Abstract
Objective:
To compare antidepressant-related adverse events (AEs), suicidality and AE-related discontinuation in double-blind, placebo-controlled trials of pediatric patients with OCD and anxiety disorders treated with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs).
Method:
MEDLINE, PubMed, Web of Science, PsycINFO and Embase were searched for peer-reviewed, English-language articles from inception through March 1, 2019. We identified prospective, randomized, SSRI and SNRI studies in patients <18 years of age with OCD, generalized, separation or social anxiety disorders. AE rates were extracted and antidepressant-placebo differences examined using Bayesian hierarchical models (BHM). Then posterior estimates of relative risk (RR) were determined for each AE by medication class and disorder.
Results:
Data were included from 18 trials (2631 patients) and 7 medications (16 SSRI and 4 SNRI trials). Compared to placebo, SSRIs were associated with a greater likelihood of AE-related discontinuation (RR: 3.59, Credible Interval [CrI]: 0.019 to 0.067, p=0.0003), activation (RR: 2.39, CrI: 0.048 to 0.125, p=0.003), sedation (RR: 1.94, CrI: 0.035 to 0.157, p=0.002), insomnia (RR: 1.93, CrI: 0.040 to 0.149, p=0.001), abdominal pain (RR: 1.53, CrI: 0.032 to 0.164, p=0.005) and headache (RR: 1.24, CrI: 0.003 to 0.139, p=0.04). Activation was more common with SSRI (vs. SNRIs, RR: 1.32, CrI: 0.018 to 0.114, p=0.007). Neither SSRIs nor SNRIs were associated with treatment-emergent suicidality.
Conclusions:
In pediatric OCD and anxiety disorders, SSRIs as compared to placebo are associated with distinct adverse events (AEs) and greater AE-related discontinuation, although their tolerability does not differ between anxiety disorders and OCD. SSRIs are more likely than SNRIs to produce activation. Class-related AEs are important for clinicians to consider, particularly in light of data suggesting differences in class-related efficacy. While SSRIs are superior to SNRIs and the treatment of choice for anxiety, for youth who become activated on SSRIs, SNRIs might represent a good second choice given their reported efficacy and lower risk of activation
Keywords: selective serotonin reuptake inhibitor (SSRI, SRI); serotonin norepinephrine reuptake inhibitor (SNRI); separation anxiety disorder (SAD); generalized anxiety disorder (GAD); obsessive compulsive disorder (OCD); side effect; activation
For youth with anxiety and obsessive compulsive disorders (OCD), antidepressant medications improve symptoms and functional outcomes.1–5 Among antidepressants, the selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) supplanted other medication classes for the treatment of youth with anxiety disorders and OCD over the past two decades.6 Unlike in major depressive disorder, nearly all randomized controlled trials find SSRIs and SNRIs to be efficacious in pediatric patients with generalized, separation and social anxiety disorders as well as OCD.1,7–11 Extant meta-analyses of these medications in anxious youth, including those with OCD, also suggest that they are efficacious;2,12–14 although, recent studies raise the possibility that, for pediatric anxiety disorders, SSRIs may be more efficacious15,16 and produce faster improvement compared to SNRIs.15
Despite the abundance of clinical trial data and meta-analyses supporting the efficacy of SSRIs and SNRIs in pediatric anxiety14,16–18 and SSRIs in OCD,13,19 meta-analytic evaluations of antidepressant tolerability focus almost entirely on discontinuation or suicidality.14,16,20,21 To our knowledge, there is only one meta-analysis of specific AE risks in this population, although some meta-analyses suggest higher rates of treatment-emergent discontinuation for antidepressants compared to placebo.16,19 Meta-analyses of common treatment-related AEs—including headaches, nausea, abdominal pain, activation, sedation and weight gain1,22–26—are virtually non-existent in pediatric anxiety disorders and OCD. Although we have previously reported on activation, gastrointestinal symptoms and suicidality in a small meta-analysis of anxious youth treated with SSRIs and SNRIs, that study had several important limitations. Only 3 AEs were examined, SSRIs and SNRIs together and complete homogeneity was assumed across studies. Clinicians selecting antidepressant medications to treat pediatric patients need comparative tolerability data for specific AEs, particularly given that AEs increase the likelihood of discontinuation, slow the trajectory of improvement and potentially limit dose adjustment to effective doses.27,28 Comparative tolerability data are critical for child and adolescent psychiatrists and other mental health clinicians who are ethically and medicolegally compelled to discuss medication side effects/AEs with patients and their parents.29
The dearth of systematic evaluations of antidepressant-related adverse events in pediatric patients is not coincidental. Categorizing, capturing and quantifying these AEs in pediatric randomized controlled trials of SSRIs and SNRIs varies considerably and may be influenced by the population being studied (e.g., OCD, anxiety disorders), the age of the patients, the antidepressant class and dose and trial characteristics (e.g., dosing schedule, method of capturing/eliciting AEs). Modeling observed and unobserved heterogeneity across studies represents a formidable analytic challenge. Examining antidepressant trials separately may fail to incorporate data across trials.
Traditional approaches to examining AEs across treatments seldom leverage prior data30,31 (e.g., variability in AE rates within a single randomized controlled trial of a particular antidepressant class in youth, variability in AEs among clinical trials of youth with a specific disorder). Yet, this prior information may provide more accurate estimates of the true risk of AEs at each level of the hierarchy (e.g., disorder, medication class, maximum medication dose).30,32 Bayesian hierarchical modeling (BHM) uses a hierarchy (e.g. disorder, medication class) to estimate the probability of an event at each level of the heirarchy. Sub-models of the hierarchy are then combined with the observed data to account for uncertainty.33
Aggregating AE data from all available trials of SSRIs and SNRIs in pediatric anxiety disorders and OCD allows the AE rates and the impact of selected medication-specific and trial-specific variables to be evaluated with greater statistical power than can be accomplished in individual trials. With these considerations in mind, we conducted a meta-analysis of antidepressant-related adverse events using BHM and posterior updating from randomized, placebo-controlled trials of SSRIs and SNRIs for the treatment of generalized, social or separation anxiety disorders as well as OCD in children and adolescents. This approach allows us to evaluate AEs separately in patients with OCD or anxiety disorders and to evaluate these AEs conjointly.
We sought to examine specific AEs that are commonly reported in antidepressant-treated pediatric patients, including activation, gastrointestinal symptoms (e.g., nausea, abdominal pain), insomnia, headache, tiredness/sedation, as well as rare, but important associations (e.g., suicidality) and discontinuation secondary to adverse events. The objectives of this meta-analysis is to examine SSRI and SNRI-related AEs in pediatric trials of (1) anxiety disorders; (2) OCD; (3) both anxiety disorders and OCD and to compare SSRI- and SNRI-related AEs.
METHOD
Search Strategy
All meta-analytic methods and sensitivity analyses were specified before conducting the meta-analysis proper, although the meta-analysis was not pre-registered. The studies included were obtained through an electronic search of PubMed (1966 through March 1, 2019) in addition to the Cochrane Database, Web of Science, Embase and PsychInfo as well as the government clinical trials registry, www.clinicaltrials.gov using the search strategy (adolescent* OR children OR pediatric OR youth) AND (anxiety OR social phobia OR social anxiety disorder OR SAD OR generalized anxiety disorder OR GAD OR separation anxiety disorder OR obsessive compulsive disorder*) AND (selective serotonin reuptake inhibitor OR SSRI OR selective serotonin norepinephrine reuptake inhibitor OR SNRI OR selective serotonin norepinephrine reuptake inhibitor OR fluoxetine OR fluvoxamine OR citalopram OR escitalopram OR fluoxetine OR paroxetine OR venlafaxine OR desvenlafaxine OR duloxetine OR vortioxetine OR vilazodone). The results of the search were then manually limited to randomized, placebo-controlled trials. The references of all eligible trials and review articles were searched for additional clinical trials.
