Abstract
We study the impact of short-term exposure to ambient air pollution on the spread and severity of COVID-19 in Germany. We combine data at the county-by-day level on confirmed cases and deaths with information on local air quality and weather conditions. Following Deryugina et al. (2019), we instrument short-term variation in local concentrations of particulate matter (PM10) by region-specific daily variation in wind directions. We find significant positive effects of PM10 concentration on death numbers from four days before to ten days after the onset of symptoms. Specifically, for elderly patients (80+ years) an increase in ambient PM10 concentration by one standard deviation between two and four days after developing symptoms increases the number of deaths by 19 percent of a standard deviation. In addition, higher levels air pollution raise the number of confirmed cases of COVID-19 for all age groups. The timing of effects surrounding the onset of illness suggests that air pollution affects the severity of already-realized infections. We discuss the implications of our results for immediate policy levers to reduce the exposure and level of ambient air pollution, as well as for cost-benefit considerations of policies aiming at sustainable longer-term reductions of pollution levels.
Keywords: COVID-19, Health, Air pollution, Germany
1. Introduction
The novel coronavirus (SARS-CoV-2) has sparked the largest public health and economic crisis in recent history. To date, with millions of confirmed cases the coronavirus disease 2019 (COVID-19) pandemic has claimed more than 1.5 million deaths globally.1 World Bank projections of economic damage range up to a 5.2 percent contraction in global GDP, which would be the deepest recession in decades (World Bank, 2020). Pressing social and economic issues that had dominated the public debate – most notably environmental issues of climate change and pollution – had almost vanished for a time and only slowly re-emerged.
In this paper, we show that environmental pollution and the COVID-19 pandemic are significantly connected: higher levels of local air pollution increase the number of deaths of COVID-19, leading to a more severe course of the pandemic. Three plausible mechanisms link air pollution to the spread and course of severe acute respiratory infections (SARI) and influenza-like illnesses (ILI). First, long-run exposure to air pollution is linked to medical pre-conditions such as illnesses of the respiratory system, which can exacerbate the course of the disease (Analitis et al., 2006; Atkinson et al., 2001; Le Tertre et al., 2002; Dominici et al., 2003; Katsouyanni et al., 2001). Second, short-term exposure to air pollution leads to inflammatory reactions and lower immune responses to new infections (Ciencewicki and Jaspers, 2007; Contini and Costabile, 2020; Martelletti and Martelletti, 2020). Third, higher levels of air pollution increase the risk of airborne infection of viruses by prolonging the time that the virus remains in open air (Cui et al., 2003; Frontera et al., 2020). Our empirical setup focusing on day-to-day changes in pollution before and after the onset of symptoms allows us to discriminate between these mechanisms.
Against this background, we add to the epidemiological literature empirically linking levels of different air pollutants to the onset and severity of SARI and ILI in general, and the new COVID-19 disease specifically. We study the interaction of short-term exposure to ambient air pollution, measured by levels of particulate matter (PM10) in German counties, and the number of newly-confirmed cases and deaths of COVID-19. We instrument levels of PM10 by exploiting region-specific relationships between wind direction and air pollution following Deryugina et al. (2019). Fixed effects for county and date keep county-specific time-invariant and national time-variant confounding variables constant. We further control for several potentially confounding variables: weather conditions, the county-specific state of the pandemic, local mobility patterns based on mobile phone movements, county-specific requirements for wearing face masks in public as well as state-specific fixed effects for the different policy regimes implemented in response to the first wave of the pandemic between spring and early summer of 2020 in Germany.
We find significant effects of higher air pollution on both the number of deaths as well as the number of confirmed cases by day and county. Effects are specifically pronounced for patients aged 80 and above. For this age group, significant effects of air pollution emerge for male patients from four days before to ten days after the onset of symptoms. A one standard deviation increase in the three-day average PM10 concentration (7.2 μg/m3) over the period between two and four days after the onset of illness increases the number of deaths among male patients by about 1.2 deaths per 100K members of the population per day, or about 19 percent of a standard deviation. Effect patterns for women are similar, yet less pronounced. Additionally, we find that air pollution just before as well as after the onset of illness leads to increased numbers of confirmed cases across the entire age distribution, which is likely explained by aggravated symptoms persuading patients to get tested.
By isolating the causal effect of air pollution on the severity of COVID-19, we complement and confirm earlier descriptive evidence based on cross-sectional and time series variation (see Bhaskar et al. (2020) for a systematic review). To the best of our knowledge, there are few studies estimating quasi-experimental causal links between air pollution and ILI or respiratory disease severity. Clay et al. (2018) exploit differential timing of the Spanish flu pandemic across U.S. cities to show that contemporary short-term air pollution levels increased the number of deaths. Moghadas et al. (2020) show that U.S. county-by-month influenza-related hospitalizations increase with higher air pollution. With respect to the COVID-19 pandemic, Persico and Johnson (2020) show that air pollution – instrumented by a rollback of environmental regulations in the U.S. – increases both the number of cases and case fatality of COVID-19. Also for the U.S., Austin et al. (2020) apply the same IV approach using county-level variation in wind directions to study the impact of fine particle concentrations on the number of cases and deaths of COVID-19.
We add to these more closely-related papers in two important ways. First, it is yet to be determined whether results from previous pandemics and/or other regional settings are generalizable to other external settings, in case of both disease and locality. To the best of our knowledge, we are the first to provide evidence on the linkage between air pollution and the spread and severity of COVID-19 for Germany. Second, we use fine-grained data on daily pollution levels, which allows us to identify critical time windows relative to the onset of the illness when pollution levels most crucially affect the mortality risk. This allows us to discriminate between different mechanisms at play. As effects materialize just before and mainly after the onset of illness, our results support mechanisms causing inflammatory reactions, reducing the immune response and aggravating symptoms. Our results do not support proposed mechanisms of higher ability of the virus to cause airborne infections due to higher air pollution. By focusing on short-term changes in the exposure to air pollution, our results do not speak to potential effects through longer-run exposure to air pollution on respiratory pre-conditions.
We discuss three groups of policy instruments that may be justified by the results of this paper: first, any policy that reduces the exposure of vulnerable patients towards ambient air pollution might lead to a reduced mortality of COVID-19; second, policies that directly reduce levels of air pollution should be considered; and third, our results confirm that policies that sustainably reduce pollution levels will have substantial health effects through reduced severity of respiratory diseases.
The remainder of this paper is organized as follows. Section 2 discusses plausible mechanisms through which higher air pollution might affect the onset and severity of COVID-19 and summarizes the related literature. Section 3 describes the data and provides a descriptive overview of the course of the pandemic in Germany. Section 4 describes our empirical approach. Section 5 presents the main results as well as additional analyses. Section 6 discusses implications for policy-makers. Section 7 concludes.
2. Background and literature
Mechanisms. The medical literature has analyzed a number of direct physiological mechanisms concerning how acute air pollution could affect the infection probabilities and severeness of respiratory virus infections such as influenza and SARS (see Ciencewicki and Jaspers, 2007, for a review). Several authors speculate that these mechanisms could be at play in the case of COVID-19 (Contini and Costabile, 2020; Martelletti and Martelletti, 2020).
Direct physiological mechanisms range from lower initial immune response, reduced ability of macrophages (cells specialized in the detection and destruction of harmful organisms, including viruses), and stronger oxidative stress.2 Specific to particulate matter (PM) concentration, mice display lower early immune responses when having been exposed to carbon black particles (Lambert et al., 2003). PM-exposed macrophages display lower abilities to devour viruses (Kaan and Hegele, 2003; Becker and Soukup, 1999). Several lab studies show that animals or cell cultures develop stronger oxidative stress and aggravated disease severity after being exposed to PM (Jaspers et al., 2005; Lee et al., 2014).
A second type of mechanisms is related to how air pollution is affecting the airborne transmission of viruses. A number of studies suggest that higher levels of air pollution – especially PM concentration – increase the ability of viruses to cause airborne infection and raise the initial viral load (Cui et al., 2003; Frontera et al., 2020). With respect to SARS-CoV-2, it has been shown that the infectious dose and initial viral load as important predictors of the severeness of cases (Zheng et al., 2020).
Besides these acute direct effects, air pollution has repeatedly been shown to be strongly linked to medical pre-conditions such as cardiovascular and respiratory diseases that have been found to crucially affect the severeness of COVID-19 (Analitis et al., 2006; Atkinson et al., 2001; Le Tertre et al., 2002; Dominici et al., 2003; Katsouyanni et al., 2001). As we focus our empirical analysis on the effect of short-term changes in the exposure to pollutants, these long-term effects are not the focus of this study.
Epidemiological Evidence. Tracing the outcome of these mechanisms in the field, a number of epidemiological studies propose empirical links between measures of air quality and case numbers and deaths of respiratory virus infections. With few exceptions, the majority of these studies rely on either pure cross-sectional or regional time series variation. Both approaches limit opportunities to identify a causal link due to potential confounders at both the time and regional level. Spatial variation likely correlates with the presence of obvious confounders, such as population density, public transport usage and age composition. Variation in air pollution over time might be plagued by simultaneity and reversed causality issues. For example, several studies have shown that the reduced economic activity that follows an outbreak has strong effects on air pollution, which reflects an obvious challenge for estimating the causal effect of the latter on the former.
Based on cross-sectional variation, a positive link between regional air pollution and the local severeness of the COVID-19 pandemic and related severe acute respiratory and influenza-like virus infections has been shown. For the early- and severely-hit region of Northern Italy, several cross-sectional studies propose a relationship between high levels of air pollution and numbers of COVID-19 cases (Contini and Costabile, 2020; Pansini and Fornacca, 2020). Others have raised concerns that the high level of domestic bio mass fuel usage in developing countries might aggravate the impact of the pandemic (Thakur et al., 2020), whereby this effect had already been foreshadowed by evidence from the interaction of biomass fuel usage and the Spanish flu pandemic (Clay et al., 2018). For countries besides Italy, Andree (2020) and Cole et al. (2020) report correlations between air pollution and the number of COVID-19 cases across Dutch municipalities. Travaglio et al. (2020) finds positive associations between markers of poor air quality and COVID-19 cases in England. For China, Yongjian et al. (2020) and Yao et al. (2020) describe positive spatial associations between PM2.5 and PM10 and COVID-19 death rates. Wu et al. (2020) find that small increases in long-term PM2.5 concentration are associated with large increases in the COVID-19 death rate, yet acknowledging the inherent limitations of their study design.
Other studies focus on (regional) time series variation in PM. Several studies show positive time series correlations between daily PM levels and ILI in Chinese regions (Liang et al., 2014; Su et al., 2019; Huang et al., 2016). Chen et al. (2018) show Granger causality between PM2.5 and weekly influenza cases with specifically strong effects on the elderly. Similar relationships have previously been found for SARS (Cui et al., 2003).
Causal Effects of Air Pollution. Few studies have attempted to identify a causal effect of air pollution on the onset and severity of respiratory infections using quasi-experimental study designs. Clay et al. (2018) show that air pollution exacerbated the impact of the Spanish flu by applying fixed effects regressions exploiting the differential timing of the pandemic across U.S. cities. Based on a similar methodological approach as our own, Moghadas et al. (2020) show that county-by-month influenza-related hospitalizations increase with higher air pollution. With respect to the COVID-19 pandemic, Persico and Johnson (2020) show that air pollution – instrumented by a rollback of environmental regulations – increases both the number of cases and case fatality of COVID-19 in the U.S. In a closely-related paper, Austin et al. (2020) also apply an instrumental variable approach exploiting daily variation in regional wind direction following Deryugina et al. (2019) using county-level data from the U.S. and also find that higher PM2.5 concentrations increase confirmed the number of cases and deaths of COVID-19.
