1) [24], [44], [45]. We assessed the role of macropinocytosis in SARS-CoV-2 spike-bearing pseudovirus infection using 5-(N-ethyl-N-isopropyl) amiloride (EIPA, an inhibitor of NHE) [46], NSC 23,766 trihydrochloride (an inhibitor of RAC1) [47], IPA-3 (an inhibitor of PAK1) [48]. All inhibitors used were tested on HEK293T-ACE2hi cells by cytotoxicity assays to obtain the proper working concentration range (Fig. S1G-I). As shown in Fig. 4A, EIPA pretreatment decreased SARS-CoV-2 spike-bearing pseudovirus infectivity, only when the concentration of EIPA reaches 80 μM. IPA-3 pretreatment exhibited no statistically significant change on the percentage of pseudovirus infection efficiency (Fig. 4B). A dose-dependent increase of SARS-CoV-2 spike-bearing pseudovirus infection was observed in the presence of NSC 23,766 trihydrochloride (Fig. 4C). The result of GFP observed under a fluorescence microscope to evaluate pseudovirus infection is similar to the result of luciferase luminescence (Fig. 4D). In summary, these results indicate that macropinocytosis is likely not involved in the mediation of the infection of SARS-CoV-2 spike-bearing pseudoviruses.
Fig. 4.
SARS-CoV-2 spike-bearing pseudoviruses infects cells independent with macropinocytosis. (A–C) HEK293T-ACE2hi cells were pre-treated with different concentrations of EIPA (n = 3) (A), IPA-3 (n = 3) (B), and NSC 23,766 trihydrochloride (n = 3) (C), and then infected with SARS-CoV-2 spike-bearing pseudoviruses at MOI of 10. The pseudoviruses infectivity was analyzed by firefly luciferase activity at 48 h post-infection. (D) Fluorescence images are shown of control, EIPA (80 μM), IPA-3 (20 μM), and NSC 23,766 trihydrochloride (40 μM) at 24 h post-infection with SARS-CoV-2 spike-bearing pseudoviruses. Scale bar indicates 200 μm. Statistical data is presented as the mean ± SD of three independent experiments with repeated three times. * P < 0.05.
3.5. SARS-CoV-2 spike-bearing pseudoviruses invades host cells in dependence on cholesterol-rich lipid rafts
Cholesterol-rich lipid rafts have been found to play a crucial role in the interaction between the SARS-CoV S protein and ACE2 [34], [49]. To investigate the involvement of cholesterol-rich lipid rafts during the initial steps of SARS-CoV-2 infection, we used methyl-β-cyclodextrin (MβCD) to deplete cholesterol from HEK293T-ACE2hi cells. The proper working concentration range of MβCD was obtained by cytotoxicity assays in HEK293T-ACE2hi cells (Fig. S1J). MβCD, which captured cholesterol and thereby sequestered cholesterol from the plasma membrane, was able to block SARS-CoV-2 spike-bearing pseudovirus infection in a dose-dependent manner (Fig. 5A and B). When the concentration of MβCD pretreatment reached 2.5 mM, the inhibition efficiency exceeded 96% compared to vehicle control treatment cells at 48 h post-infection with the SARS-CoV-2 spike-bearing pseudoviruses. As negative control, the infection of VSV-G pseudoviruses was not affected by cholesterol depletion (Fig. 5A). HEK293T-ACE2hi cells were treated with 2.5 mM MβCD at different time points and inoculated with SARS-CoV-2 spike-bearing pseudoviruses for viral infection. As shown in Fig. 5C, when MβCD was pre-treated to cells (pre-2 h, pre-1 h, pre-0.5 h, 0 h), pseudovirus infection was significantly reduced by MβCD pretreatment; whereas MβCD treatment at 1 h post infection had no effect on viral infection. This indicated that cholesterol mainly affected the process before the virus entered the cells. The infection and infection of SARS-CoV-2 spike-bearing pseudoviruses could be restored by supplement of exogenous cholesterol. MβCD-treated HEK293T-ACE2hi cells with exogenous cholesterol (100 μg/ml) showed almost twice the total number of luciferase luminescence compared to cells treated with pseudoviruses only. (Fig. 5D and E). In addition, we also tested the effect of supplementing exogenous cholesterol on pseudovirus infection, and found that different concentrations of cholesterol all increased pseudovirus infection (Fig. 5F). Hence, it demonstrates that the anti-SARS-CoV-2 spike-bearing pseudovirus infection of MβCD is dependent on cholesterol depletion. The clathrin-independent carriers (CLIC) and GPI-anchored-protein-enriched endosomal compartments (GEEC) pathway is an important endocytic pathway for cholesterol-rich lipid raft components [14], [50]. The CLIC/GEEC pathway has been demonstrated to be regulated by GRAF1, CDC42 and Arf1 [51], [52], [53]. We tried to preliminarily assess the role of CLIC/GEEC pathway in SARS-CoV-2 spike-bearing pseudovirus infection using MLL141 (an inhibitor of CDC42) [54], [55]. The MLL141 was tested on HEK293T-ACE2hi cells by cytotoxicity assays to obtain the proper working concentration range (Fig. S1K). The result showed that MLL141 (10 μM) pretreatment exhibited no statistically significant change on the pseudovirus infection (Fig. 5G), suggesting that CDC42-involved CLIC/GEEC pathway probably might not participate in the infection of SARS-CoV-2.