Criteria for Inclusion of Studies
Studies were included if they were prospective, randomized, parallel-group, placebo-controlled trials that evaluated SSRI or SNRIs in the treatment of social, generalized and/or separation anxiety disorder or OCD in children or adolescents and systematically captured adverse events. Exclusionary criteria were adapted from a recent meta-analysis of SSRIs in pediatric patients with major depressive disorder34 as well as the pediatric anxiety disorders.2,15 As such, clinical trials were excluded if they met the following criteria: included adults (age >18 years); used a cross-over design; did not study an SSRI or SSRI; were not randomized; were not placebo-controlled; provided adjunctive psychotherapy to active or control group; or included <10 patients per treatment group. The focus on OCD and anxiety disorders is largely related to the common psychopharmacologic treatment approaches, the similar ages of onset, common co-morbidity, although we recognize that the disorder could influence tolerability which is why we also evaluated the disorders separately. Specific phobia was not included given that behavioral therapies (e.g., exposure-based treatment) represent the preferred treatment29 and panic disorder was not included given the absence of prospective trials of SSRIs or SNRIs in pediatric patients with panic disorder. Trials of SSRIs or SNRIs in youth with major depressive disorder were not included as these trials have considerable methodologic variability and as the depressive pathology may influence specific symptoms (e.g., fatigue/anergia, hypersomnia).
Data Extraction
As previously described,35 data were extracted into an Excel (Microsoft, Redmond, WA) spreadsheet. Additional data related to the methods, demographics, duration of the trial, and other relevant aspects and results of the studies were collected (e.g., number of study sites, funding source). The individual AEs included abdominal pain, activation, diarrhea, nausea, insomnia, headache, sedation as well as discontinuation due to an adverse event. The rates of each of these AEs (or events in the case of suicidality and discontinuation secondary to an adverse event) from each included trial was determined relative to the intent-to-treat population randomized to medication or placebo. All AEs were extracted from the trials and then manually reviewed by the authors. Then, AEs (with the exception of activation) were classified using the Medical Dictionary for Regulatory Activities (MedDRA, v. 22.0, www.medra.org) system organ class and high level group terms and activation-cluster symptoms, as previously described49 to ensure consistency among the categorization of AEs. For each AE to be included in the meta-analysis, ≥2 trials from each antidepressant class were required to report results. Because of heterogeneity in reporting terminology and definitions of AEs, terms were pre-ranked (in order of preference) for specific AEs. For example “activation” consisted of activation then restlessness then impulsivity then irritability.22,36 For abdominal pain, “abdominal pain” represented the preferred AE term; however, if this was not reported then the frequency of “abdominal discomfort” was used and if neither was reported, the rate of “gastric distress” was used. Establishing this hierarchy of preferred terms for our AE analysis a priori decreases the likelihood of inflation and reporting bias and is consistent with approaches used in meta-analyses of the efficacy of these medications in pediatric anxiety disorders.16,35
Statistical Methods
A BHM was applied to evaluate AE risk to allow for heterogeneity across studies given that the incidence of AEs is related across studies (Figure 1). In BHMs, the degree of heterogeneity across studies is assumed to be unknown and estimated by the model. From the BHM posterior simulation samples, we determined the relative risk (RR) for each AE (or AE-related discontinuation) and report this relative risk with the 95% credible interval (CrI). Relative risk values greater than one indicate a greater risk for medication relative to placebo. A more thorough explanation of this approach and the MCMC estimation procedure for the BHM, including examples and executable code, is available at https://github.com/tszanalytics/Juliacon2019.33,37,38
Figure 1.
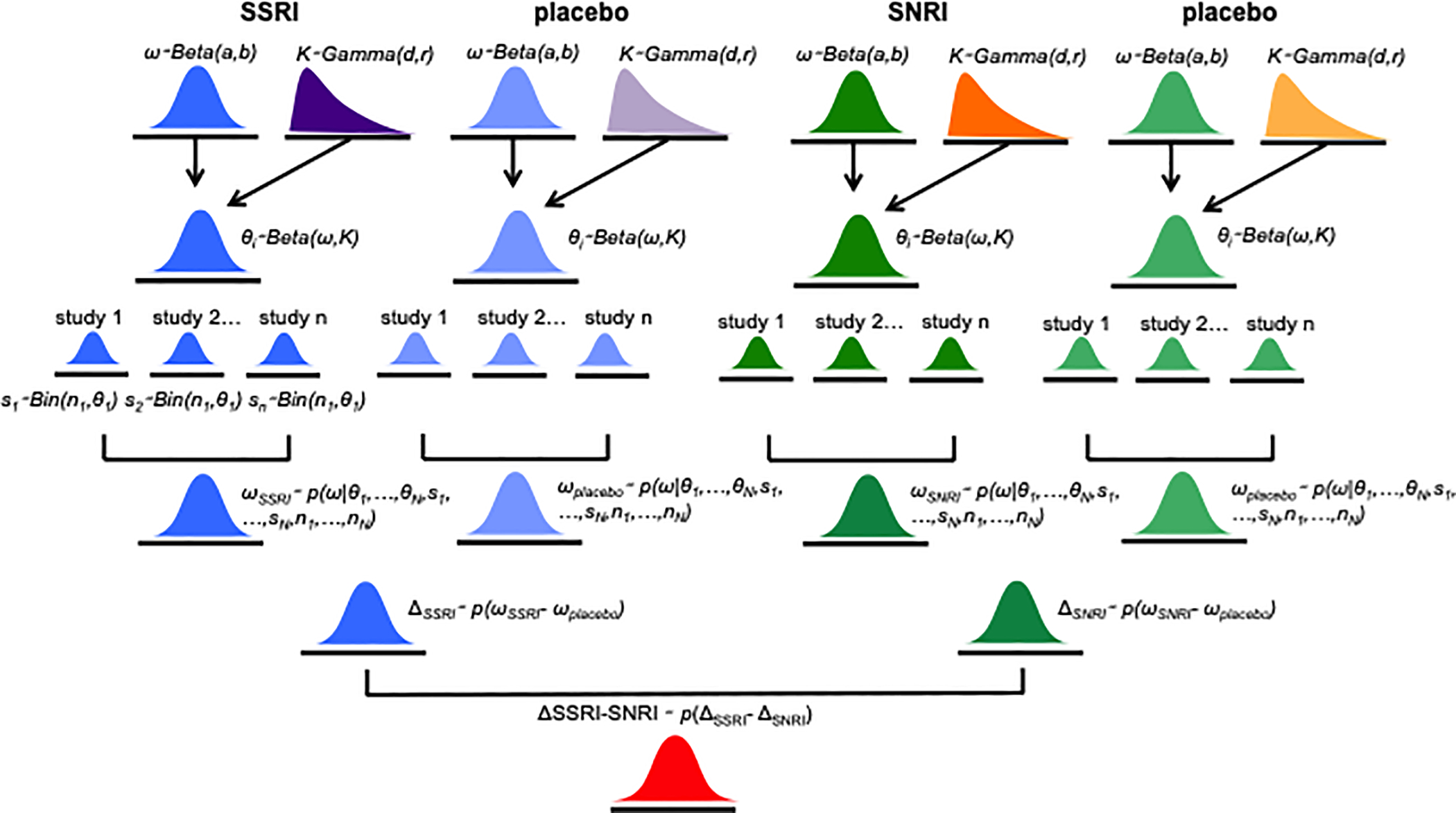
Hierarchical dependencies for the data from trials of SSRIs and SNRIs in pediatric patients with OCD and anxiety disorders.