3. Data and descriptives
COVID-19 Cases and Deaths. We collect data on the number of confirmed cases and deaths of COVID-19 by German counties (Kreise) from the official German COVID-19 reporting database by the Robert-Koch-Institut (RKI). In accordance with the Infection Protection Act (Infektionsschutzgesetz), the RKI collects daily reports from county-level public health offices on newly-detected cases and deaths. Case reports are transmitted to the RKI by 0:00 a.m. on the respective day. The records contain the exact date on which the local public health office became aware of the case and recorded it electronically. For most cases, the data contains additional information on when the patient became ill with clinical symptoms according to the patient's own statement or according to the statement of the treating physician (illness onset). Cases are separately recorded by gender and age group (0–34, 35–59, 60–79, 80+). Daily case counts are regularly updated based on delayed lab confirmations and deaths of earlier-recorded cases. The sample period of our data contains confirmed cases between February 1 and May 26, 2020. We use population counts of demographic groups to normalize case numbers and deaths to 100K inhabitants by county, gender and age.
Table 1 displays the average number of day-by-county cases and deaths per 100K population members, as well as cell population shares for gender × age groups over the entire window of observation from February 1 until May 26, 2020. The bottom row displays the average number of cases and deaths across all age × gender groups. On average, we observe 1.73 confirmed cases and 0.09 deaths per day per county, with a case fatality rate (i.e. the probability of dying of COVID-19 conditional on being a confirmed case) of 5.2 percent. Germany's comparably low case fatality rate compared to European hotspots such as Spain (14.5 percent) or Italy (11.5 percent)3 has been explained by several arguments, including higher numbers of intensive care beds, higher test intensity and demographic factors such as a younger population living largely separated from their parents' generation (Bayer and Kuhn, 2020).
Table 1.
Descriptives: COVID-19 cases and deaths (by county and day).
| Cases per 100K |
Deaths per 100K |
Pop. share |
|||||
|---|---|---|---|---|---|---|---|
| Age range | Gender | Mean | SD | Mean | SD | Mean | SD |
| 0–34 | Men | 1.28 | 3.56 | 0 | .03 | .19 | .02 |
| 0–34 | Women | 1.39 | 3.42 | 0 | .03 | .17 | .02 |
| 35–59 | Men | 1.91 | 4.28 | .02 | .25 | .18 | .01 |
| 35–59 | Women | 2.17 | 4.69 | .01 | .16 | .17 | .01 |
| 60–79 | Men | 1.67 | 4.57 | .19 | 1.28 | .11 | .01 |
| 60–79 | Women | 1.37 | 3.89 | .07 | .65 | .12 | .02 |
| 80+ | Men | 2.86 | 11.55 | 1.04 | 6.14 | .03 | 0 |
| 80+ | Women | 3.11 | 13.52 | .74 | 4.77 | .04 | .01 |
| All | All | 1.73 | 3.14 | .09 | .36 | 1 | 0 |
Notes: This table summarizes means and standard deviations of confirmed cases and deaths of COVID-19, as well as average population shares of demographic groups separated by age and gender. All numbers averaged across counties.
Source: RKI and Statistical Office.
There is considerable heterogeneity in the number of deaths and confirmed cases by age and gender. Below the age of 80, case prevalence varies little by age and gender between 1.3 and 2.2 cases per 100K population members in a respective demographic group. We only observe a higher prevalence of 2.86 cases per 100K population for males and 3.11 cases for females among the very old aged 80 and above. Deaths due to COVID-19 are also concentrated among the very old. Below the age of 60, we observe close to zero deaths per 100K per demographic group. Among male patients between 60 and 79, we observe 0.19 daily deaths per 100K population. The resulting case fatality rate in this demographic group is about eleven percent. Female patients of the same age group display a significantly lower case fatality rate of just 5.1 percent. For very old patients above 80 years, deaths are substantially higher: per 100K population, we observe about one death per day per county for male and about 0.74 deaths per day per county for female patients. Associated case fatality rates are 36 percent for male and 24 percent for female patients.
To understand the limits of interpreting the data, it is important to discuss the testing regime in place over the period under consideration. COVID-19 cases are confirmed after a patient has been positively tested. During the peak of the pandemic, patients in Germany mostly were eligible for scarce testing capacities after having developed symptoms (acute respiratory symptoms and/or a loss of sense of taste/smell) or after having been in contact with a confirmed case. Thus, it can be expected that the majority of asymptomatic cases will have remained undetected. Early estimates of the share of symptomatic cases based on Chinese data from the city of Wuhan range from 5 to 9.2 percent (Read et al., 2020; Nishiura et al., 2020). A population study in the county of Heinsberg in North Rhine-Westphalia, Germany, estimates a five-fold higher number of infected individuals than the number of officially-reported cases (Streeck et al., 2020). We later discuss that the effect of air pollution on the number of confirmed cases likely results from aggravating the symptoms of already-realized infections (instead of increasing the likelihood of infection itself). This interpretation relies on the implicit assumption that the ratio of detected symptomatic vs. undetected asymptomatic cases is the same over districts, which appears plausible given the similar testing regimes across federal states. By only focusing on confirmed symptomatic cases, the results may display a lower bound of the total effect of air pollution on the severity of COVID-19.
Timeline of the Pandemic. The first cases of COVID-19 in Germany were registered and contained near Munich, Bavaria, at the end of January 2020. After that, additional cases – which were related to the outbreak in Italy – were only detected in in the state of Baden-Wuerttemberg on February 25. A first major cluster emerged at the beginning of March in the county of Heinsberg in the state of North Rhine-Westphalia. Shortly after, additional clusters broke out all over the country. German disease control initially reacted with local containment strategies, and federal and state governments stressed that the country was well prepared for a larger outbreak.
On March 11, 2020, on the day that the World Health Organization announced the pandemic status of the COVID-19 outbreak, Chancellor Angela Merkel asked the German population to do everything to avoid the further spread of the virus. Schools and public child care as well as national borders were closed on March 15. On March 16, closures of bars, restaurants, churches and shops followed. Federal states reacted with severe mandatory social distancing measures from March 21 onward. Groups outside were restricted to no more than two persons if from different households. In some states, leaving one's home was only allowed for important or recreational activities. From March 30 onward, visits to hospitals and homes for elderly were prohibited. After numbers of newly-infected decreased again, counter-measures were slowly retracted starting on April 15.
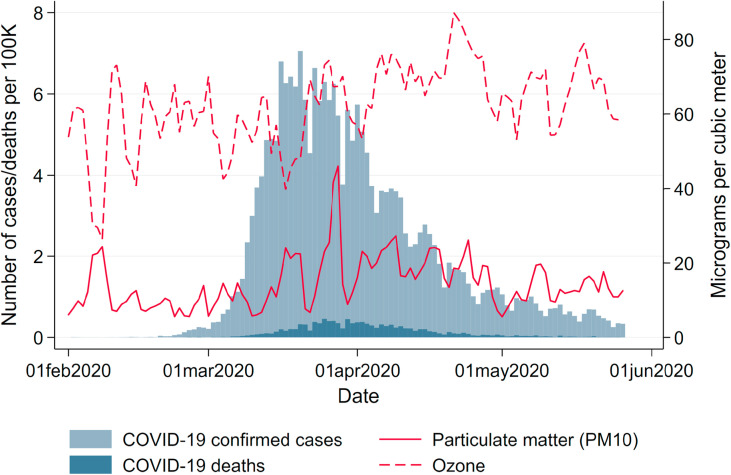
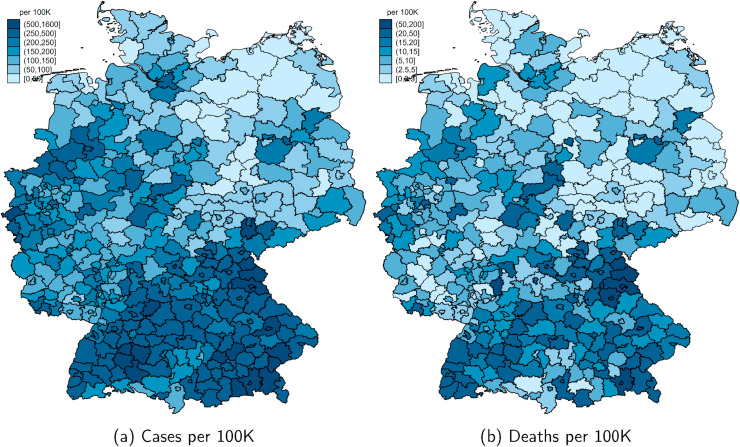
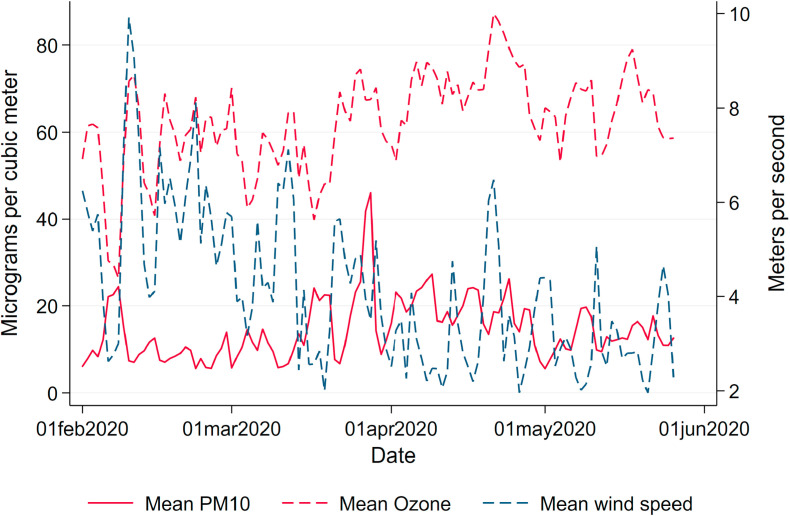
Fig. 1 summarizes the course of the pandemic over our window of observation from February to May 2020. The number of registered cases is displayed in light-blue, and deaths in dark-blue bars. By June 8, 184,193 cases, 8674 deaths and about 169,600 recoveries had been reported to the RKI. The pandemic has hit German regions differentially. Fig. A.1 in the Appendix in the Appendix shows the total number of confirmed cases and deaths per 100K population as of May 26, 2020. Following the first clusters that emerged in the states of North Rhine-Westphalia, Bavaria and Baden-Wuerttemberg, these states remained the hotspots of the pandemic. Northern and especially Eastern states (with the exception of the city of Berlin) were hit much less severely, with some counties experiencing fewer than 50 cumulative cases per 100K population up to the beginning of June.
Fig. 1.
Daily variation in new confirmed cases and deaths and mean air pollution. Note: This graph shows the total number of new confirmed cases and deaths of COVID-19 in Germany as well as the mean concentration of particulate matter (PM10) and ozone (O3) by date.
Source: RKI and UBA.