Fig. 5.
The infection of SARS-CoV-2 spike-bearing pseudoviruses is cholesterol dependent. (A) HEK293T-ACE2hi cells were pre-treated with MβCD at different concentrations (n = 3), and then infected with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) or the control pseudoviruses VSV-G (n = 3). The infection was measured by firefly luciferase activity at 48 h post-infection. (B) Fluorescence and brightfield images are shown of control and pretreatment with different concentrations of MβCD at 4 h post-infection. Scale bar indicates 200 μm. (C) Time frame of MβCD treatment. HEK293T-ACE2hi cells were treated with 2.5 mM MβCD at indicated time points and infected with SARS-CoV-2 spike-bearing pseudoviruses at MOI of 10. The infectivity was analyzed by firefly luciferase activity at 48 h post-infection. (D) HEK293T-ACE2hi cells were pre-treated with MβCD for 1 h, and then different concentrations of supplement of exogenous cholesterol were added for 1 h, followed by the incubation of SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) for 4 h. The infectivity was analyzed by firefly luciferase activity at 48 h post-infection. (E) Fluorescence and brightfield images are shown of control, pretreatment with MβCD and MβCD + cholesterol at 48 h post-infection with pseudoviruses. Scale bar indicates 200 μm. (F) HEK293T-ACE2hi cells were pre-treated with cholesterol at different concentrations (n = 4) and then infected with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10). (G) HEK293T-ACE2hi cells were pre-treated with ML141 (10 μM) (n = 3) for 1 h and infected with SARS-CoV-2 spike-bearing pseudoviruses (MOI = 10) for 4 h. The infection was then measured by firefly luciferase activity at 24 h post-infection. Statistical data is presented as the mean ± SD of three independent experiments with repeated three times. ** P < 0.01, *** P < 0.001.
4. Discussion
The SARS-CoV-2 virus infection initiated with binding of viral particles to host surface ACE2 receptors, is a crucial step for effective viral infection. Using SARS-CoV-2 pseudoviruses we could show that cholesterol-rich membrane lipid raft plays a major role in the infection and not the classical endocytosis pathways, such as clathrin-, caveolin-, endophilinA2-mediated endocytosis and macropinocytosis.
SARS-CoV-2 is a positive-stranded RNA virus belonging to the β coronavirus genus. The structure of this new coronavirus is mainly composed of single-stranded RNA, membrane (M), nucleocapsid (N), envelope (E), and S proteins [56]. The N protein, one of the most abundant viral proteins, combines with viral genomic RNA to form a ribonucleoprotein (RNP) complex [57]. It showed that N protein mainly involved in viral mRNA transcription and replication and cytoskeletal and immune regulation of host cells [58]. The M protein of SARS-CoV-2 can inhibit IFN-β promoter activation and participate in evading host anti-viral immunity [59]. E protein relates to the virus pathogenicity and may activate the host's inflammatory response. In some coronaviruses, the E protein deletion will reduce the virus's toxicity [60]. S protein directly binds to the host cell membrane receptor to mediate SARS-CoV-2 entry [61]. Pseudoviruses are useful virological tools because of their safety and versatility, and the pseudovirus expressing S-protein has been shown to be used to study the mechanism of SARS-CoV-2 entry [5], [62], [63] and neutralization assays [64].