We conducted sensitivity analyses in which the posterior densities of differences in risk rates were examined for SSRI or SNRI trials in patients with OCD or anxiety disorders. The extent to which the estimated unobserved heterogeneity across trials influenced our findings was examined by comparing the BHM with a model assuming complete homogeneity (i.e., patients being treated as if they had all come from a single study).
The mean probability of each AE, standard deviations and credible intervals were then computed directly from the simulated posterior sample, along with the posterior density ratio (PDR) representing posterior odds against the null hypothesis of no difference and a Bayesian posterior p-value.33,37,38 Analyses were conducted in R (version: 3.4.3)41 and Julia (version: 1.1.0).42 Means are represented ±their standard deviations (SD) and precision is expressed as 95% CrIs. Regarding multiple comparisons, a Bayesian inferential approach was selected given its advantages over the frequentist approach. Specifically, the Bayesian approach allows direct probability statements and comparisons for the unknown parameters, which enables simulation across potential dependencies. Additionally, the Bayesian approach obviates the need to adjust for multiple comparisons given the sequential relationship between inference and hypothesis testing.38
Study quality was rated using the Cochrane Risk of Bias Assessment Tool.44 Studies were classified as having the following: (1) “low risk of bias” if no domain was rated as high risk of bias and 3 domains were classified as “unclear risk;” (2) “high risk of bias” if >1 domain was rated as high risk of bias or no domain was rated as high risk of bias but >3 domains were rated as unclear risk; and (3) “uncertain risk of bias” if other combinations of bias across domains were present.
RESULTS
Selection of Studies and Study Characteristics
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram45 illustrating the selection procedure—which yielded 18 studies—is shown in Figure 2. Overall, 10,370 citations were identified by the searches (see Table S1, available online) and 612 potentially eligible articles were retrieved. The included studies—involving 2631 children and adolescents—included 1347 patients who received active treatment and 1284 who received placebo. Five different SSRIs were evaluated in these randomized controlled trials: fluoxetine (κ=6),46–50 fluvoxamine (κ=3),11,51 paroxetine (κ=2) 26,52 and sertraline (κ=4).1,4,9,53 Three SNRIs were evaluated: atomoxetine (κ=1), venlafaxine (κ=3 [2 studies were reported as pooled results),8,10 duloxetine (κ=1).7 The median duration of the acute treatment was 11 weeks (interquartile range [IQR] 9–12) and the majority of studies were multi-center (N=13; 72%). Ten of the studies (56%) were federally-funded, with the remaining 8 studies funded by industry and all studies were conducted in the outpatient setting. The characteristics of the included studies are summarized in Table S2 (available online).
Figure 2.
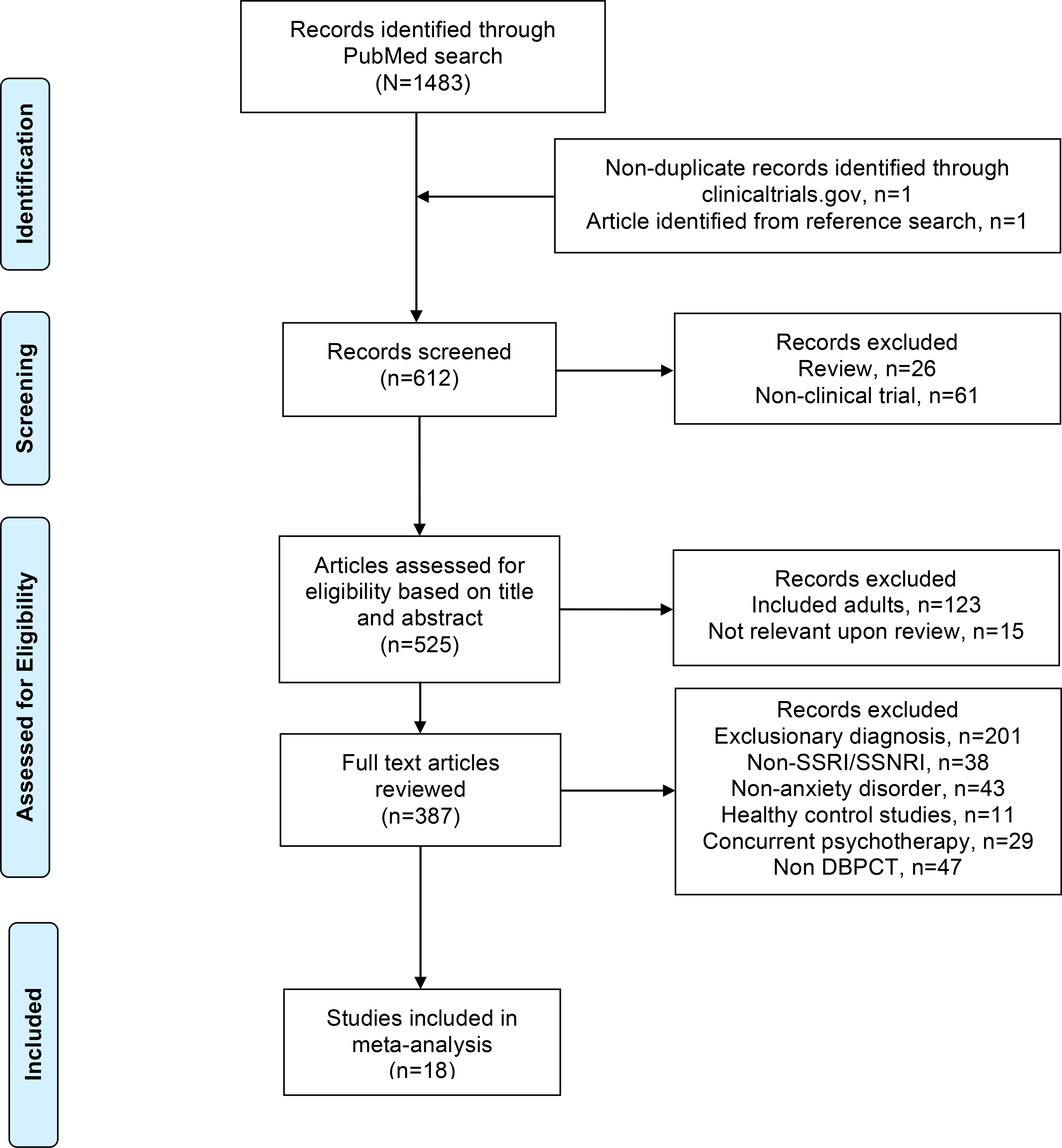
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Risk of bias was generally low or unclear with few exceptions (see Table S3, available online). Among the studies included, those that were categorized as “high risk of bias” included studies where patients had limited interaction with personnel not blinded to treatment (i.e., a non-assessing nurse monitoring for AEs),50 a large difference between groups in dropout rate or reasons for dropout (i.e., lack of efficacy or adverse events),50 and inconsistency in data reporting. High bias risk was present in small studies and common reasons for unclear risk of bias in studies were statements regarding the study being “double blind” or “randomized” without specifying how subjects were randomized or who was blinded representing unclear risk of bias in random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
Heterogeneity across studies
Heterogeneity measures for comparisons with placebo for AE-related discontinuation in SSRI studies of anxiety + OCD were as follows: Q=14.83 (p=0.318), I2=12.38, τ2=0.002. For non-OCD studies of SSRIs, Q=3.99 (p=0.550), I2=0, τ2=0 while for non-OCD studies of SNRIs, Q=0.74 (p=0.862), I2=0, τ2=0. Finally, for SSRI studies in OCD, Q=10.49 (p=0.162), I2=0.01, τ2=0.012. For other adverse events and suicidality, Q, I2 and τ2 did not suggest heterogeneity (see Table S4, available online)
Adverse Events (AEs)
In the BHM of anxiety disorders comparing SSRIs to placebo, abdominal pain (p=0.003), activation (p=0.005), headaches (p=0.027) and sedation (p=0.024) were more likely with SSRIs compared to placebo. The BHM of SNRIs in patients with anxiety disorders revealed SNRIs to be associated with a likelihood of nausea (p=0.002) compared to placebo. In trials of OCD, SSRIs were associated with a higher likelihood of activation (p<0.001), diarrhea (p=0.013), insomnia (p<0.001) and sedation (p=0.012) (Table 1).