Air Pollution. We assess local levels of air pollution by PM, which measures the concentration of small airborne particles including dust, dirt, soot, smoke and liquid droplets. PM may be emitted by natural sources such as bush fires, dust storms, pollens and sea spray, or anthropogenic (“man-made”) sources like motor vehicle emissions and industrial processes.
In our main empirical analysis, we focus on PM10, i.e. the concentration of particles up to a diameter of 10 μm. We later corroborate our results using PM2.5. Unfortunately, the coverage of PM2.5 measurements in Germany is significantly scarcer than for PM10. For the window of analysis, measurements of these smaller particulates are only available for a selective set of counties. The measures are strongly correlated and lead to similar aforementioned physical reactions of the human body. Levels of PM10 are strongly correlated with levels of further pollutants such like NO2, SO2 and CO, which are associated with similar inflammatory reactions. Nonetheless, these pollutants are less well covered by measurement stations.
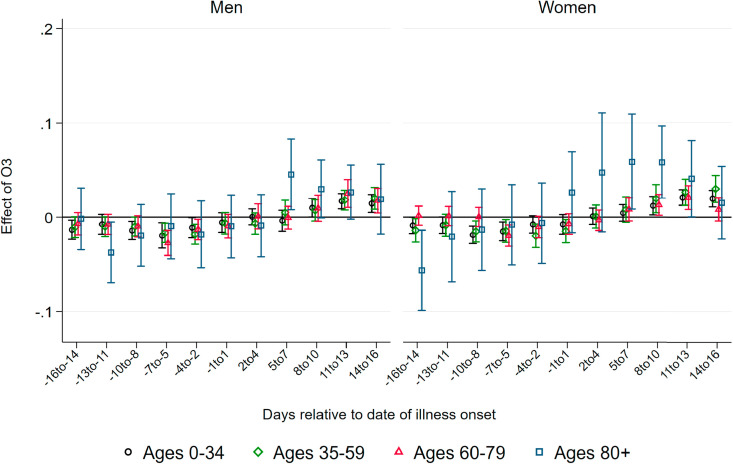
In addition, we assess levels of ozone (O3) as a second dimension of air pollution. Ozone levels are in general negatively correlated with PM levels, yet they again lead to similar inflammatory reactions (Ciencewicki and Jaspers, 2007). Ozone arises from reactions under sunlight of nitrogen oxide with so-called ‘reactive organic substances’. These substances mainly stem from motor vehicle exhausts and aviation. As we focus on the effect of PM10, we treat ozone as a confounder in our regressions. We separately show patterns of the effect of ozone on the onset and severity of the disease in the additional analyses in the Appendix.
Data on PM10 and O3 are provided on a daily basis by the air pollution monitoring system of the German Federal Environment Agency (Umweltbundesamt, UBA). Data is available at the geo-coded monitor level, which allows us to assign levels of air pollution to counties. Specifically, we compute a county's daily pollution level as the inverse-distance weighted mean of all monitors within a radius of 25 km around the county centroid as a proxy for the population center. Fig. A.2 displays the coverage of counties through monitors.4
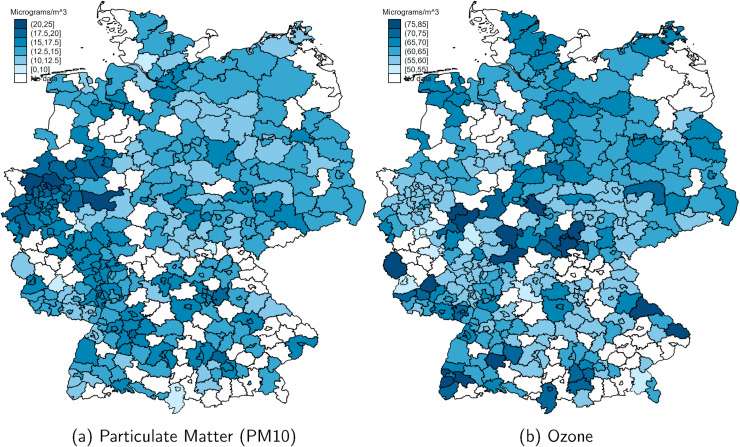
Table 2 summarizes county levels of daily and three-day averages of air pollution. All pollutants are measured in units of μg/m 3. As with the number of confirmed COVID-19 cases per population, Fig. A.3 in the Appendix in the Appendix shows strong heterogeneity in air pollution across counties. PM10 is closely associated with industry agglomeration and population density, with the highest levels in Western Germany, concentrated in the highly-populated and industrialized regions in North Rhine-Westphalia, Rhineland-Palatinate and Baden-Wuerttemberg following the rivers Rhine, Main and Ruhr, which connected the former heavily industrialized regions of Western Germany. Interpreting simple spatial correlations between average levels of air pollution and the local onset and severity of the COVID-19 pandemic might lead to erroneous claims about a causal effect of the former on the latter. We will instead focus our empirical analysis on within-county changes in short-term exposure to air pollution to circumvent this identification problem.
Table 2.
Descriptives: Air pollution and weather (by county and day).
| Mode of Transport | Unit | Avg. Period | Mean | SD | Min | Max | |
|---|---|---|---|---|---|---|---|
| Air pollution | PM10 | μg/m3 | 1 day | 14.5 | 8.5 | 1 | 95 |
| PM10 | μg/m3 | 3 days | 14.5 | 7.2 | 1 | 55.3 | |
| Ozone | μg/m3 | 1 day | 62.3 | 14.8 | 2.1 | 132.5 | |
| Ozone | μg/m3 | 3 days | 62.3 | 12.8 | 2.3 | 125.8 | |
| PM2.5 | μg/m3 | 1 day | 8.7 | 5.5 | .4 | 50.6 | |
| PM2.5 | μg/m3 | 3 days | 8.7 | 4.6 | .7 | 41.1 | |
| Weather | Precipitation | mm/m2 | 1 day | 2 | 3.9 | 0 | 52.1 |
| Wind speed | m/s | 1 day | 4.1 | 2 | .9 | 16.5 | |
| Temperature | ○ C | 1 day | 8.4 | 4.3 | −3.5 | 20.8 |
Notes: This table summarizes means and standard deviations of average pollution and weather measures across counties.
Source: UBA and DWD.
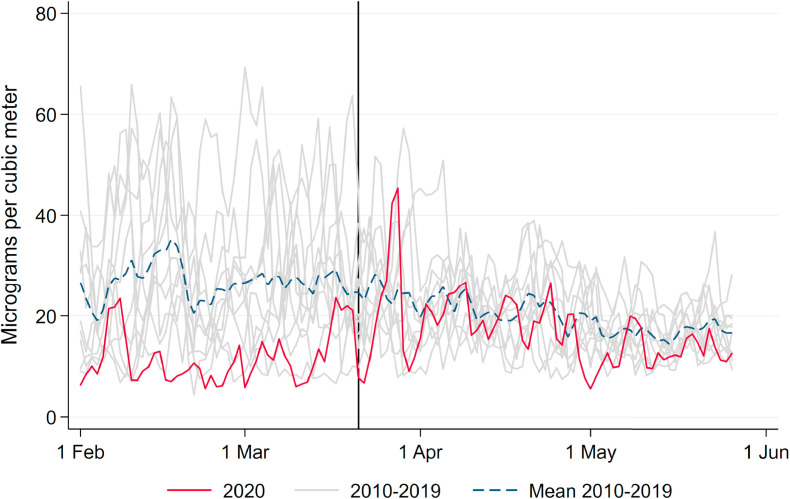
Solid and dashed red lines in Fig. 1 display average levels of our main independent of interest, namely levels of PM10 and O3. Both variables display significant day-to-day variation over time. While PM10 pollution levels have in general declined by the reduced economic activity during the pandemic (Venter et al., 2020), we do not observe respective reductions in Germany; rather, levels of PM10 even reached a maximum during the peak of the crisis. The reasons for this particular development of air pollution in Germany lie in several sources. First and foremost, the lockdown coincided with a period of extremely low precipitation, reducing the amount of PM washed out of the air by rain (see below). Second, the German lockdown was less severe than in other countries. While economic activity was reduced overall, economic sectors hit hardest by the lockdown – such as the hospitality and entertainment industries and retail – are characterized by rather low levels of emissions. Strongly polluting industries in the manufacturing sector kept up their production during the lockdown. Industrial processes, heating and agriculture – which are arguably less affected by the lockdown – contribute up to about 70 percent.5 Taken together, these facts led to above-average levels of pollution during the lockdown for German standards, as can be seen in Appendix Fig. A.4, which depicts pollution levels in spring 2020 compared to pollution levels over the past ten years.
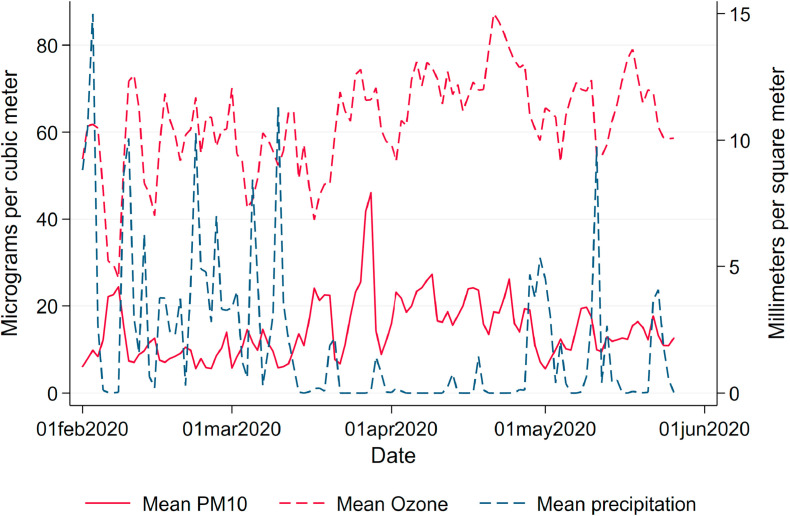
Weather.Weather conditions drastically changed in Germany almost simultaneously with the lockdown, leading to particularly high levels of pollution. Levels of air pollution are heavily influenced by local weather conditions. Wind speed and precipitation reduce the level of air pollution by “washing away” PM. Given that wind speed and precipitation may also have a direct effect on the virus spread by affecting contact probabilities and virus survival in the air, we control for these variables as potential confounders. We use daily local measurements of weather conditions provided by the German Meteorological Services (Deutscher Wetterdienst, DWD) as controls in our regressions. Daily county means of weather conditions (precipitation in mm/m 2, windspeed in m/s and temperature in degree Celsius) are listed in Table 2. and Fig. A.5, Fig. A.6 in the Appendix highlight how both average precipitation and wind speed dropped to extremely low levels over the entire observation window, partially explaining the high levels of air pollution coinciding with the pandemic.