TMPRSS2 have been shown to cleave SARS-CoV-2 S protein and enhance membrane fusion [6], [65]. The camostat mesylate (an inhibitor of TMPRSS2) can partially block SARS-CoV-2 infection of the Caco-2 cells (TMPRSS2+), while it did not interfere with SARS-CoV-2 infection of the Vero and HEK293T cells (TMPRSS2−) [5], [65], [66]. In contrast, the entry of SARS-CoV-2 mainly depends on the endosomal pathway in Vero and HEK293T cells that do not express TMPRSS2, and can be inhibited by E-64d (cathepsins B/L inhibitor) [5], [66]. Consistent with these findings, Zhu et al [6] showed that SARS-CoV-2 entered cells via two pathways. One pathway was the membrane fusion pathway that relied on proteases (e.g., TMPRSS2), and the other pathway was the endosomal pathway, which is less efficient than the plasma membrane fusion pathway. In the present study, we focused on the cell endosomal pathways, which remained poorly understood by now, using the SARS-CoV-2 spike-bearing pseudoviruses and HEK293T cell line (TMPRSS2−) as research tools.
Previous studies have shown that SARS-CoV S protein and its interaction with the cell receptor ACE2 is essential for SARS-CoV entry and membrane fusion [67], [68]. Recent studies also highlighted the important role of ACE2 in mediating the SARS-CoV-2 entry [69], [70]. Compared with SARS-CoV, the ACE2 receptor binding domain of SARS-CoV-2 differs on several key amino acid residues which leads to a stronger binding affinity with receptor and may account for the greater pathogenicity of SARS-CoV-2 [56], [71]. In this study, we also confirmed the important role of ACE2 in SARS-CoV-2 infection.
The chloroquine and hydroxychloroquine have been demonstrated to inhibit SARS-CoV-2 effectively in vitro [38], [72]. Howerve, some subsequent studies have found that chloroquine targets the viral activation which is not actived in lung cells and is unlikely to prevent the infection of SARS-CoV-2 in related patients, while the use of chloroquine was associated with an increased risk of denovo ventricular arrhythmia during hospitalisation [73], [74]. We found that NH4Cl and bafilomycin A1 also had inhibitory effect to SARS-CoV-2 pseudovirus infection as chloroquine, and bafilomycin A1 had a better inhibitory effect than chloroquine in HEK293T-ACE2hi cells. Our data provide additional information that inhibition of endosome acidification can be used for anti-SARS-CoV-2 infections drug design.
Lipid rafts represent foci for mediating signal transduction and protein trafficking and increasing evidence shows that lipid rafts are essential in the replication cycle of different viruses [29], [75]. Biochemical fractionation and confocal imaging confirmed that ACE2 colocalized with lipid raft marker ganglioside monosialotetrahexosylganglioside1 (GM1) and upon cholesterol depletion by MβCD treatment inhibited infectivity of SARS-CoV pseudoviruses by 90% [34]. Cholesterol depletion with MβCD inhibited the infectivity and the receptor binding ability of Newcastle disease virus (NDV) [76]. Cholesterol-rich lipid rafts are required for release of infectious human respiratory syncytial virus (RSV) [77]. Strong data also suggest that virus budding can occur within membrane lipid rafts [3], [29], [31]. It has been reported that the budding of HIV virions through lipid rafts whereby host cell cholesterol, sphingolipids, and GPI-linked proteins within these domains are incorporated into the viral envelope [78]. Host lipid rafts play a critical role in binding, endocytosis, assembly and budding of influenza A virus (IAV) [79].