TABLE 1.
Bayesian Hierarchical Modeling of Antidepressant-Related Adverse Events (AEs)
| Anxiety | OCD | OCD vs Anxiety | Anxiety + OCD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AE/ Outcome | SSRI vs. pbo | SNRI vs pbo | SSRI vs. SNRI | SSRI vs. pbo | SSRI vs. pbo | SSRI vs. pbo | SNRI vs. pbo | SSRI vs. SNRI | |
| Abdominal pain | Mean±SD | 0.149±0.049 | 0.031+0.032 | 0.119±0.059 | 0.042±0.044 | 0.107±0.066 | 0.096±0.034 | 0.031+0.032 | 0.066±0.047 |
| PDR | 103.45:1 | 1.68:1 | 8.01:1 | 1.54:1 | 3.76:1 | 54.88:1 | 1.68:1 | 2.84:1 | |
| p-value | 0.0026 | 0.326 | 0.043 | 0.338 | 0.101 | 0.005 | 0.326 | 0.151 | |
| Credible interval | 0.005 to 0.248 | −0.031 to 0.094 | 0.004 to 0.235 | −0.046 to 0.129 | −0.022 to 0.236 | 0.032 to 0.164 | −0.031 to 0.094 | −0.025 to 0.158 | |
| Headache | Mean±SD | 0.100±0.045 | −0.003±0.041 | 0.102±0.061 | 0.015±0.060 | 0.085±0.075 | 0.076±0.037 | −0.003±0.041 | 0.079±0.055 |
| PDR | 11.49:1 | 1.02:1 | 4.31:1 | 1.02:1 | 1.92:1 | 7.32:1 | 1.02:1 | 2.69:1 | |
| p-value | 0.027 | 0.937 | 0.093 | 0.804 | 0.253 | 0.040 | 0.937 | 0.153 | |
| Credible interval | 0.011 to 0.188 | −0.083 to 0.077 | −0.018 to 0.221 | −0.103 to 0.132 | −0.062 to 0.233 | 0.003 to 0.149 | −0.083 to 0.077 | −0.028 to 0.187 | |
| Activation | Mean±SD | 0.085±0.031 | 0.020±0.014 | 0.065±0.034 | 0.083±0.024 | −0.002±0.007 | 0.086±0.020 | 0.020±0.014 | 0.065±0.024 |
| PDR | 43.66:1 | 2.94:1 | 6.46:1 | 425.45:1 | 1.01:1 | 2180.3:1 | 2.94:1 | 35.28:1 | |
| p-value | 0.0053 | 0.152 | 0.054 | 0.0007 | 0.831 | 0.0001 | 0.152 | 0.007 | |
| Credible interval | 0.025 to 0.146 | −0.007 to 0.048 | −0.001 to 0.133 | 0.045 to 0.122 | −0.013 to 0.015 | 0.048 to 0.125 | −0.007 to 0.048 | 0.018 to 0.114 | |
| Sedation/Drowsiness | Mean±SD | 0.077±0.035 | 0.050±0.029 | 0.028±0.045 | 0.112±0.045 | 0.034±0.057 | 0.094±0.031 | 0.050±0.029 | 0.044±0.042 |
| PDR | 12.22:1 | 4.55:1 | 1.23:1 | 23.42:1 | 1.20:1 | 106.26:1 | 4.55:1 | 1.75:1 | |
| p-value | 0.024 | 0.080 | 0.539 | 0.012 | 0.540 | 0.002 | 0.080 | 0.295 | |
| Credible interval | 0.011 to 0.147 | −0.006 to 0.107 | −0.061 to 0.117 | 0.026 to 0.203 | −0.076 to 0.147 | 0.035 to 0.157 | −0.006 to 0.107 | −0.039 to 0.127 | |
| Insomnia | Mean±SD | 0.042+0.032 | <2 SNRI studies | <2 SNRI studies | 0.149+0.040 | 0.107+0.051 | 0.094+0.028 | <2 SNRI studies | <2 SNRI studies |
| PDR | 2.23:1 | 364.4:1 | 9.60:1 | 194.3:1 | |||||
| p-value | 0.188 | 0.0004 | 0.032 | 0.001 | |||||
| Credible interval | −0.020 to 0.104 | 0.071 to 0.228 | 0.009 to 0.209 | 0.040–0.149 | |||||
| Suicidality | Mean ± SD | 0.037 ±0.009 | 0.010±0.012 | −0.007±0.015 | 0.008±0.009 | 0.004±0.013 | 0.006±0.006 | 0.010±0.012 | −0.006±0.015 |
| PDR | 1.02:1 | 1.42:1 | 1.06:1 | 1.40:1 | 1.03:1 | 1.74:1 | 1.42:1 | 1.11:1 | |
| p-value | 0.669 | 0.394 | 0.655 | 0.389 | 0.741 | 0.284 | 0.394 | 0.663 | |
| Credible interval | −0.013 to 0.022 | −0.014 to 0.036 | −0.037 to 0.023 | −0.010 to .027 | −0.022 to 0.030 | −0.005 to 0.018 | −0.014 to 0.036 | −0.037 to 0.023 | |
| AE-related discontinuation | Mean±SD | 0.034±0.015 | 0.005±0.016 | 0.029±0.022 | 0.051±0.193 | −0.017±0.025 | 0.043±0.012 | 0.005±0.016 | 0.038± 0.020 |
| PDR | 13.06:1 | 1.10:1 | 2.21:1 | 42.13:1 | 1.30:1 | 1297:1 | 1.10:1 | 5.60:1 | |
| p-value | 0.022 | 0.753 | 0.191 | 0.0075 | 0.501 | 0.0003 | 0.753 | 0.063 | |
| Credible interval | 0.005 to 0.066 | −0.027 to 0.037 | −0.014 to 0.072 | 0.014 to 0.090 | −0.065 to 0.031 | 0.019 to 0.067 | −0.027 to 0.037 | −0.002 to 0.077 | |
| Nausea | Mean ± SD | 0.010±0.033 | 0.081±0.026 | −0.072+0.043 | 0.057±0.035 | −0.047±0.048 | 0.034±0.025 | 0.081±0.026 | −0.048±0.037 |
| PDR | 1.08:1 | 89.94:1 | 4.09:1 | 3.75:1 | 1.57:1 | 2.52:1 | 89.94:1 | 2.43:1 | |
| p-value | 0.764 | 0.002 | 0.099 | 0.107 | 0.326 | 0.170 | 0.002 | 0.191 | |
| Credible interval | −0.055 to 0.075 | 0.029 to 0.133 | −0.155 to −0.014 | −0.012 to 0.126 | −0.141 to 0.048 | −0.014 to 0.084 | 0.029 to 0.133 | −0.120 to 0.025 | |
| Diarrhea | Mean±SD | −0.010±0.026 | <2 SNRI studies | <2 SNRI studies | 0.071±0.028 | 0.081±0.038 | 0.031±0.019 | <2 SNRI studies | <2 SNRI studies |
| PDR | 1.10:1 | 22.91:1 | 9.79:1 | 3.19:1 | |||||
| p-value | 0.683 | 0.013 | 0.030 | 0.108 | |||||
| Credible interval | −0.062 to 0.039 | 0.051 to 0.125 | 0.008 to 0.154 | −0.006 to 0.069 | |||||
SD, standard deviation; H0, null hypothesis; pbo, placebo. PDR, posterior density ratio against the null hypothesis (H0). The mean represents the mean posterior probability of each AE.