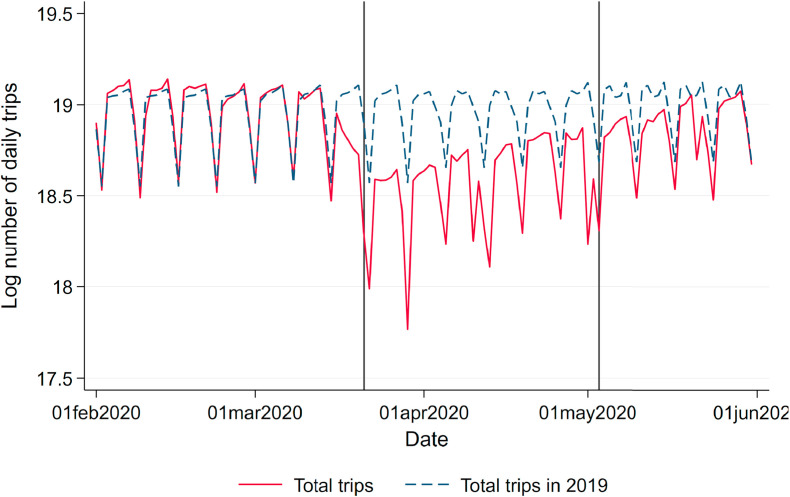
Mobility Patterns. As several studies have shown, the reduced economic activity during the lockdown has triggered lower levels of air pollution and simultaneously led to lower numbers of cases. To estimate the effect of air pollution in light of these simultaneous effects, we keep mobility patterns constant by controlling for mobile phone mobility. We use commercial data on daily levels of mobile phone mobility provided by Teralytics, which allows measuring the daily frequencies of trips within and across counties for about one-third of Germany's mobile phone market. Fig. A.7 in the Appendix in the Appendix displays the sharp reduction of mobility during the lockdown.
4. Empirical approach
To estimate the effect of higher air pollution levels on the number of COVID-19 cases and deaths, we compare changes in case and death numbers in relation to changes in short-term exposure to air pollution. To account for potential issues of simultaneity through lockdown measures affecting both cases and pollution, we instrument local changes by regional effects of wind directions on pollution. Our aim is to estimate the following relationship:
| (1) |
where is the dependent variable, i.e. the number of deaths or confirmed cases in county i for the date of illness onset t, normalized to 100K population by gender-age group g. Fixed effects for the county (α i) and date (μ t) keep time-invariant confounding variables at the county level and nationwide homogeneous time-variant factors constant.
Our main independent variable of interest is a three-day average of particulate matter, PM i,t−l, measured in lags or leads l relative to the date of illness onset t.6 We consider lags and leads about two weeks before and after the date of illness onset to analyze at which stages of the disease air pollution has an effect on the severity of COVID-19. This window of lags and leads encompasses the typical course of the disease: The median incubation period between infection and the onset of illness has been reported to range between five to six days (WHO, 2020). Several studies report a median time between the onset of illness until hospitalization of four days (Docherty et al., 2020; Chen et al., 2020). The time between hospitalization and intensive care is estimated as about one day (ISARIC, 2020). Other studies report the median time between the onset of illness and pneumonia as four days, and eight days for acute lung failure (Guan et al., 2020; Li and Ma, 2020). Based on data from New York, on average deceased patients were hospitalized for nine days (Cummings et al., 2020).7
We remain ex ante agnostic about when exactly to expect the effects during the course of the illness.8 For the number of deaths, plausible effects could happen both during and before the onset of the infection. Higher initial viral loads – e.g. through higher ability of the virus to cause airborne infection at high levels of air pollution – have been shown to lead to more severe progressions later in the course of the disease. However, effects after the onset of illness are also plausible, when higher air pollution causes inflammatory reactions and additional burden for already-stressed immune systems, thus increasing the severeness of symptoms.
For the number of cases, at first glance it appears only plausible to expect effects up to the date of onset of illness (the latest point where an infection could have happened), but not after. Nonetheless, the incomplete testing that hampers the data collection allows for a less obvious channel of air pollution affecting the number of cases even after their onset of illness: as case confirmations rely on infected individuals to seek testing, symptoms aggravated through air pollution after infection and the onset of illness can plausibly lead to higher numbers of confirmed cases.
In regression model (1), we control for a number of confounding variables for air pollution as well as factors that affect the dynamics of the epidemic. In particular, we control for three-day averages of local weather conditions W i,t−l (temperature, wind speed, precipitation). In addition, W i,t−l contains three-day averages of ozone as a potential confounding variable. While the concentration of PM is typically strongly and positively correlated with other criteria air pollutants (like nitrogen oxides, sulfur dioxide, carbon monoxide, etc.) and therefore measurements of PM also pick up variation in these other pollutants, the correlation between PM and ozone is typically negative as ozone is generated from the chemical interaction of nitrogen oxides, volatile organic compounds (VOCs) under heat and sunlight. Therefore, ozone is typically very high in summer when concentrations of PM and other fossil-fuel related air pollutants are typically lower. Hence, ozone concentrations are typically negatively correlated with PM. Since ozone also irritates the respiratory system of the human body and may also make COVID-19 more severe, it acts as a confounding variable in the PM analysis and thus we keep it constant.
Additional control variables are represented by the vector X i,t−l. To further control for differing local states of the pandemic as well as local counter-measures, this includes the number of active cases as the aggregate number of new confirmed cases net of confirmed deaths over the past three weeks.9 To control for potential confounding effects of avoiding behavior through masks (see the later discussion in Section 5.2), we control for local mask requirements in public transport and shops at the county level collected by Mitze et al. (2020). Finally, to keep mobility patterns potentially coinciding or triggering reductions in air pollution statistically constant, we control for several mobility indicators based on mobile phone mobility as described in Section 3. The vector X i,t−l also represents controls for county-specific mobility based on mobile phone data. We also include state-specific fixed effects for the main phases of the pandemic throughout the period under investigation.10 Standard errors ɛ it are clustered at the county level.
The ambient concentration of PM may be endogenous when lockdown measures, reduced economic activity and social distancing have simultaneous effects on both COVID-19 case numbers and air pollution. We therefore employ an instrumental variable approach exploiting plausibly exogenous local variation in wind directions. Wind direction may serve as a strong predictor of levels of pollution as particles may be transported in ambient air over long distances. Hence, depending on the wind direction, a location may be more or less exposed to air pollution stemming from specific sources. On the other hand, wind direction is plausibly exogenous to the severity and case numbers of COVID-19. This IV strategy closely follows the recent contribution by Deryugina et al. (2019).
