Abstract
We report on the development of minimal change disease (MCD) with nephrotic syndrome and acute kidney injury (AKI), shortly after first injection of the BNT162b2 COVID-19 vaccine (Pfizer-BioNTech). A 50-year-old previously healthy man was admitted to our hospital following the appearance of peripheral edema. Ten days earlier, he had received the first injection of the vaccine. Four days after injection, he developed lower leg edema, which rapidly progressed to anasarca. On admission, serum creatinine was 2.31 mg/dL and 24-hour urinary protein excretion was 6.9 grams. As kidney function continued to decline over the next days, empirical treatment was initiated with prednisone 80 mg/d. A kidney biopsy was performed and the findings were consistent with MCD. Ten days later, kidney function began to improve, gradually returning to normal. The clinical triad of MCD, nephrotic syndrome, and AKI has been previously described under a variety of circumstances, but not following the Pfizer-BioNTech COVID-19 vaccine. The association between the vaccination and MCD is at this time temporal and by exclusion, and by no means firmly established. We await further reports of similar cases to evaluate the true incidence of this possible vaccine side effect.
Index Words: Nephrotic syndrome (NS), Acute kidney injury (AKI), Coronavirus 2019 (COVID-19), COVID-19 vaccine, Side effect, Adverse effect, Minimal change disease (MCD), Acute tubular injury, Renal function, Kidney biopsy, Prednisone, Case report
Introduction
The global coronavirus 2019 (COVID-19) pandemic has caused extensive morbidity and mortality worldwide during the past year. The advent of novel vaccines seems currently to be altering the course of events in a favorable direction. Along with clear benefits stemming out of vaccination programs in many countries, side effects of these vaccines remain a concern that must be addressed. Major side effects seem to be uncommon.
We report on the development of minimal change disease (MCD) with full-blown nephrotic syndrome and acute kidney injury (AKI), starting a few days after administration of the first injection of the BNT162b2 vaccine (Pfizer-BioNTech mRNA-based vaccine against severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]).
Case Report
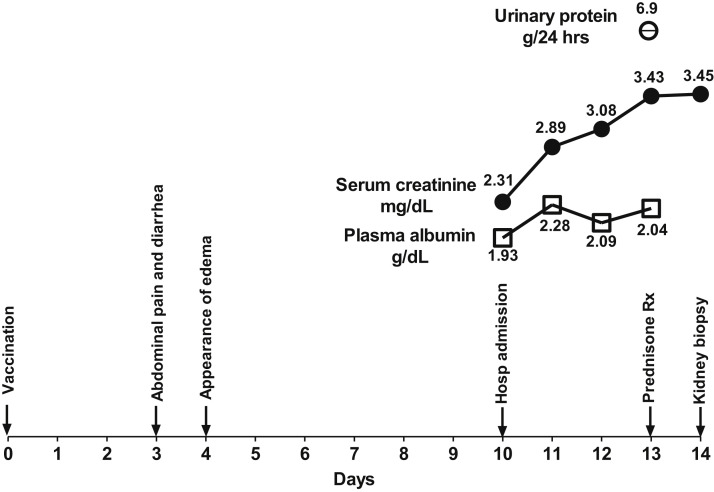
A 50-year-old previously healthy man was admitted to our medical center following the appearance of peripheral edema. Ten days earlier, he had received the first injection of the Pfizer-BioNTech COVID-19 vaccine. The timeline of clinical events is shown in Fig 1 . He initially reported pain in the injection area. On the third day after the injection, he developed abdominal pain and diarrhea. One day later, he noticed swelling of the lower extremities, which gradually worsened over the next 6 days. On day 10 after vaccination, he presented to the Emergency Department with anasarca. Laboratory tests 10 months earlier had been normal, with serum creatinine 0.78 mg/dL and normal urinalysis. The patient denied use of nonsteroidal anti-inflammatory drugs before or after the vaccination.
Figure 1.
Timeline of clinical events from time of vaccination and until the kidney biopsy was performed.
On admission, blood pressure was 170/110 mm Hg and heart rate 80 beats/min. Physical examination revealed pitting edema in the lower extremities, palms of hands, abdominal wall, and penis. The abdomen was distended. There were rales at both lung bases. Laboratory tests revealed serum creatinine 2.31 mg/dL, serum urea nitrogen 78 mg/dL, albumin 1.93 g/dL, cholesterol 484 mg/dL, and triglycerides 166 mg/dL. Testing for hepatitis B surface antigen and antibodies to hepatitis C virus gave negative results; levels of complement C3 and C4 were within reference ranges; and tests for antineutrophil cytoplasmic antibody (ANCA), anti–glomerular basement membrane (GBM) antibodies, and antinuclear antibody (ANA) also gave negative results. Polymerase chain reaction and serology testing for SARS-CoV-2 (Cobas Elecsys Anti-SARS-CoV-2 [Roche Diagnostics]) gave negative results. However, the titer of immunoglobulin G antibodies to Spike protein subunits S1 and S2, which was determined in this case to detect antibodies that develop once a person has received the COVID-19 vaccination, was 38.9 absorbance units per milliliter, a positive result for the assay. Urinalysis revealed protein (4+) and urinary sediment showed 3-5 red blood cells per high-power field (some dysmorphic), fatty casts, and oval fat bodies. Twenty-four-hour urinary collection revealed proteinuria of 6.9 grams. Chest radiograph showed bilateral pleural effusions. Computed tomography examination without contrast demonstrated kidneys of normal size and no evidence of urinary tract obstruction.