In the BHM of anxiety + OCD trials, SSRIs were associated with abdominal (p=0.005), activation (p=0.001) diarrhea (p=0.004), insomnia (p=0.001), headache (p=0.040), sedation (p=0.00) compared to placebo. Additionally, in the BHM of anxiety + OCD trials, SSRIs were associated more activation compared to SNRIs (p=0.007).
The relative risk and CrI for each AE, AE-related discontinuation and treatment-emergent suicidality are shown in Figure 3.
Figure 3. Relative Risk of Antidepressant-Related Adverse Events (AEs), Suicidality and Discontinuation Secondary to Adverse Events.
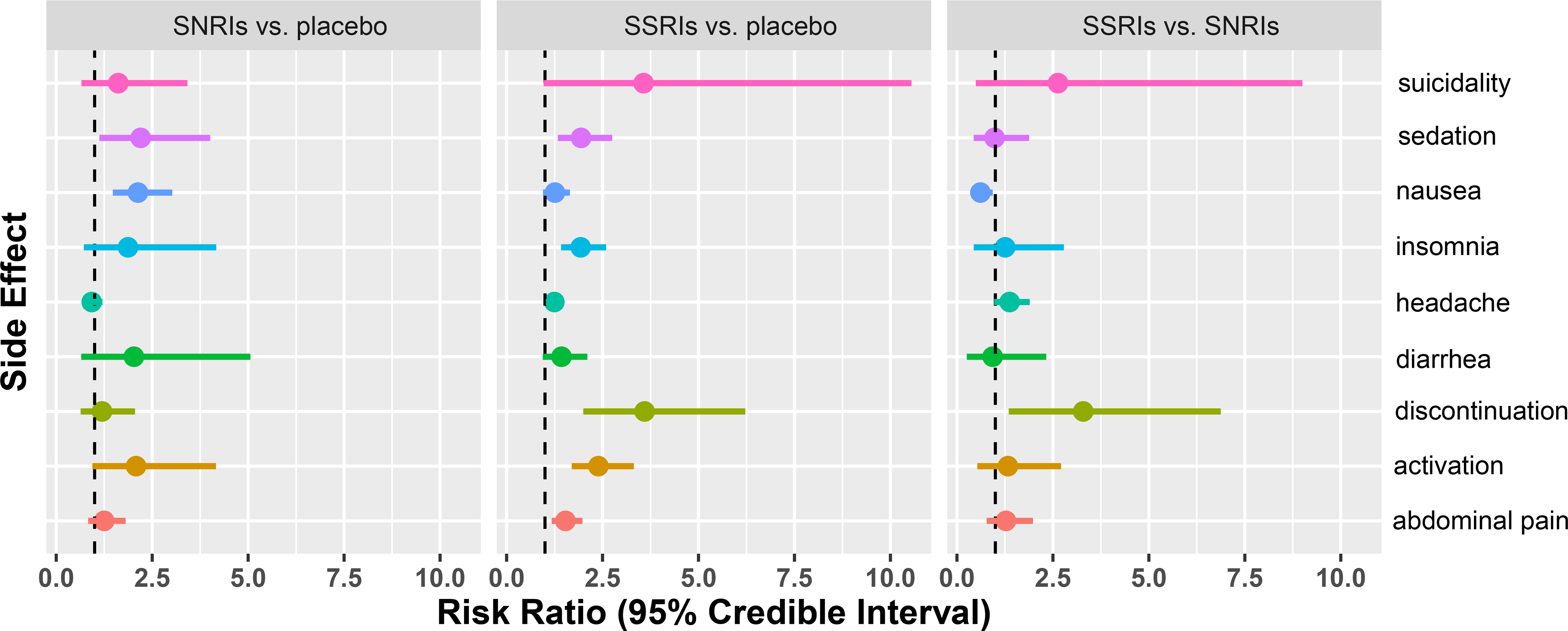
The relative risk of each AE is show in addition to the 95% credible interval. The large interval estimates for AE-related discontinuation and suicidality relate to the small number of occurrences relative to number of patients leading to a skewed distribution when converting the estimated relative probabilities to the log odds scale, rather than an indication of large potential chances of these outcomes.
Suicidality
Treatment emergent suicidality did not significantly differ between SSRIs and placebo in BHMs of anxiety disorders, OCD or anxiety + OCD. Additionally, no differences between SSRIs and SNRIs with regard to the likelihood of treatment emergent suicidality were identified (Table 1). Results of the BHM for suicidality did not differ from models assuming homogeneity across studies (see Table S5, available online))
AE-related discontinuation
In the BHM of SSRIs and SNRIs in anxiety disorders, SSRIs were associated with greater AE-related discontinuation compared to placebo (p=0.022); however, SNRIs were not (p=0.73). SSRIs did not differ from SNRIs in terms of the likelihood of AE-related discontinuation (p=0.191). For the BHM model of SSRIs in OCD, SSRIs were more likely to cause AE-related discontinuation compared to placebo (p=0.008). In the BHM for anxiety + OCD, adverse event-related discontinuation was greater in SSRIs compared to placebo (p=0.001) and was greater for SSRIs compared to SNRIs, albeit not at a 5% significance threshold (p=0.063). Results of the BHM for adverse event-related discontinuation did not differ from models assuming homogeneity across studies with the exception of the model involving anxiety + OCD in which AE-related discontinuation was higher in SSRI-treated patients compared to those receiving SNRIs (p=0.012) (Table 1).
Sensitivity analysis
Pre-specified sensitivity analyses were conducted in which the BHM was compared to a model that assumed homogeneity across studies (see Table S5, available online). No additional AEs were identified in the BHM beyond those identified in the model that assumed homogeneity across studies.
Specifically, for the model assuming homogeneity, in trials of anxiety disorders, SSRIs as compared to placebo were associated with greater abdominal pain (p<0.001), activation (p<0.001), headaches (p=0.009) and sedation (p<0.01) whereas SNRIs only differed from placebo in terms of the likelihood of nausea (p<0.001) and drowsiness (p=0.018). In trials of anxiety disorders, SSRIs were associated with a higher likelihood of abdominal pain (p=0.021), activation (p=0.022) and headaches (p=0.019), but were less likely to be associated with nausea (p=0.020) (see Table S5, available online). Similarly, in trials of OCD, SSRIs were associated with activation (p<0.001), diarrhea (p=0.004), insomnia (p<0.001) and sedation (p=0.004) (see Table S5, available online).