We use data from the DWD and assign three-day averages of wind directions at the county level, WindDir i,t−l, by taking the wind direction measured at the nearest wind monitor to the county centroid and transform wind direction into four wind direction bins d defined according to the four quadrants of a wind rose: North-East, South-East, South-West and North-West. Our instrumental variable is a series of binary indicators resulting from interactions with the four wind direction bins d and a binary indicator for county i being part of region r. Regions are defined as federal states, where out of all sixteen states the three city-states of Berlin, Hamburg and Bremen as well as the small state of Saarland are merged with their larger neighboring states, yielding twelve regions in total. Using the most frequent wind direction bin (South-West) as the baseline, this gives us (D − 1) × R = 3 × 12 = 36 indicator variables that we use as instruments for PM i,t−l. Hence, the first-stage regression equation reads as:
| (2) |
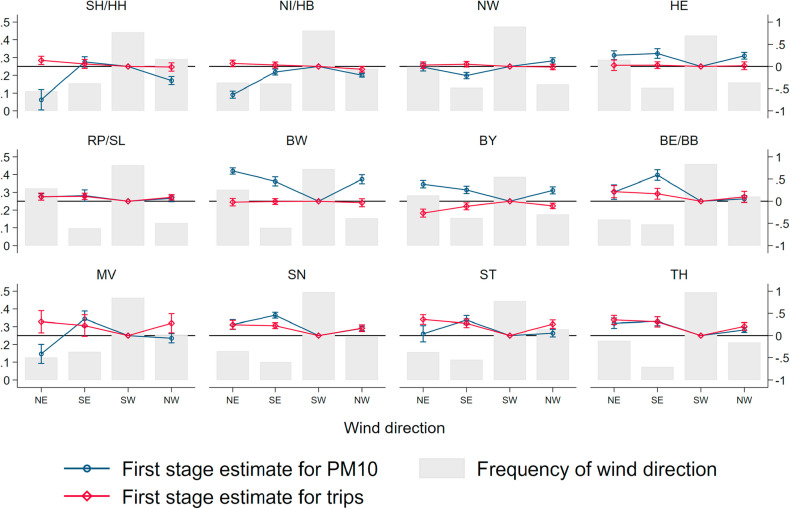
This flexible specification allows us to remain agnostic about which wind direction causes higher air pollution in a specific region, which may depend on the relative direction of major pollutant sources. Fig. A.8 in the Appendix in the Appendix summarizes the results of the first stage. Gray bars indicate relative frequencies of wind directions, which are more or less homogeneous across regions. During the window of observation, wind from the South-West was the most common wind direction. The effects of wind direction on pollution differ strongly between regions. For example, wind from the North-East and North-West reduces levels of particulate pollution in the northern coastal states of Schleswig-Holstein/Hamburg (SH/HH), Niedersachsen/Bremen (NI/HB) and Mecklenburg-Vorpommern (MV), as it transports relatively clean air from the North and Baltic Sea. In other states, the same wind direction leads to higher air pollution relative to days with the wind blowing from the South-West as it carries particles suspended from inland emission sources.11
5. Results
5.1. Effects of PM10 on COVID-19 cases and deaths
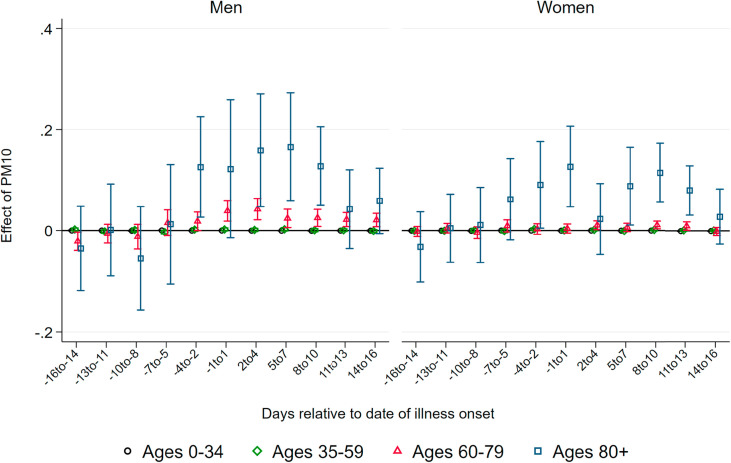
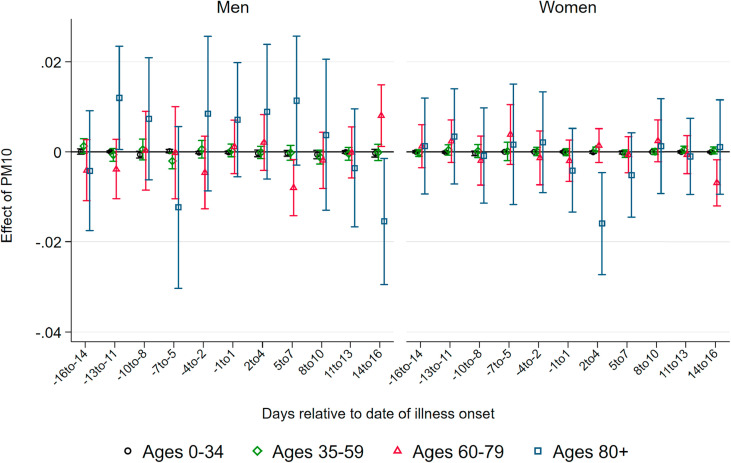
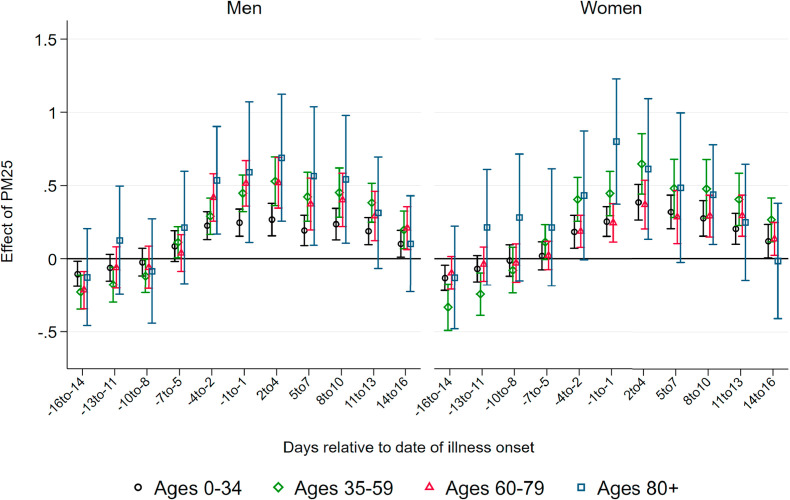
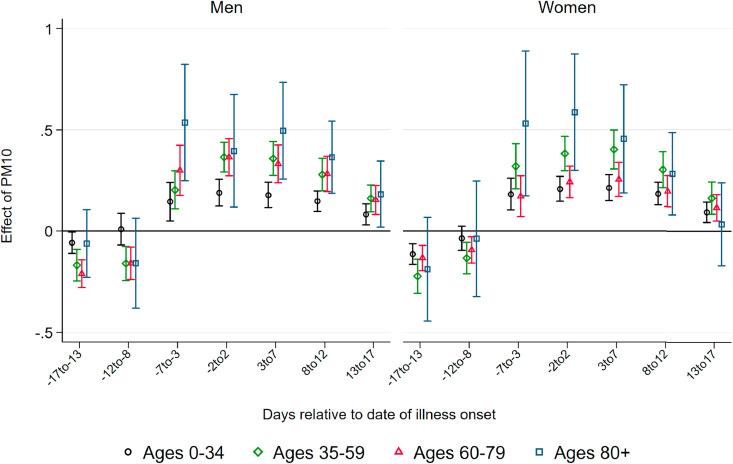
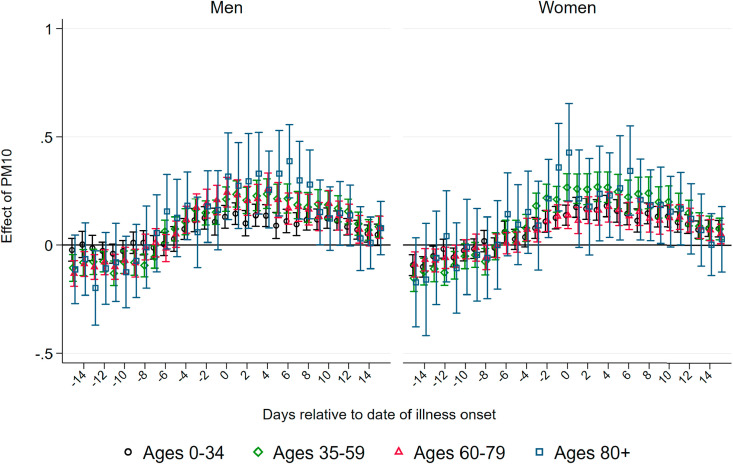
Effect of PM10 on Deaths.Fig. 2 displays coefficients of three-day averages of PM10 for lags and leads surrounding the day of the onset of illness on the number of deaths per 100K population members in a respective demographic group. The estimation is based on equation (1). Each coefficient stems from a separate regression of the number of deaths in a particular age and gender group on the respective lag/lead predicted pollution levels. Pollution appears to be unrelated to the severity of the pandemic if it occurs before infection. From four days before the onset of symptoms, higher levels of PM10 display significant effects, which are heterogeneous by age group. This implies that becoming infected with SARS-CoV-2 while exposed to higher levels of ambient air pollution (before the onset of symptoms) increases the likelihood of a fatal course of the disease, most likely by reducing the body's initial immune response. In addition, exposure to pollution in the further course of the disease (after developing symptoms) also raises the likelihood of not recovering from the infection. This is particularly the case for older patients.
Fig. 2.
Effect of PM10 on new deaths from COVID-19. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM10 concentration on the number of deaths from COVID-19per 100K population . Each coefficient results from a separate regression of deceased patients on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
For patients below the aged of 60, effect patterns are precisely estimated and remain close to zero. For male patients aged 60–79 years, we observe significant effects from the onset of illness onward, with the stronger effects just after symptoms occurred: a one μg/m 3 increase in PM10 two to four days after the onset of illness leads to 0.042 additional deaths per 100K population. Translated to standard deviations as reported in Table 1, Table 2, a one standard deviation increase in air pollution two to four days after the onset of illness leads on average to 0.3 additional deaths per 100K population. This effect is sizable in relative terms and accounts for an increase of about 24 percent of a standard deviation in the fatality rate of this demographic group. For women, the effect is similar in relative terms, but it is only marginally significant with an effect of about 0.01 additional deaths for a one μg/m 3 increase in PM10 two to four days after the onset of symptoms.
For men aged 80 years and older, we observe significant positive effects from four days before to ten days after the onset of symptoms. The effect is strongest between five and seven days after the onset of illness. At this point, a one μg/m 3 increase in PM10 leads to about 0.16 additional deaths per 100K population members. This implies an effect of a one standard deviation increase in air pollution of 1.2 additional deaths per 100K population on average, corresponding to about 19 percent of a standard deviation in the fatality rate per 100K population members of this demographic group. Due to the small sample sizes in this age group, effect sizes are rather imprecisely estimated, with 95 percent confidence intervals ranging from 0.05 to 0.27 additional deaths per one μg/m 3 increase. For women, the effect pattern is similar: the effect of a one standard deviation increase in PM10 concentration exactly around the onset of illness for female patients aged 80 and above accounts for a similar increase of about 0.9 additional deaths, corresponding to 19 percent of a standard deviation in the number of deaths.
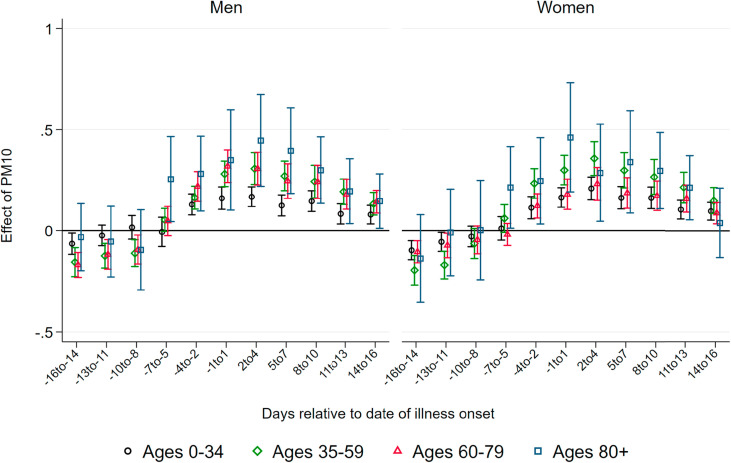
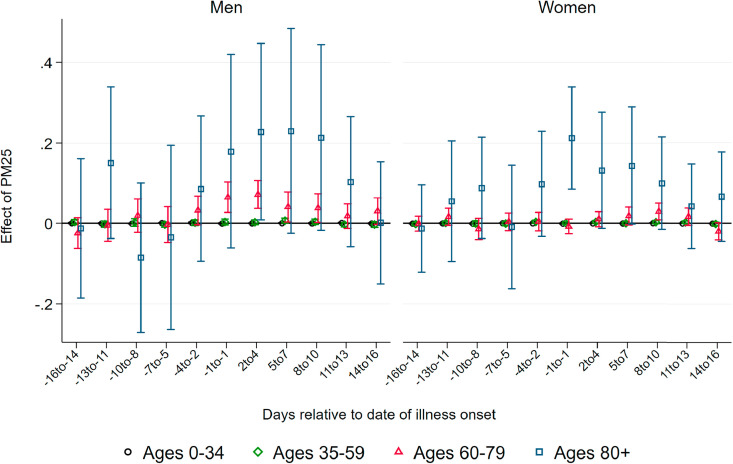
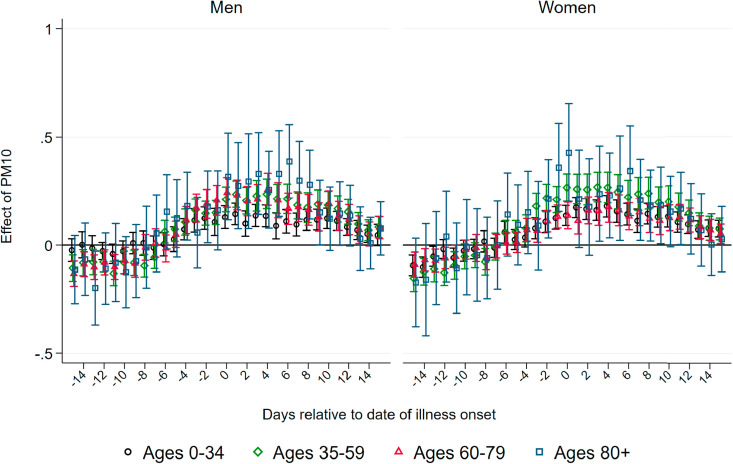
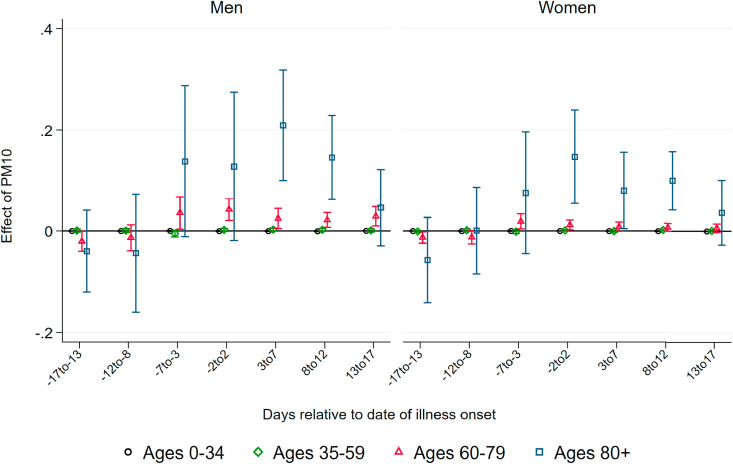
Effect of PM10 on Confirmed Cases.Fig. 3 summarizes coefficients from regressions of the number of confirmed cases per 100K population on three-day averages of PM10 surrounding the onset of illness, based on separate samples split by age and gender. We do not observe effects of higher air pollution until about one week before the onset of illness. Starting at seven to five days before the onset of illness – which roughly coincides with the median incubation period of five to six days – we observe statistically significant positive effects of PM10 pollution on new confirmed cases for all demographic groups.
Fig. 3.