Therefore, a role for cholesterol has attracted increasing attention for the understanding of the SARS-CoV-2 infection process [62], [80], [81]. It has been reported that cholesterol 25‐hydroxylase (CH25H) inhibits SARS‐CoV‐2 and other coronaviruses by depleting membrane cholesterol in vitro and in COVID‐19‐infected patients [62]. Observational studies on COVID-19 individuals with underlying cardiovascular disease (CVD) showed that they increased the severity of the disease and mortality [82]. The Chinese Center for Disease Control and Prevention published the case series to date of COVID-19 in mainland China (72,314 cases, updated through February 11, 2020), with an overall case-fatality rate (CFR) was 2.3%. Importantly, the CFR with underlying CVD patients was the highest among those with preexisting comorbid conditions, increased to 10.5% (7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer) [83]. Statins are lipid-lowering therapeutics with favorable anti-inflammatory profiles and have been proposed as an adjunct therapy for COVID-19 [84], [85]. The retrospective study on 13,981 patients with COVID-19 in Hubei Province, China, among which 1,219 received statins showed that the risk for 28-day all-cause mortality was 5.2% and 9.4% in the matched statin and non-statin groups respectively [84]. Combined with the results of this study, statins may inhibit SARS-CoV-2 infections by lowering cholesterol. In this study, we proved that SARS-CoV-2 spike-bearing pseudovirus infection are associated with cholesterol-rich lipid rafts. Meanwhile, we preliminarily studied the CLIC/GEEC pathway, which is an important lipid raft-related endocytic pathway. Adeno-associated virus 2 (AAV2) infection had been shown to require endocytosis through the CLIC/GEEC pathway [86]. The infection of AAV2 is dependent on membrane cholesterol and can be blocked by inhibition of the three main effectors of CLIC/GEEC pathway, GRAF1, CDC42 and Arf1 [86]. However, our result showed that inhibition of CDC42 had no effect on SARS-CoV-2 spike-bearing pseudovirus infection. Therefore, it remains not clear how cholesterol-rich lipid raft regulates SARS-CoV-2 binding or entry, which need to be studied further.
In conclusion, the present study provides evidence that the infection of SARS-CoV-2 spike-bearing pseudoviruses to HEK293T-ACE2hi cells is via ACE2 receptor-dependent, pH-sensitive endocytosis and can be blocked by inhibitors for endosomal acidification. Moreover, classical endocytosis pathways, such as clathrin-, caveolin-, endophilinA2-mediated endocytosis and macropinocytosis are not involved in SARS-CoV-2 spike-bearing pseudovirus infection. Instead, cholesterol-rich membrane lipid raft may play a major role in the infection of SARS-CoV-2 spike-bearing pseudoviruses to HEK293T-ACE2hi cells (Fig. 6).
Fig. 6.
Graphical summary of SARS-CoV-2 spike-bearing pseudovirus infection.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Funding: The work was supported by grants from the National Natural Science Foundation of China (81671629, 81970029, 81930096 and 32070913), Shaanxi Province Natural Science Foundation, China (2020JQ082, 2021JG-393 and 2018JM7057), the Fundamental Research Funds for the Central Universities, China (xzy032020042 and xjj2018159), and Qinnong Bank-XJTU special project for COVID-19, China (qnxjtu-12).
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Author contributions: All authors contributed to the discussion, writing, and revising of the manuscript. X. L. and W.Z. designed the research, performed the experiments, including acquiring data and analyzing data, and wrote the manuscript. M. F. performed siRNA assay. J. Z. helped with cytotoxicity assay, analyzed the data, and revised the manuscript. Y. P. and F. H. performed some pseudovirus infection assays and analyzed the data. R.H. designed the research and revised the manuscript. L.M. and S.L. designed the research, analyzed the data, revised the manuscript, supervised and took the overall responsibility of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2021.04.001.
Contributor Information
Liesu Meng, Email: mengliesu@xjtu.edu.cn.