DISCUSSION
This meta-analysis of randomized, double-blind, placebo-controlled trials of SSRIs and SNRIs in pediatric patients with anxiety disorders and OCD is the first to examine medication class-specific AEs and the first to use Bayesian hierarchical modeling to explore differences in antidepressant tolerability between disorders. SSRIs are associated with greater activation and sedation regardless of whether the medications are used to treat OCD or an anxiety disorder, and are associated with more abdominal pain, headaches and adverse event-related discontinuation compared to placebo. SNRIs do not differ from placebo in terms of AE-related discontinuation. Finally, SSRIs and SNRIs did not differ from placebo in terms of their likelihood of treatment-emergent suicidality, consistent with other meta-analyses.2,14,16,54
Pediatric patients are more likely to exhibit activation with SSRIs compared to placebo and compared to SNRIs. Activation increases the likelihood of medication discontinuation and, in a prospective study of sertraline in pediatric patients with OCD, activation decreased treatment response.27 Prior antidepressant meta-analyses in pediatric patients only observed trends toward greater activation associated with SSRIs and SNRIs (OR: 1.86, CI: 0.98 to 3.53, p=0.054)17 despite individual clinical trials identifying medication-placebo differences in activation.22,25,46 The prior meta-analysis that evaluated activation and used a similar definition examined antidepressants in aggregate (i.e., SSRIs and SNRIs), thus potentially obscuring SSRI-specific activation.17 Additionally, SSRI-related activation in pediatric patients is correlated with dose36 and blood level in several studies; however, the relationship between dose and exposure (e.g., blood level)22 as well as other factors that increase the risk of activation (e.g., age) is complex. In the Child/Adolescent Anxiety Multimodal Study,1 activation-related adverse events were more common in sertraline-treated children compared to adolescents (disinhibition: 5.8% vs. 0%; increased motor activity 4.1% vs. 0.8%; restless/fidgety: 2.8% vs. 1.6%; significance thresholds for individual events not reported). Regarding the relationship between dose and activation, higher plasma concentrations of SSRIs as well as rapid increases in SSRI plasma concentrations may represent a risk factor for activation-related adverse events. In a randomized controlled trial of fluvoxamine for pediatric anxiety disorders22 in which 10 of 22 patients (45%) showed symptoms of activation compared to only 1 of 23 (4%) who received placebo, differences in plasma fluvoxamine concentrations were noted between those patients with activation-related adverse events and those who did not experience activation. Specifically, plasma fluvoxamine concentrations (at endpoint, 8 weeks), in this randomized controlled trial of flexibly-dosed fluvoxamine, were higher in patients who exhibited an activation-related adverse event (381.7±232.0 ng/mL) compared to those who did not (127.5±68.5 ng/mL; p=0.04). However, medication dose between patients who experienced activation and those who did not was not significantly different (activation: 134±22 mg/day; no activation 150±22 mg/day, p=0.63). Regarding this absence of a direct dose-exposure-activation relationship, a prospective trial of escitalopram in adolescents with GAD, found CMAX and AUC to be higher in patients with activation cluster symptoms compared to those without activation (p=0.040). Also, in es/citalopram-treated children and adolescents, the number of activation symptoms that patients exhibited (insomnia, irritability, hyperactivity, and impulsivity) was related to the CYP2C19 metabolizer status, with slower metabolizers experiencing more activation symptoms than faster metabolizers (p=0.019) in addition to the maximum dose of es/citalopram (p<0.001).36
Gastrointestinal symptoms have been associated with antidepressants in pediatric patients,55 but are also influenced by the presence and severity of an anxiety disorder.56 Yet, in general, gastrointestinal symptoms improve with active treatment, whether psychopharmacologic or psychotherapeutic.56 Mechanistically, SSRI-associated gastrointestinal symptoms are related to central and peripheral (e.g., direct action at serotonin receptors in the gut that modulate gastrointestinal motility) actions. Our meta-analysis examined the presence of these symptoms across treatments, however, we are unable to determine whether these symptoms emerged and then resolved or remained a persistent problem for patients. Additionally, there is significant heterogeneity in the reporting and categorizing of gastrointestinal symptoms (e.g., nausea vs. dyspepsia vs. nausea+abdominal pain vs. abdominal pain). In this study, we examined nausea as well as abdominal pain and diarrhea. However, with regard to nausea and abdominal pain, the two often co-occur and have been classified as a single adverse event in some studies. Thus, heterogeneity related to classification or reporting may have influenced our findings and underscores the importance of systematic and standardized terminology that is increasingly common in clinical trials (e.g., Medical Dictionary for Regulatory Activities [MedDRA])
Sleep disturbances are common in both youth with anxiety disorders57,58 and OCD.59–61 As with gastrointestinal symptoms and gastrointestinal AEs, the causal relationship with regard to treatment and/or the underlying disorder is complex. Given that insomnia and tiredness/sedation were both more likely in SSRI-treated patients, the two may be related. In this regard, individuals who report initial or middle insomnia may subsequently show more daytime tiredness resulting from decreased sleep efficiency or a reduction in sleep duration. Consistent with this possibility, studies with greater rates of insomnia also reported more tiredness and sedation. However, sedation and tiredness may also relate to the underlying receptor profiles of the medications, and in particular the SSRIs of which several (e.g., fluvoxamine, paroxetine) have either anticholinergic or antihistaminergic effects.
The lack of treatment-related differences in treatment-emergent suicidality compared to placebo or between SSRIs and SNRIs replicates most2,16,54 but not all prior meta-analyses.14,62 However, accumulating data suggest that multiple factors influence the likelihood of treatment-emergent suicidality, including specific medication (e.g., venlafaxine),16,21,63 baseline suicidality, clinical factors63 and the type of disorder being treated.54 Regarding the SNRIs, dose may also influence AEs which would be consistent with one prior report of more adverse events in patients with higher venlafaxine plasma concentrations (i.e., exposure) and is relevant in that, at low doses and with slower CYP2D6 metabolism, venlafaxine may behave more like an SSRI than an SNRI. The current analysis which incorporates the type of disorder as well as the medication class and reveals no increase in risk should be interpreted with caution in that many of the factors described above are difficult to incorporate into these meta-analytic evaluations of tolerability. However, they highlight the need to synthesize data from prospective trials, network meta-analyses and BHMs to determine clinically relevant risk predictions.
In our BHM analysis, discontinuation due to adverse events was similar to relative risk estimates derived from recent network meta-analyses that focused primarily on efficacy and compared multiple medication classes (e.g., SSRIs, SNRIs, TCAs, benzodiazepines, α2 agonists, etc.).14,16 In this regard, we previously observed that SNRIs were superior to α2 agonists, benzodiazepines, 5-HT1A agonists and SSRIs in terms of adverse event-related discontinuation. Consistent with the results of the present analysis, SNRIs were not associated with an increased risk of treatment-emergent adverse event-related discontinuation. However, one prior study examining three SNRI trials in depressed youth suggests greater discontinuation compared to placebo (κ=3, RR: 2.95, p<0.001).14 While we examined specific AEs that differ among classes of antidepressants across pediatric patients with OCD and anxiety disorders, we lack data on which specific adverse event resulted in AE-related discontinuation. Finally, given that SSRIs may be poorly tolerated in some patients but (at least in anxiety disorders) are more effective compared to SNRIs,35 these analyses may aid clinicians who are trying to balance efficacy and tolerability.28 For example, in a patient who has experienced an SSRI-related side effect, should a clinician try an SNRI which may be associated with less efficacy or try another SSRI which may be associated with a higher risk of certain side effects?