Effect of PM10 on new confirmed cases of COVID-19. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM10 concentration on the number of new confirmed cases of COVID-19 per 100K population members. Each coefficient results from a separate regression of newly-confirmed cases on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Coefficient sizes again increase with the age of the patients, albeit less strongly compared to the effect on the number of deaths. A one μg/m 3 increase in three-day average PM10 concentration causes between 0.2 and 0.45 additional confirmed cases per day per 100K population members, corresponding to increases between 13 and 18 percent relative to the respective demographic group's mean (see Table 1). For the critical age group of patients aged 80 and above, we find that a one μg/m 3 higher level of PM10 around and shortly after the onset of illness increases the number of confirmed cases for male and female patients by about 0.45 cases per 100K population members. This translates into an effect of a one standard deviation increase in PM10 of more than three additional confirmed cases, corresponding to an increase of around 25 percent of a standard deviation. The effect sizes remain statistically significant but substantially decrease in magnitude for periods more than one week after the onset of symptoms.
The results of air pollution after the onset of symptoms increasing the number of confirmed cases imply that air pollution aggravates symptoms of already-realized infections. During the peak of the pandemic, asymptomatic cases remained largely undetected as predominantly symptomatic patients were tested. Symptoms aggravated by higher levels of air pollution increase the number of symptomatic compared to asymptomatic cases, swaying people to seek testing, and thus also increasing the number of confirmed cases. In addition, significant effects before the onset of symptoms are in line with the notion that air pollution makes the human body more susceptible to an infection due to irritation of the airways.
The significant effect of air pollution on the number of confirmed cases has important implications for interpreting case fatality rates, i.e. deaths divided by cases per population, which are an important and often-used indicator for the severity of the pandemic. Higher air pollution positively affects both the nominator and denominator of such an indicator for all age groups. Thus, an effect of air pollution on the severity of COVID-19 measured in case fatality rates would be downward-biased if higher air pollution after the onset of illness aggravates symptoms and reduces the number of asymptomatic and thus potentially undetected cases. Fig. A.9 in the Appendix in the Appendix supports this argument: case fatality rates for age groups aged 60 and above are indeed reduced in response to higher air pollution after the onset of illness.
5.2. Additional analyses and discussion
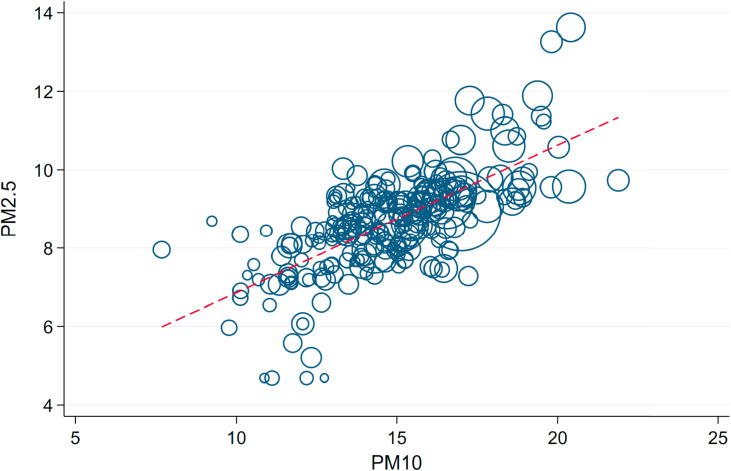
Effects of PM2.5. Some of the related literature on the effect of air pollution has focused on PM2.5 – particulate matter with a diameter up to a fourth of PM10. PM2.5 indeed has a stronger potential to enter deeper into the human respiratory system and might lead to more severe inflammatory reactions. Unfortunately, the measurement of PM2.5 in Germany is less widespread and universal, and data is not as readily available as in the case of PM10. Additionally, as Fig. A.10 shows, the two measurements are strongly correlated, and thus we believe that the effect of PM10 already gives a sufficient approximation of the effect of smaller particulates.
We further estimate the effect of PM2.5 for those counties for which we were able to secure data access. and Fig. A.11, Fig. A.12 in the Appendix summarize the results of these regressions. The results mainly confirm the patterns that we have found for PM10 in the main results, yet in a less pronounced way. Because we cannot rule out selectivity in the counties providing PM2.5 measurements, we draw our main conclusions from the results on PM10 described above.
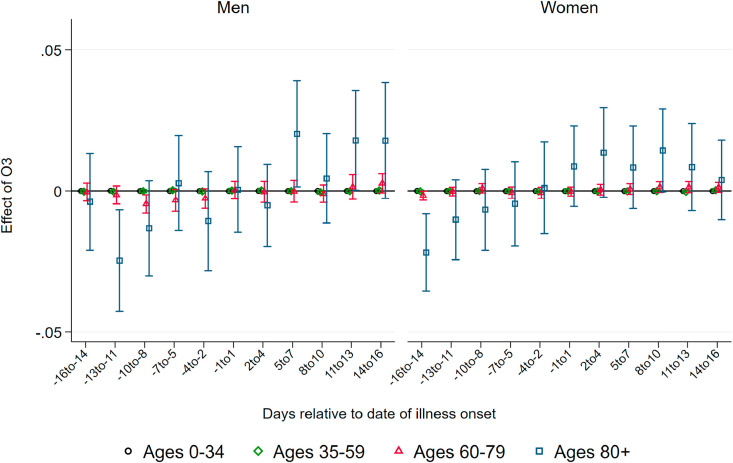
Effects of Ozone. As several studies show (see Ciencewicki and Jaspers, 2007, for an overview), ozone itself has adverse respiratory health effects. While we consider ozone as a confounder to be kept constant in our main regressions summarized above, it is informative to look at coefficient patterns of ozone as an additional mechanism of how air pollution can affect the number of deaths by and confirmed cases of COVID-19. and Fig. A.13, Fig. A.14 in the Appendix summarize the respective coefficient patterns resulting from the same regressions as summarized above. In contrast to the strong effects exhibited by PM10, we only observe very small effects of ozone arising a few days after the onset of illness and mainly for the oldest age group above 80 years. This effect is only marginally significant for male patients. Similarly, we observe effects of ozone after five days on the number of confirmed cases for both male and female patients of the oldest age group and to a much lesser extent for younger age groups. Taken together, it appears that ozone leads to qualitatively similar but quantitatively less pronounced effects on the onset and severity of COVID-19. Ozone arguably does not add to the proposed mechanism of increasing the ability of the virus to cause airborne infection. The observed effect on the number of cases and severity therefore supports the notion that our results are mainly explained through additional inflammatory reactions by which higher air pollution aggravates COVID-19 infections. However, one has to keep in mind that throughout the period under investigation ozone levels remained relatively low, since ozone concentrations are typically much more elevated during the summer, which is beyond our period of investigation until late-May.
Masks as Avoidance Behavior. Face masks have become one of the main non-pharmaceutical interventions to slow down the spread of the novel coronavirus by reducing the number of virus-carrying aerosols suspended to the air. At the same time, covering the mouth and nose may also affect individual exposure to particulate pollution of ambient air (Pacitto et al., 2019). Our results will display lower bounds of the overall physiological effect of pollution if individuals respond to higher pollution by covering their faces with masks or scarfs. In the following, we lay out two arguments against mask-wearing as a serious concern for the interpretation of our results.
First, effectiveness towards reducing inhaling particulate pollution is questionable for the specific kind of face masks that has been predominantly applied during the COVID-19 pandemic. Even for medical masks that are most effective in reducing the spread of infections, little is known about their effectiveness against polluted air (Huang and Morawska, 2019). Commercially available face masks may not provide adequate protection against PM, primarily due to poor facial fit (Cherrie et al., 2018). Cloth masks – which have been the most widely-used form of mouth and nose covering during the COVID-19 pandemic in Germany – are only marginally beneficial in protecting individuals from particulate pollution because they are unsuitable to prevent the inhalation of fine particles (Shakya et al., 2017). Given this evidence, the extent to which wearing face masks effectively reduces individual exposure to air pollution is rather limited.
Second, unlike in many Asian societies, the wearing of face masks in public – for avoiding infections or pollution – was virtually inexistent prior to the COVID-19 pandemic in Germany, except for medical staff at work. Ambient air pollution in general does not reach levels that would warrant wearing masks. The adoption of face masks only became widespread long after the onset of the COVID-19 pandemic. Mask requirements were not part of the very first non-pharmaceutical policy interventions implemented at the very beginning of the spread of the novel coronavirus in Germany starting in early-/mid-March. Indeed, for a long time, the RKI as the federal pandemic prevention authority even cautioned against the usage of face masks as they would provide a false sense of protection. Wearing face masks only became mandatory in public transportation and shops as well as many other indoor settings where distancing is difficult (workplaces, schools, etc.) only in late-April.12
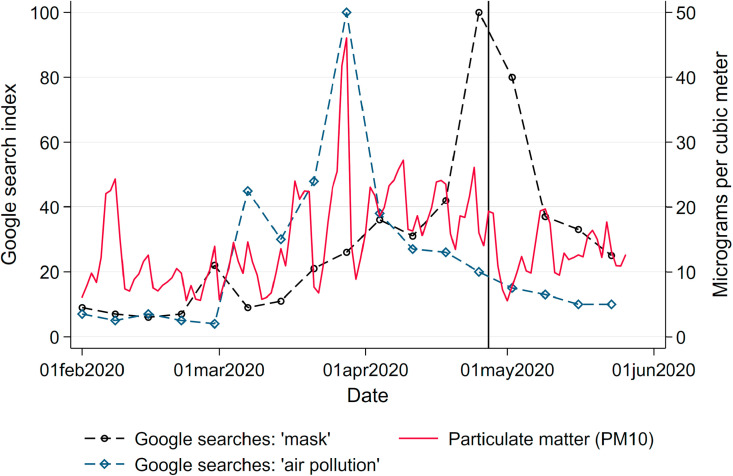
To corroborate these arguments with observed behavior, we relate the frequency of internet searches for the term “mask” (in German: Maske) and “air pollution” (in German: Luftverschmutzung) during our window of observation to levels of PM10 (Fig. A.15 in the Appendix).13 Searches started to increase slowly during April and spiked around the date when masks became mandatory in most states. The search frequency remained unresponsive to levels of PM10 pollution. Spikes in the search volume for the term “air pollution” are triggered by episodes of high PM10 concentrations across the country. This implies that while Germans seem to notice episodes of elevated levels of air pollution, they do not seem to respond with mask-wearing as a form of avoidance behavior reducing exposure to poor air quality. This again contrasts against behavior in locations where people are frequently exposed to extremely high levels of air pollution, such as Chinese industrial cities. For example, Zhang and Mu (2018) document significant increases of face mask purchases during extreme pollution episodes in China.
6. Policy implications
Our results point to significant effects of higher ambient air pollution on the severity of COVID-19 cases. Especially for the more vulnerable patients, we observe significantly higher mortality rates if patients are exposed to higher air pollution a few days after case confirmation. These health effects display a considerable additional economic cost of contemporary air pollution. Thus, our results have far-reaching implications for immediate policy responses to the ongoing as well as future pandemics of respiratory diseases that interact with ambient air pollution.
In the following, we discuss three groups of policy instruments that may be justified by the results of this paper: measures reducing short-term exposure to pollution, measures reducing short-term levels of pollution, and measures reducing the level and exposure to pollution in the longer term, beyond the immediate concerns of the current pandemic.