Shemin Lu, Email: lushemin@xjtu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrock E., Fujimura E., Kula T. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020 doi: 10.1126/science.abd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercer J., Schelhaas M., Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 4.Smith A.E., Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181. 2020:271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y., Feng F., Hu G. A genome-wide CRISPR screen identifies host factors that regulate SARS-CoV-2 entry. Nat Commun. 2021;12:961. doi: 10.1038/s41467-021-21213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., Yang P., Liu K. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan R., Zhang Y., Li Y. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteil V., Kwon H., Prado P. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 181. 2020:905–913 e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennighausen L., Lee H.K. Activation of the SARS-CoV-2 receptor Ace2 through JAK/STAT-dependent enhancers during pregnancy. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandvig K., Kavaliauskiene S., Skotland T. Clathrin-independent endocytosis: an increasing degree of complexity. Histochem Cell Biol. 2018;150:107–118. doi: 10.1007/s00418-018-1678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelkmans L., Helenius A. Insider information: what viruses tell us about endocytosis. Curr Opin Cell Biol. 2003;15:414–422. doi: 10.1016/s0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Oliveira C., Freire J.M., Conceicao T.M. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol Rev. 2015;39:155–170. doi: 10.1093/femsre/fuu004. [DOI] [PubMed] [Google Scholar]
- 14.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 15.Anderson H.A., Chen Y., Norkin L.C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelkmans L., Puntener D., Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 17.Pelkmans L., Kartenbeck J., Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 18.Ewers H, Romer W, Smith AE, et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat Cell Biol 12; 2010: 11-18; sup pp 11-12. https://doi.org/10.1038/ncb1999. [DOI] [PubMed]
- 19.Matveev S., Li X., Everson W. The role of caveolae and caveolin in vesicle-dependent and vesicle-independent trafficking. Adv Drug Deliv Rev. 2001;49:237–250. doi: 10.1016/s0169-409x(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 20.Boucrot E., Ferreira A.P., Almeida-Souza L. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517:460–465. doi: 10.1038/nature14067. [DOI] [PubMed] [Google Scholar]
- 21.Chen S.L., Liu Y.G., Zhou Y.T. Endophilin-A2-mediated endocytic pathway is critical for enterovirus 71 entry into caco-2 cells. Emerg Microbes Infect. 2019;8:773–786. doi: 10.1080/22221751.2019.1618686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gowrisankaran S., Houy S., Del Castillo J.G.P. Endophilin-A coordinates priming and fusion of neurosecretory vesicles via intersectin. Nat Commun. 2020;11:1266. doi: 10.1038/s41467-020-14993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renard H.F., Simunovic M., Lemiere J. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015;517:493–496. doi: 10.1038/nature14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercer J., Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 25.Liberali P., Kakkonen E., Turacchio G. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 2008;27:970–981. doi: 10.1038/emboj.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amstutz B., Gastaldelli M., Kalin S. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008;27:956–969. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicola A.V., Hou J., Major E.O. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeed M.F., Kolokoltsov A.A., Albrecht T. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chazal N, Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev 67; 2003: 226-237, table of contents. https://doi.org/10.1128/mmbr.67.2.226-237.2003. [DOI] [PMC free article] [PubMed]
- 30.Manes S., del Real G., Martinez A.C. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 31.Bavari S., Bosio C.M., Wiegand E. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manie S.N., de Breyne S., Vincent S. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J Virol. 2000;74:305–311. doi: 10.1128/jvi.74.1.305-311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y.E., Cassese T., Kielian M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J Virol. 1999;73:4272–4278. doi: 10.1128/JVI.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem Biophys Res Commun. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raff A.B., Woodham A.W., Raff L.M. The evolving field of human papillomavirus receptor research: a review of binding and entry. J Virol. 2013;87:6062–6072. doi: 10.1128/JVI.00330-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandenburg B., Lee L.Y., Lakadamyali M. Imaging poliovirus entry in live cells. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q., Liu Q., Huang W. Current status on the development of pseudoviruses for enveloped viruses. Rev Med Virol. 2018;28 doi: 10.1002/rmv.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N., Han S., Liu R. Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus. Phytomedicine. 2020;79 doi: 10.1016/j.phymed.2020.153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosoki K., Chakraborty A., Sur S. Molecular mechanisms and epidemiology of COVID-19 from an allergist's perspective. J Allergy Clin Immunol. 2020;146:285–299. doi: 10.1016/j.jaci.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Gou W., Kim D.S. Clathrin-mediated endocytosis of alpha-1 antitrypsin is essential for its protective function in islet cell survival. Theranostics. 2019;9:3940–3951. doi: 10.7150/thno.31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L.H., Rothberg K.G., Anderson R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macia E., Ehrlich M., Massol R. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Harper C.B., Popoff M.R., McCluskey A. Targeting membrane trafficking in infection prophylaxis: dynamin inhibitors. Trends Cell Biol. 2013;23:90–101. doi: 10.1016/j.tcb.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Saeed M.F., Kolokoltsov A.A., Freiberg A.N. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S., Huang X., Huang Y. Entry of a novel marine DNA virus, Singapore grouper iridovirus, into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner. J Virol. 