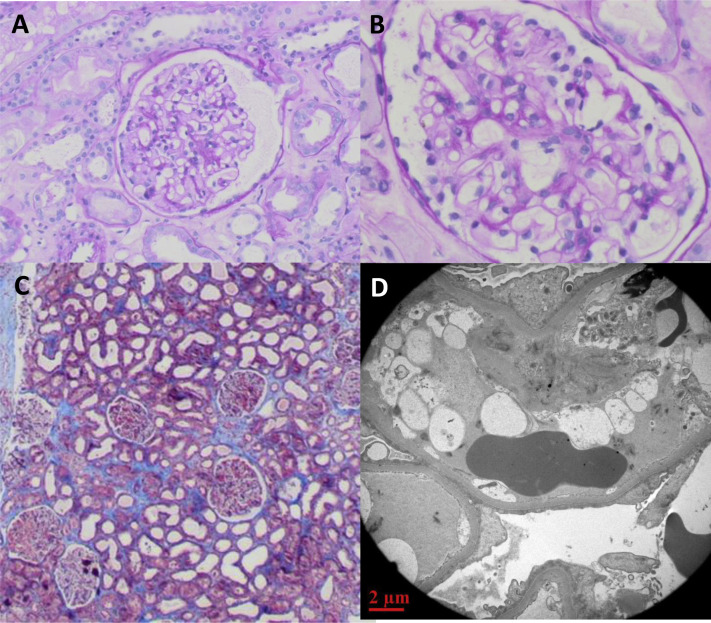
During the first few days after admission, kidney function continued to decline, with the serum creatinine increasing to 3.43 mg/dL. Empirical treatment with prednisone 80 mg/d was initiated on day 4 after admission. A percutaneous kidney biopsy was performed the next day. The results are shown in Fig 2 . Twenty-four glomeruli were detected, 1 with global sclerosis and the others normal. There was mild congestion in the glomerular capillaries and no thickening of the GBM. The interstitium was notable for small lymphocytic infiltrates. There was extensive acute tubular injury, with beginning of calcifications in the medulla. Immunofluorescence revealed linear membranous and mesangial albumin, IgG (trace), mesangial IgM (1+), vascular C3 (1+), and tubular IgG (trace). Two glomeruli were examined by electron microscopy. The GBM thickness ranged from 360 to 550 nm with diffuse flattening of the podocyte foot processes over approximately 90% of the glomerular volume. There were no electron-dense deposits but there were foci of microvillus transformation. These findings were consistent with MCD with acute tubular injury.
Figure 2.
Kidney biopsy findings. Light microscopy of representative glomerulus stained by (A) hematoxylin-eosin (original magnification, ×100) and (B) periodic acid–Schiff (original magnification, ×200), and of (C) the renal cortex stained by Masson trichrome (original magnification, ×40). (D) Electron microscopy of glomerulus (original magnification, ×1,200), demonstrating extensive effacement of foot processes and absence of electron-dense deposits.
Steroid therapy was continued and treatment for hypertension was initiated. The clinical course showed a further rise in serum creatinine levels to a maximum of 6.6 mg/dL. Five days after initiation of corticosteroid therapy, urine output increased, there was a short polyuric phase with loss of 7 kg in body weight, and peripheral edema gradually disappeared. In parallel, kidney function began to improve and serum creatinine gradually declined to 1.25 mg/dL. The patient was discharged after 17 days in hospital with continued steroid treatment. One week later, serum creatinine was 0.97 mg/dL, plasma albumin 3.2 g/dL and urinary albumin-creatinine ratio was 155 mg/g.
Discussion
The clinical triad of MCD, nephrotic syndrome, and AKI has been previously described under a variety of circumstances,1 , 2 including at least 2 cases following the influenza vaccine.3 , 4 To the best of our knowledge, such occurrence has not been previously reported following the Pfizer-BioNTech or other COVID-19 vaccines.
The clinical appearance of MCD in our case, with nephrotic syndrome and severe AKI, is somewhat atypical. Usually, the major presenting clinical features in MCD are related to nephrotic syndrome, with overt peripheral edema, massive proteinuria with hypoalbuminemia, and no major disturbance in glomerular filtration rate.4 AKI has been described, however, as a complication of nephrotic syndrome,2 including in the setting of MCD.1 The explanation for the occurrence of AKI in our case may be found in the results of the kidney biopsy, demonstrating extensive acute tubular injury. This is indeed atypical for MCD, in which the pathologic hallmark is absence of visible alterations by light microscopy.