In the short run, any policy that reduces the exposure of vulnerable patients towards ambient air pollution might lead to a reduced mortality of COVID-19. These policies may contain technical counter-measures such like air purifiers to be used to reduce levels of indoor pollution. The effectiveness and beneficial health effects of such purifiers have been demonstrated – among others – by Chen et al. (2015) and Karottki et al. (2015). Public health messages could be broadcasted to raise the public's awareness about the interaction of air pollution and the severity of COVID-19 to further encourage staying indoors on days with high air pollution, as discussed by Kelly et al. (2012), D'Antoni et al. (2017) and Barwick et al. (2019).
Further, the added benefits of reducing local air pollution levels identified in this paper should be taken into account in careful cost-benefit analyses of more comprehensive policies to reduce local pollutant emissions (traffic shutdowns (Pestel and Wozny, 2019; Davis, 2008), speed limits (Bel and Rosell, 2013), or restrictions by vehicle type (Barahona et al., 2020)), or sustainable long-term policies reducing levels of pollution, such as industrial regulations (Persico and Johnson, 2020) and investments in public transport (Li et al., 2019; Gallego et al., 2013).
Measures aimed at reducing levels and exposure of air pollution might have an additional indirect effect by reducing the number and severity of further respiratory diseases. Reducing the exposure and level of pollution not only reduces the number of severe COVID-19 cases but also the number of patients in intensive care and under ventilator usage with further respiratory diseases (Ciencewicki and Jaspers, 2007; Graff Zivin et al., 2020), thus reducing the likelihood of an overburdened health care system.
The policy implications discussed above might hold particular importance for less-advanced economies. These are in general characterized by higher levels of air pollution resulting from excess usage of fossil fuels in heating and cooking, as well as lower levels of pollution control (Mannucci and Franchini, 2017). At the same time, developing economies provide less-developed health care infrastructure, a lower number of intensive care beds and ventilators. This combination might likely increase the effectiveness of policy measures reducing levels and exposure to pollution in reducing the mortality of COVID-19 and related diseases.
7. Conclusions
This paper studies the effect of short-term changes in the exposure to air pollution – measured by levels of particulate matter PM10 – on the onset and severity of COVID-19. We base our analysis on comprehensive data on the number of confirmed cases and deaths from the official reporting provided by the Robert-Koch Institute, the German disease control authority. We merge this data with county × three-day averages of pollutants and potentially confounding weather conditions as well as a number of control variables related to the course of the pandemic. To isolate the effect of air pollution from confounding factors, we instrument for PM10 concentrations by exploiting local variation in daily wind directions following Deryugina et al. (2019), while keeping constant time-variant global confounders and time-invariant confounders at the county level.
Our findings suggest a strong impact of short-term variation in ambient air pollution on the severity of the COVID-19 pandemic. Specifically, we find that higher particulate pollution between four days before and ten days after the onset of symptoms significantly increases both the number of deaths as well as the number of confirmed cases by day and county. We also find that air pollution just before as well as after the onset of illness leads to increased numbers of confirmed cases across the entire age distribution, which is likely explained by aggravated symptoms swaying patients to get tested.
These results are qualitatively in line with cross-sectional correlations between air pollution and COVID-19 severity that have been documented so far (see among others Cole et al. (2020), Becchetti et al. (2020) and Wu et al. (2020)). Bhaskar et al. (2020) conclude that 27 out of 28 systematically-reviewed studies point to positive and significant associations.14
We further complement two quasi-experimental studies using within-region short-term fluctuations in PM to identify effects on COVID-19 case numbers and severity in U.S. settings. Austin et al. (2020) instrument daily levels of PM2.5 using the same instrumental strategy that we employ in this paper. They find that an one μg/m 3 increase in PM2.5 increases the same-day death rate by three percent from the mean. This rather modest effect stands in contrast to the substantially larger effect by Persico and Johnson (2020) of a one μg/m 3 increase in predicted PM2.5 doubling the conditional death rate from COVID-19. Our data does not contain the exact day of death but the day of onset of symptoms. As we estimate effects of PM levels relative to the start of symptoms, our results imply that PM levels matter most around the average span between the onset of symptoms and death, complementing the U.S. results described.15
Our empirical design allows us to causally interpret the estimated effects. Our result patterns largely confirm previous cross-sectional and time series-based studies and show that previous results are likely to hold when unobserved confounders are taken into account. We extend beyond these studies by relying on fine-grained daily data on the onset of illness, which allows us to discriminate between transmitting mechanisms: The timing of the estimated effects – only affecting severity after the onset of illness – supports mechanisms of increased inflammatory reactions causing additional stress for the immune system.
Further, our results show substantial effects in a setting of modest pollution levels in a Western society with a sufficient supply of high-quality health care, intensive care units and ventilators. There is reason to fear – and a need for additional research to confirm – that the effect of air pollution on the severity of COVID-19 will be intensified when the pandemic reaches less-developed world regions where PM pollution and associated health risks hold much stronger importance, e.g. through the more widespread (indoor) use of fossil fuels for cooking and heating, and where the supply of high-quality medical care is constrained.
Declaration of competing interest
The authors have no financial or other conflicts of interest to disclose.
Acknowledgments
We are grateful to Steffen Künn, Juan Palacios, Nicolas R. Ziebarth, seminar participants at IZA as well as the editor and two anonymous referees for extremely helpful comments and suggestions. Dajan Baischew, Eva Justenhoven and Marc Lipfert provided excellent research assistance. Funding by the German Research Foundation (DFG) through CRC TR 224 (Project A02) is gratefully acknowledged. An earlier version of this paper is available as IZA Discussion Paper No. 13418.
Footnotes
Numbers taken from the John Hopkins University Coronavirus Resource Center: https://coronavirus.jhu.edu/map.html (last updated: 11 December 2020).
Oxidative stress describes a situation where the production of free radicals exceeds the metabolism's ability to counteract or detoxify and contributes to the pathogenesis of respiratory infections (Liu et al., 2017).
Fatality rates are from https://ourworldindata.org/coronavirus/(last accessed June 23, 2020).
Ground-level monitor data may suffer from issues of low coverage and interpolation from single monitors to counties. Nonetheless, these issues hold limited importance in a densely-populated country such as Germany. Usage of alternative satellite or simulated data is unfeasible for assessing day-to-day variations in pollution (Sullivan and Krupnick, 2018; Christopher and Gupta, 2010; Sorek-Hamer et al., 2016; Muller and Mendelsohn, 2007).
Calculations based on https://www.umweltbundesamt.de/daten/luft/luftschadstoff-emissionen-in-deutschland#ermittlung-der-emissionsmengen (last accessed: June 22, 2020).
Defining lags and leads based on daily changes or longer five-day averages leads to qualitatively similar patterns (Fig. A.16, Fig. A.17, Fig. A.18, Fig. A.19).
See also the SARS-CoV-2 briefing note of the RKI at https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html (last accessed: June 26, 2020).
This sets us apart from a common event study design since we cannot rely on a pre-treatment period as a comparison group, as we do not ex ante know when the treatment occurs. Instead, as a comparison period we use time windows where no effect is yet to be expected (before infection) or no longer expected (after the illness).
To calculate the number of active cases, we rely on an estimated duration to recovery of three weeks. This may overestimate the time to recovery for the majority of mildly symptomatic cases, but may underestimate the time to recovery of the minority of strongly symptomatic cases.
The main phases are (1) before the national lockdown with comprehensive social distancing requirements implemented on March 21, (2) the lockdown phase from March 22 to May 3, and (3) the phase of relaxation of social distancing requirements after May 3.
A potential threat to the validity of the instrument would arise if mobility were directly affected by weather conditions correlated with local wind direction, if these weather conditions were not fully picked up by our controls for precipitation, temperature and wind speed. Indeed, we find that residuals from a regression of total mobile phone movements per day and county on weather controls (wind speed, temperature, and precipitation) as well as further controls and fixed effects (date and county) as in equations (1), (2) are not systematically or meaningfully related to variation in wind directions.
Only few individual counties made face masks mandatory as of early-April, which has proven very effective in substantially slowing down the spread of the virus (Mitze et al., 2020).
Similar patterns are found for related searches “Mundschutz” (face cover), “Mund-Nasen-Schutz” (mouth-nose-cover) and “Maskenpflicht” (mandatory mask-wearing), as well as “Luftqualit’ (air quality).
Our research design substantially differs from most of these studies by using within-variation in municipalities as well as allowing for effects to materialize at different points surrounding the day of confirmed infection. A quantitative comparison in magnitudes to these studies is unfeasible.
Again, our results are not straightforward to compare in magnitude, as we focus on heterogeneous results by age group. For the affected vulnerable patients aged 80 and over, our results strongly exceed the average effects documented by Austin et al. (2020) and are not as large as the average effects found by Persico and Johnson (2020).
A. Appendix
Fig. A.1.
Total confirmed cases and deaths of COVID-19 by county. Note: These maps show the total number of confirmed of COVID-19 cases and deaths per 100K population across counties (Kreise) as of May 26, 2020.
Source: RKI.
Fig. A.2.
Air pollution and weather monitor locations. Note: This map shows the locations of air pollution and weather monitors across Germany as well as the geographic centroids of counties (Kreise).
Source: UBA and DWD.
Fig. A.3.
Mean concentration of air pollutants by county. Note: These maps show the mean concentration of particulate matter (PM10) and ozone across counties (Kreise) over the period 1 Feb to 26 May 2020.
Source: UBA.
Fig. A.4.
PM10 pollution compared to previous years. Note: This figure shows daily mean PM10 over the period of analysis as well as the same period in previous years from 2010 to 2019. The vertical line indicates the start of the nationwide lockdown in Germany (March 21, 2020).
Source: UBA and DWD.
Fig. A.5.
Air pollution and precipitation. Note: This figure shows daily mean levels of precipitation, ozone and PM10 over the period of analysis.
Source: UBA and DWD.
Fig. A.6.
Air pollution and windspeed. Note: This figure shows daily mean wind speed, ozone and PM10 over the period of analysis.
Source: UBA and DWD.
Fig. A.7.
Total number of daily trips based on mobile phone data. Note: This figure shows the log number of total daily trips based on mobile phone mobility data over the period of analysis as well as the average total number of trips on the same day of the week in the same month of 2019. The vertical lines indicate the start of the nationwide lockdown in Germany (March 21, 2020) and the relaxation of restrictions (May 4, 2020).
Source: Teralytics.
Fig. A.8.
First stage: Region-specific wind directions and PM pollution. Note: This figure shows the results for the first-stage regression model outlined in equation (1).
Source: UBA and DWD. Gray bars indicate relative frequencies of wind directions, which are more or less homogeneous across regions (left y axis). Red and blue coefficients display region-specific estimates of effects of wind direction on PM levels (right y axis). Regions: SH/HH Schleswig-Holstein / Hamburg, NI / HB Niedersachsen / Bremen, NW Nordrhein-Westfalen, HE Hessen, RP/SL Rheinland-Pfalz / Saarland, BW Baden-Württemberg, BY Bayern, BE/BB Berlin/Brandenburg, MV Mecklenburg-Vorpommern, SN Sachsen, ST Sachsen-Anhalt, TH Thüringen.
Fig. A.9.
Effect of PM10 on case fatality rate of COVID-19. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM10 concentration on the case fatality rate of COVID-19. Each coefficient results from a separate regression of the case fatality rate on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.10.
Correlation between PM2.5 and PM10. Note: This graph visualizes the relationship between measures of PM2.5 and PM10 weighted by county population size.