2014;88:13047–13063. doi: 10.1128/JVI.01744-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu B.Y., Shang B.Y., Du Y. A new HDAC inhibitor cinnamoylphenazine shows antitumor activity in association with intensive macropinocytosis. Oncotarget. 2017;8:14748–14758. doi: 10.18632/oncotarget.14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Lu Y.F., Li C.L. Involvement of Rac1 signalling pathway in the development and maintenance of acute inflammatory pain induced by bee venom injection. Br J Pharmacol. 2016;173:937–950. doi: 10.1111/bph.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viaud J., Peterson J.R. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther. 2009;8:2559–2565. doi: 10.1158/1535-7163.MCT-09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glende J., Schwegmann-Wessels C., Al-Falah M. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381:215–221. doi: 10.1016/j.virol.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferreira A.P.A., Boucrot E. Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 2018;28:188–200. doi: 10.1016/j.tcb.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Sabharanjak S., Sharma P., Parton R.G. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 52.Lundmark R., Doherty G.J., Howes M.T. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol. 2008;18:1802–1808. doi: 10.1016/j.cub.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumari S., Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10:30–41. doi: 10.1038/ncb1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong L., Kenney S.R., Phillips G.K. Characterization of a Cdc42 protein inhibitor and its use as a molecular probe. The Journal of biological chemistry. 2013;288:8531–8543. doi: 10.1074/jbc.M112.435941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanin G., Shenhar-Tsarfaty S., Yayon N. Competing targets of microRNA-608 affect anxiety and hypertension. Hum Mol Genet. 2014;23:4569–4580. doi: 10.1093/hmg/ddu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X., Geng M., Peng Y. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng Y., Du N., Lei Y. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design. EMBO J. 2020;39 doi: 10.15252/embj.2020105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ni L., Ye F., Cheng M.L. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977 e973. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Y., Zhuang M.W., Han L. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct Target Ther. 2020;5:299. doi: 10.1038/s41392-020-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mandala V.S., McKay M.J., Shcherbakov A.A. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat Struct Mol Biol. 2020;27:1202–1208. doi: 10.1038/s41594-020-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin (vol 579, pg 270, 2020), Nature 588; 2020: E6-E6. https://doi.org/10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed]
- 62.Wang S, Li W, Hui H, et al. Cholesterol 25-Hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol. EMBO J 2020; e106057. https://doi.org/10.15252/embj.2020106057. [DOI] [PMC free article] [PubMed]
- 63.Zang R., Case J.B., Yutuc E. Cholesterol 25-hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion. PNAS. 2020;117:32105–32113. doi: 10.1073/pnas.2012197117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nie J., Li Q., Wu J. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zang R., Gomez Castro M.F., McCune B.T. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffmann M., Kleine-Weber H., Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779–784 e775. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan Y., Cao D., Zhang Y. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song W., Gui M., Wang X. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lan J., Ge J., Yu J. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 70.Walls A.C., Park Y.J., Tortorici M.A. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gheblawi M., Wang K., Viveiros A. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffmann M., Mosbauer K., Hofmann-Winkler H. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585:588–590. doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 74.Mehra M.R., Desai S.S., Ruschitzka F. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Bukrinsky M.I., Mukhamedova N., Sviridov D. Lipid rafts and pathogens: the art of deception and exploitation. J Lipid Res. 2020;61:601–610. doi: 10.1194/jlr.TR119000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin J.J., Holguera J., Sanchez-Felipe L. Cholesterol dependence of Newcastle Disease Virus entry. Biochim Biophys Acta, Mol Cell Biol Lipids. 1818;2012:753–761. doi: 10.1016/j.bbamem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang T.H., Segovia J., Sabbah A. Cholesterol-rich lipid rafts are required for release of infectious human respiratory syncytial virus particles. Virology. 2012;422:205–213. doi: 10.1016/j.virol.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen D.H., Hildreth J.E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verma D.K., Gupta D., Lal S.K. Host lipid rafts play a major role in binding and endocytosis of influenza A virus. Viruses. 2018;10 doi: 10.3390/v10110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Radenkovic D., Chawla S., Pirro M. Cholesterol in relation to COVID-19: should we care about it? J Clin Med. 2020;9 doi: 10.3390/jcm9061909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bailly C., Vergoten G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Ther. 2020;214 doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 5; 2020: 811–818. https://doi.org/10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed]
- 83.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 323; 2020: 1239–1242. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 84.Zhang X.J., Qin J.J., Cheng X. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187 e174. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodrigues-Diez R.R., Tejera-Munoz A., Marquez-Exposito L. Statins: Could an old friend help in the fight against COVID-19? Br J Pharmacol. 2020 doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nonnenmacher M., Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10:563–576. doi: 10.1016/j.chom.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.