The Bayesian hierarchical approach lends important strengths to meta-analyses such as this. This approach calibrates the meta-analytic results64 and, for each comparison, preserves the relationship between treatment-specific (as opposed to pooled) variance and treatment effects. This process frees us from assuming that trials are exchangeable—a significant limitation in traditional meta-analyses. Trials vary considerably in methodology (e.g., fixed-dose, randomization ratio), sample characteristics (e.g., age distribution, co-morbidity patterns) and these factors may influence their results. At one extreme, studies may be seen as “identical replications of each other”65 while, at the other extreme, studies may be seen as “so different that the results of any one study provide no information about the results of any of the others.”32 In reality, and particularly in the studies included in our report, most studies are comparable; however, there are studies with high placebo response and studies with high medication response. The BHM calibrates the degree of unobserved heterogeneity across studies by estimation of the hierarchical parameters. This facilitates the meta-analytic approach without assuming either extreme of complete homogeneity or independence of studies. As such, the inter-trial differences are incorporated into the response model, attenuating the influence of assumptions related to homoegeneity.
Limitations
While this is the first meta-analytic evaluation of antidepressant class and disorder in children and adolescents with anxiety disorders, OCD and anxiety disorders + OCD, there are several important limitations. First, despite the general similarity of studies and our use of Bayesian hierarchical modeling to address the influence of exchangeability assumptions, unobserved factors may still affect the likelihood of adverse events described in this report. Second, just as placebo-response has received considerable attention in pediatric mood and anxiety disorders, the impact of placebo-related adverse events, which affects our ability to quantify medication-related adverse events, may vary as a result of patient expectation, clinician-specific factors (e.g., experience with the disorder under study, expertise in the clinical trial population)66 or trial design (e.g., capture of AEs). In this regard, by choosing to examine OCD and anxiety disorders (rather than major depressive disorder), we have selected conditions with lower placebo response rates66–68 which may minimize the influence of high placebo response on AE reporting. Third, although OCD and anxiety disorders were examined separately with the BHM, we also examined AEs in combined OCD and anxiety. Some might argue that these disorders should not be combined; however, the separation of OCD and anxiety disorders represents a recent development (2013). Anxiety disorders and OCD were classified as “anxiety disorders” in the DSM-IV, have similar treatment responses, age of onset distribution and overlapping neurobiology. Fourth, trial duration varied between 8 and 16 weeks and trial duration could influence the probability of AEs as there are more reporting opportunities in a longer trial. However, most trials (78%), were between 8–12 weeks and AEs typically emerge early in the course of treatment which may mitigate the influence of trial duration on AE rates. The two trials that lasted 16 weeks were balanced with regard to medication class (i.e., 1 SSRI and 1 SNRI trial) and both were trials of anxiety disorders. Fifth, electrocardiographic and laboratory parameters as well as vital signs were inconsistently reported across studies and were confounded by age and sex distributions of the sample. These were frequently reported as “no significant changes were observed” or were reported as a mean change from baseline without reporting baseline measures. As such, we were unable to evaluate changes in vital signs or electrocardiographic parameters across trials. Finally, the severity of AEs was infrequently reported and thus our analyses were unable to examine the severity of AEs across trials.
Across disorders, SSRIs are more likely to produce activation, abdominal pain, sedation/drowsiness and AE-related discontinuation compared to placebo, and SSRI tolerability is similar in pediatric patients with OCD and anxiety disorders. This analysis raises the possibility that SSRIs are more likely to produce activation compared to SNRIs. For clinicians, these findings demonstrate class-related AE differences that are important, particularly in light of data suggesting differences in class-related efficacy for pediatric anxiety disorders. Finally, this analysis demonstrates the feasibility of BHM derived estimates of AE risk that could help clinicians to reconcile disparate AE estimates from individual studies that vary in dosing, medication, design and disorder.
Supplementary Material
Acknowledgments
Funding Support: Support was received from the National Institute of Mental Health (NIMH) through Grant K23MH106037 and from the Yung Family Foundation.
Disclosures: Dr. Strawn has received research support from the National Institutes of Health (NIMH/NIEHS) as well as Allergan and Neuronetics. He has received material support from Assurex Health and receives royalties from the publication of two texts (Springer) and serves as an author for UpToDate and an Associate Editor for Current Psychiatry
Key words and abbreviations:
- SSRI, SRI
selective serotonin reuptake inhibitor
- SSNRI, SNRI
selective serotonin norepinephrine reuptake inhibitor
- SAD
anxiety disorders, separation anxiety disorder
- GAD
social phobia, social anxiety disorder, generalized anxiety disorder
REFERENCES
- 1.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344(17):1279–1285. [DOI] [PubMed] [Google Scholar]
- 4.Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. [DOI] [PubMed] [Google Scholar]
- 5.Alderman J, Wolkow R, Chung M, Johnston HF. Sertraline treatment of children and adolescents with obsessive-compulsive disorder or depression: pharmacokinetics, tolerability, and efficacy. J Am Acad Child Adolesc Psychiatry. 1998;37(4):386–394. [DOI] [PubMed] [Google Scholar]
- 6.Bushnell GA, Compton SN, Dusetzina SB, et al. Treating pediatric anxiety: Initial use of SSRIs and other antianxiety prescription medications. J Clin Psychiatry. 2018;79(1):pii:16m11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283–293. [DOI] [PubMed] [Google Scholar]
- 8.Rynn MA, Riddle MA, Yeung PP, Kunz NR. Efficacy and Safety of Extended-Release Venlafaxine in the Treatment of Generalized Anxiety Disorder in Children and Adolescents: Two Placebo-Controlled Trials. Am J Psychiatry. 2007;164:290–300. [DOI] [PubMed] [Google Scholar]
- 9.Rynn MA, Siqueland L, Rickels K. Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry. 2001;158(12):2008–2014. [DOI] [PubMed] [Google Scholar]
- 10.March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A Randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry. 2007;62(10):1149–1154. [DOI] [PubMed] [Google Scholar]
- 11.Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. [DOI] [PubMed] [Google Scholar]
- 12.Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry Allied Discip. 2008;49(5):489–498. [DOI] [PubMed] [Google Scholar]
- 13.Geller DA, Biederman J, Stewart SE, et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry. 2003;160(11):1919–1928. [DOI] [PubMed] [Google Scholar]
- 14.Locher C, Koechlin H, Zion SR, et al. Efficacy and safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine reuptake inhibitors, and placebo in common psychiatric disorders a meta-analysis in children and adolescents. JAMA Psychiatry. 2017;74(10):1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strawn JR, Mills JA, Sauley BA, Welge JA. The Impact of antidepressant dose and class on treatment response in pediatric anxiety disorders: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2018;57(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy in pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. [DOI] [PubMed] [Google Scholar]