The inevitable question that arises is whether the appearance of MCD with nephrotic syndrome and AKI is coincidental to or causally related to the vaccination. The association between COVID-19 vaccination and the appearance of this triad, as described in this report, can only be based on timing and by exclusion of other inciting factors, as there are currently no other conclusive means to prove causality.
The association between the timing of vaccination and the development of nephrotic syndrome and AKI raises questions as to the mechanisms involved. The underlying pathology, as evidenced by the biopsy results, is MCD, a disease in which T cell–mediated podocyte injury appears to predominate in the pathogenesis.5 The question arises whether it is plausible that the COVID-19 vaccine elicits a cell-mediated response as early as 3-4 days from its administration and could account thereby for the first putative signs of side effects of the vaccine, including gastrointestinal symptoms and the appearance of peripheral edema. From the clinical/observational perspective, a review of published reports of MCD postvaccination delineates a range of 4 days to 4 months from time of vaccination to onset of clinical symptoms.6 One report of MCD in a 65-year-old patient within 4 days after the influenza vaccination is consistent with our current report.4 From the immunological viewpoint, evidence suggests that during viral infection, a cellular immune response can be observed within approximately 1 week from infection, but T-cell activation can occur 2-3 days earlier.7 , 8 We can therefore speculate that T cells could potentially be activated by the Pfizer-BioNTech vaccine fast enough—within a matter of days—to account for development of MCD, suggesting that early T cell–mediated injury in response to the vaccine is a possibility. Further studies, however, are needed to continue exploring the early immune response to the vaccine, particularly in relation to side effects, a task beyond the scope of the current report.
In addition to anaphylaxis, an uncommon life-threatening side effect of the COVID-19 vaccination, our case report raises the possibility of another major side effect, which at this time cannot be conclusively attributed to the COVID-19 vaccine. Of significance is that this side effect is treatable with corticosteroids, with prompt and apparently complete resolution. We suggest, therefore, that patients who develop nephrotic syndrome and AKI within days to weeks following COVID-19 vaccination should undergo a kidney biopsy. If MCD is confirmed, prompt initiation of treatment with oral corticosteroids, such as prednisone at a dosage of approximately 1 mg/kg over several weeks, should be considered, as it seemed to be helpful in our patient.
By this time, many millions of doses of the various available COVID-19 vaccines (including Moderna, Oxford-AstraZeneca, Johnson & Johnson, Novavax, and Sputnik V) have been administered worldwide. We await further reports of similar cases to evaluate the true incidence of this possible major side effect of the vaccine.
Article Information
Authors’ Full Names and Academic Degrees
Larissa Lebedev, MD, Marina Sapojnikov, MD, Alexander Wechsler, MD, Ronen Levy Varadi, MD, Doron Zamir, MD, Ana Tobar, MD Nomy Levin-Iaina, MD, Shlomo Fytlovich, PhD, and Yoram Yagil, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
The authors wish to acknowledge Prof. Eli Magen for consulting on immunological issues during the discussions that led to drafting of this report.
Patient Protections
The authors declare that they have obtained consent from the patient reported in this article for publication of the information about him that appears within this Case Report.
Peer Review
Received March 6, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from the Pathology Editor and a Deputy Editor. Accepted in revised form March 30, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Oyama Y., Iwafuchi Y., Morioka T., Narita I. Acute kidney injury associated with minimal change nephrotic syndrome in an elderly patient successfully treated with both fluid management and specific therapy based on kidney biopsy findings. Case Rep Nephrol Dial. 2020;10(1):42–50. doi: 10.1159/000507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron J.S. The nephrotic syndrome and its complications. Am J Kidney Dis. 1987;10(3):157–171. doi: 10.1016/s0272-6386(87)80170-1. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez S., Dotto B., Petiti J.P., et al. Minimal change disease following influenza vaccination and acute renal failure: just a coincidence? Nefrologia. 2012;32(3):414–415. doi: 10.3265/Nefrologia.pre2012.Feb.11370. [DOI] [PubMed] [Google Scholar]
- 4.Kielstein J.T., Termuhlen L., Sohn J., Kliem V. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol. 2000;54(3):246–248. [PubMed] [Google Scholar]
- 5.Vivarelli M., Massella L., Ruggiero B., Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clajus C., Spiegel J., Brocker V., Chatzikyrkou C., Kielstein J.T. Minimal change nephrotic syndrome in an 82 year old patient following a tetanus-diphteria-poliomyelitis-vaccination. BMC Nephrol. 2009;10:21. doi: 10.1186/1471-2369-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao H., Hollenbaugh J.A., Zand M.S., et al. Quantifying the early immune response and adaptive immune response kinetics in mice infected with influenza A virus. J Virol. 2010;84(13):6687–6698. doi: 10.1128/JVI.00266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]