- 17.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, SH W, Sim L, et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: A systematic review and meta-analysis. JAMA Pediatr. 2017;171(11):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: Early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851–859.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166(1):42–49. [DOI] [PubMed] [Google Scholar]
- 21.Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881–890. [DOI] [PubMed] [Google Scholar]
- 22.Reinblatt SP, DosReis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luft MJ, Lamy M, DelBello MP, McNamara RK, Strawn JR. Antidepressant-induced activation in children and adolescents: risk, recognition and management. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safer DJ, Zito JM, DJ S, JM Z. Treatment-Emergent Adverse Events from Selective Serotonin Reuptake Inhibitors by Age Group: Children versus Adolescents. J Child Adolesc Psychopharmacol. 2006;16(1–2):159–169. [DOI] [PubMed] [Google Scholar]
- 25.Rynn MA, Walkup JT, Compton SN, et al. Child/Adolescent anxiety multimodal study: evaluating safety. J Am Acad Child Adolesc Psychiatry. 2015;54(3):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner KD, Berard R, Stein MB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61(11):1153–1162. [DOI] [PubMed] [Google Scholar]
- 27.Reid AM, McNamara JPH, Murphy TK, et al. Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial. J Psychiatr Res. 2015;71:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strawn JR, Mills JA, Croarkin PE. Switching selective serotonin reuptake inhibitors in adolescents with selective serotonin reuptake inhibitor-resistant major depressive disorder: balancing tolerability and efficacy. J Child Adolesc Psychopharmacol. 2019;29(4):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–283. [DOI] [PubMed] [Google Scholar]
- 30.Quintana M, Viele K, Lewis RJ. Bayesian analysis: Using prior information to interpret the results of clinical trials. JAMA. 2017;318(16):1605–1606. [DOI] [PubMed] [Google Scholar]
- 31.Lewis RJ, Angus DC. Time for Clinicians to Embrace Their Inner Bayesian? JAMA. 2018;320(21):2208–2210. [DOI] [PubMed] [Google Scholar]
- 32.Gelman A, Hwang J, Vehtari A. Understanding predictive information criteria for Bayesian models. Stat Comput. 2014;24:997–1016. [Google Scholar]
- 33.McGlothlin AE, Viele K. Bayesian Hierarchical Models. JAMA. 2018;320(22):2365–2366. [DOI] [PubMed] [Google Scholar]
- 34.Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, Bloch MH. Systematic review and meta-analysis: Early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557–564. [DOI] [PubMed] [Google Scholar]
- 35.Strawn JR, Mills JA, Sauley BA, Welge JA. The Impact of antidepressant dose and class on treatment response in pediatric anxiety disorders: A Meta-Analysis. J Am Acad Child Adolesc Psychiatry. 2018;57(4):235–244.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 metabolizer status on escitalopram/citalopram tolerability and response in youth with anxiety and depressive disorders. Front Pharmacol. 2019;10:99. doi: 10.3389/fphar.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills JA, Strawn JR. Probabilistic Biostatistics with Julia. In: JuliaCon Proceedings; 2019:1–6. [Google Scholar]
- 38.Kruschke JK. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan, Second Edition; 2014. [Google Scholar]
- 39.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JPT. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 41.R Development Core Team. R: A language and enviroment for statistical computing. 2008.
- 42.Bezanson J, Edelman A, Karpinski S, Shah VB. Julia: A Fresh Approach to Numerical Computing. 2014;59(1):65–98. [Google Scholar]
- 43.Ge H, Xu K, Ghahramani Z. Turing: A Language for Flexible Probabilistic Inference. Proc Twenty-First Int Conf Artif Intell Stat. 2018;84:1682–1690. [Google Scholar]
- 44.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol Vers5.1.0.; 2011. doi:http://handbook.cochrane.org/. [Google Scholar]
- 45.Moher D, Liberati ATJ and AD. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 46.Birmaher B, Axelson DA, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. [DOI] [PubMed] [Google Scholar]
- 47.Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622–1632. [DOI] [PubMed] [Google Scholar]
- 48.Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry. 2001;40(7):773–779.. [DOI] [PubMed] [Google Scholar]
- 49.Riddle MA, Scahill L, King RA, Mardin MT, et al. Double-blind, crossover trial of fluoxetine and placebo in children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1062–1069. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L22349220%0Ahttp://wx7cf7zp2h.search.serialssolutions.com?sid=EMBASE&issn=08908567&id=doi:&atitle=Double-blind%252C+crossover+trial+of+fluoxetine+and+placebo+in+children+and+adolesce. [DOI] [PubMed] [Google Scholar]
- 50.da Costa CZG, de Morais RMCB, Zanetta DMT, et al. Comparison Among Clomipramine, Fluoxetine, and Placebo for the Treatment of Anxiety Disorders in Children and Adolescents. J Child Adolesc Psychopharmacol. 2013;23(10):687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: A randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry. 2001;40(2):222–229. [DOI] [PubMed] [Google Scholar]
- 52.Geller DA, Wagner KD, Emslie G, et al. Paroxetine treatment in children and adolescents with obsessive-compulsive disorder: A randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2004;43(11):1387–1396. [DOI] [PubMed] [Google Scholar]
- 53.March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752–1756. [DOI] [PubMed] [Google Scholar]
- 54.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. [DOI] [PubMed] [Google Scholar]
- 55.Rynn MA, Walkup JT, Compton SN, et al. Child/Adolescent Anxiety Multimodal Study: Evaluating Safety. J Am Acad Child Adolesc Psychiatry. 2015;54(3):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crawley SA, Caporino NE, Birmaher B, et al. Somatic complaints in anxious youth. Child Psychiatry Hum Dev. 2014;45(4):398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):224–232. [DOI] [PubMed] [Google Scholar]
- 58.Caporino NE, Read KL, Shiffrin N, et al. Sleep-Related Problems and the Effects of Anxiety Treatment in Children and Adolescents. J Clin Child Adolesc Psychol. 2017;46(5):675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alfano CA, Kim KL. Objective sleep patterns and severity of symptoms in pediatric obsessive compulsive disorder: A pilot investigation. J Anxiety Disord. 2011;25(6):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Storch EA, Murphy TK, Lack CW, Geffken GR, Jacob ML, Goodman WK. Sleep-related problems in pediatric obsessive-compulsive disorder. J Anxiety Disord. 2008;22(5):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sevilla-Cermeño L, Andrén P, Hillborg M, Silverberg-Morse M, Mataix-Cols D, Fernández de la Cruz L. Insomnia in pediatric obsessive–compulsive disorder: prevalence and association with multimodal treatment outcomes in a naturalistic clinical setting. Sleep Med. 2019;56:104–110. [DOI] [PubMed] [Google Scholar]
- 62.Sharma A, Guski LS, Freund N, Gøtzsche PC. Suicidality and aggression during antidepressant treatment: Systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brent DA, Emslie GJ, Clarke GN, et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166(4):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williamson J Bayesian Nets and Causality : Philosophical and Computational Foundations. Oxford University Press; 2005. [Google Scholar]
- 65.Andrews M, Baguley T. Bayesian data analysis. In: The Cambridge Encyclopedia of Child Development. Vol 2. CRC press; Boca Raton, FL; 2017:165–169. [Google Scholar]
- 66.Strawn JR, Dobson ET, Mills JA, et al. Placebo Response in Pediatric Anxiety Disorders: Results from the Child/Adolescent Anxiety Multimodal Study. J Child Adolesc Psychopharmacol. 2017;27(6):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dobson ET, Strawn JR. Placebo Response in Pediatric Anxiety Disorders: Implications for Clinical Trial Design and Interpretation. J Child Adolesc Psychopharmacol. 2016;26(8):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walkup JT. Antidepressant Efficacy for Depression in Children and Adolescents: Industry- and NIMH-Funded Studies. Am J Psychiatry. 2017;174(5):1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