Source: UBA.
Fig. A.11.
Effect of PM2.5 on new confirmed cases of COVID-19. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM2.5 concentration on the number of new confirmed cases of COVID-19. Each coefficient results from a separate regression of the number of newly-confirmed cases on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.12.
Effect of PM2.5 on new deaths from COVID-19. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM2.5 concentration on the number of new deaths from COVID-19. Each coefficient results from a separate regression of the number of deceased patients on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.13.
Effect of Ozone on new deaths from COVID-19. Note:This graph shows the point estimates and the 95 percent confidence intervals of the effect of ozone concentration on the number of new deaths from COVID-19. Each coefficient results from a separate regression of the number of deceased patients on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.14.
Effect of Ozone on new confirmed cases of COVID-19. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of ozone concentration on the number of new confirmed cases of COVID-19. Each coefficient results from a separate regression of the number of newly-confirmed cases on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.15.
Google searches for mask and air pollution. Note: This figure shows the frequency of internet searches for the terms “mask” (in German: Maske) and “air pollution” (in German: Luftverschmutzung) during our window of observation on a weekly level next to daily levels of PM10 concentration. The vertical line indicates the date when face masks became mandatory in shops and public transportation in most states (April 27, 2020).
Source: UBA and Google Trends.
Fig. A.16.
Effect of PM10 on confirmed cases of COVID-19 – Daily concentrations. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM10 concentration on the number of confirmed cases from COVID-19 based on daily measures. Each coefficient results from a separate regression of the number of deceased patients on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.17.
Effect of PM10 on confirmed cases of COVID-19 – Five-day averages. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM10 concentration on the number of confirmed cases from COVID-19 based on five-day averages. Each coefficient results from a separate regression of the number of deceased patients on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.18.
Effect of PM10 on new deaths from COVID-19 – Daily concentrations. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM10 concentration on the number of new deaths from COVID-19 based on daily measures. Each coefficient results from a separate regression of the number of deceased patients on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
Fig. A.19.
Effect of PM10 on new deaths from COVID-19 – Five-day averages. Note: This graph shows the point estimates and the 95 percent confidence intervals of the effect of PM10 concentration on the number of new deaths from COVID-19 based on five-day averages. Each coefficient results from a separate regression of the number of deceased patients on the respective lag/lead of predicted levels of PM10 relative to the onset of illness.
Source: RKI, UBA and DWD.
References
- Analitis A., Katsouyanni K., Dimakopoulou K., Samoli E., Nikoloulopoulos A.K., Petasakis Y., Touloumi G., Schwartz J., Anderson H.R., Cambra K., et al. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. 2006;17(2):230–233. doi: 10.1097/01.ede.0000199439.57655.6b. [DOI] [PubMed] [Google Scholar]
- Andree B.P.J. The World Bank; 2020. Incidence of COVID-19 and Connections with Air Pollution Exposure: Evidence from the Netherlandss. Policy Research Working Paper Series 9221. [Google Scholar]
- Atkinson R.W., Ross Anderson H., Sunyer J., Ayres J., Baccini M., Vonk J.M., Boumghar A., Forastiere F., Forsberg B., Touloumi G., et al. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Am. J. Respir. Crit. Care Med. 2001;164(10):1860–1866. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- Austin W., Carattini S., Gomez Mahecha J., Pesko M. 2020. Covid-19 Mortality and Contemporaneous Air Pollution. CESifo Working Paper No. 8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahona N., Gallego F.A., Montero J.-P. Vintage-specific driving restrictions. Rev. Econ. Stud. 2020;87(4):1646–1682. [Google Scholar]
- Barwick P.J., Li S., Liguo L., Zou E. 2019. From Fog to Smog: the Value of Pollution Information; p. 78. National Bureau of Economic Research w26541. [Google Scholar]
- Bayer C., Kuhn M. Institute of Labor Economics (IZA); 2020, April. Intergenerational Ties and Case Fatality Rates: A Cross-Country Analysis. IZA Discussion Papers 13114. [Google Scholar]
- Becchetti L., Conzo G., Conzo P., Salustri F. 2020. Understanding the Heterogeneity of Adverse Covid-19 Outcomes: the Role of Poor Quality of Air and Lockdown Decisions. Available at SSRN 3572548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Soukup J.M. Exposure to urban air particulates alters the macrophage-mediated inflammatory response to respiratory viral infection. J. Toxicol. Environ. Health Part A. 1999;57(7):445–457. doi: 10.1080/009841099157539. [DOI] [PubMed] [Google Scholar]
- Bel G., Rosell J. Effects of the 80km/h and variable speed limits on air pollution in the metropolitan area of barcelona. Transport. Res. Transport Environ. 2013;23:90–97. 08034. [Google Scholar]
- Bhaskar A., Chandra J., Braun D., Cellini J., Dominici F. 2020. Air Pollution, Sars-Cov-2 Transmission, and Covid-19 Outcomes: A State-Of-The-Science Review of a Rapidly Evolving Research Area. medRxiv. [Google Scholar]
- Chen C.W., Hsieh Y.-H., Su H.-C., Wu J.J. Causality test of ambient fine particles and human influenza in Taiwan: age group-specific disparity and geographic heterogeneity. Environ. Int. 2018;111:354–361. doi: 10.1016/j.envint.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., Li F., Xu Q., Zhang Y., Xu S., et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Zhao A., Chen H., Zhao Z., Cai J., Wang C., Yang C., Li H., Xu X., Ha S., Li T., Kan H. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J. Am. Coll. Cardiol. 2015;65(21):2279–2287. doi: 10.1016/j.jacc.2015.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrie J., Apsley A., Cowie H., Steinle S., Mueller W., Lin C., Horwell C.J., Sleeuwenhoek A., Loh M. Effectiveness of face masks used to protect Beijing residents against particulate air pollution. Occup. Environ. Med. 2018;75(6):446–452. doi: 10.1136/oemed-2017-104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher S.A., Gupta P. Satellite remote sensing of particulate matter air quality: the cloud-cover problem. J. Air Waste Manag. Assoc. 2010;60(5):596–602. doi: 10.3155/1047-3289.60.5.596. [DOI] [PubMed] [Google Scholar]
- Ciencewicki J., Jaspers I. Air pollution and respiratory viral infection. Inhal. Toxicol. 2007;19(14):1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- Clay K., Lewis J., Severnini E. Pollution, infectious disease, and mortality: evidence from the 1918 Spanish influenza pandemic. J. Econ. Hist. 2018;78(4):1179–1209. [Google Scholar]
- Cole M.A., Ozgen C., Strobl E. Institute of Labor Economics (IZA); 2020. Air Pollution Exposure and COVID-19. IZA Discussion Papers 13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D., Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere. 2020;11(4):377. [Google Scholar]
- Cui Y., Zhang Z.-F., Froines J., Zhao J., Wang H., Yu S.-Z., Detels R. Air pollution and case fatality of SARS in the People's Republic of China: an ecologic study. Environ. Health. 2003;2(1):15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antoni D., Smith L., Auyeung V., Weinman J. Psychosocial and demographic predictors of adherence and non-adherence to health advice accompanying air quality warning systems: a systematic review. Environ. Health. 2017;16(1):100. doi: 10.1186/s12940-017-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L.W. The effect of driving restrictions on air quality in Mexico city. J. Polit. Econ. 2008;116(1):38–81. [Google Scholar]
- Deryugina T., Heutel G., Miller N.H., Molitor D., Reif J. The mortality and medical costs of air pollution: evidence from changes in wind direction. Am. Econ. Rev. 2019, December;109(12):4178–4219. doi: 10.1257/aer.20180279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G. Features of 20133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F., McDermott A., Zeger S.L., Samet J.M. Airborne particulate matter and mortality: timescale effects in four US cities. Am. J. Epidemiol. 2003;157(12):1055–1065. doi: 10.1093/aje/kwg087. [DOI] [PubMed] [Google Scholar]
- Frontera A., Martin C., Vlachos K., Sgubin G. Regional air pollution persistence links to COVID-19 infection zoning. J. Infect. 2020;81(2) doi: 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego F., Montero J.P., Salas C. The effect of transport policies on car use: evidence from Latin American cities. J. Publ. Econ. 2013;107:47–62. [Google Scholar]
- Graff Zivin J.S., Neidell M.J., Sanders N.J., Singer G. National Bureau of Economic Research; 2020. When Externalities Collide: Influenza and Pollution. Working Paper 27982. [Google Scholar]
- Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., Liu L., Shan H., Lei C.-l., Hui D.S., Du B., Li L.-j., Zeng G., Yuen K.-Y., Chen R.-c., Tang C.-l., Wang T., Chen P.-y., Xiang J., Li S.-y., Wang J.-l., Liang Z.-j., Peng Y.-x., Wei L., Liu Y., Hu Y.-h., Peng P., Wang J.-m., Liu J.-y., Chen Z., Li G., Zheng Z.-j., Qiu S.-q., Luo J., Ye C.-j., Zhu S.-y., Zhong N.-s. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Zhou L., Chen J., Chen K., Liu Y., Chen X., Tang F. Acute effects of air pollution on influenza-like illness in Nanjing, China: a population-based study. Chemosphere. 2016;147:180–187. doi: 10.1016/j.chemosphere.2015.12.082. [DOI] [PubMed] [Google Scholar]
- Huang W., Morawska L. Face masks could raise pollution risks. Nature. 2019;574(7776):29–30. doi: 10.1038/d41586-019-02938-1. [DOI] [PubMed] [Google Scholar]
- ISARIC . 2020. COVID-19 Report: 08 June 2020. Technical report. [Google Scholar]
- Jaspers I., Ciencewicki J.M., Zhang W., Brighton L.E., Carson J.L., Beck M.A., Madden M.C. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol. Sci. 2005;85(2):990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- Kaan P.M., Hegele R.G. Interaction between respiratory syncytial virus and particulate matter in Guinea pig alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2003;28(6):697–704. doi: 10.1165/rcmb.2002-0115OC. [DOI] [PubMed] [Google Scholar]
- Karottki D.G., Spilak M., Frederiksen M., Andersen Z.J., Madsen A.M., Ketzel M., Massling A., Gunnarsen L., Møller P., Loft S. Indoor and outdoor exposure to ultrafine, fine and microbiologically derived particulate matter related to cardiovascular and respiratory effects in a panel of elderly urban citizens. Int. J. Environ. Res. Publ. Health. 2015;12(2):1667–1686. doi: 10.3390/ijerph120201667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouyanni K., Touloumi G., Samoli E., Gryparis A., Le Tertre A., Monopolis Y., Rossi G., Zmirou D., Ballester F., Boumghar A., et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2project. Epidemiology. 2001;12(5):521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Kelly F.J., Fuller G.W., Walton H.A., Fussell J.C. Monitoring air pollution: use of early warning systems for public health. Respirology. 2012;17(1):7–19. doi: 10.1111/j.1440-1843.2011.02065.x. [DOI] [PubMed] [Google Scholar]
- Lambert A.L., Mangum J.B., DeLorme M.P., Everitt J.I. Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression. Toxicol. Sci. 2003;72(2):339–346. doi: 10.1093/toxsci/kfg032. [DOI] [PubMed] [Google Scholar]
- Le Tertre A., Medina S., Samoli E., Forsberg B., Michelozzi P., Boumghar A., Vonk J., Bellini A., Atkinson R., Ayres J., et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J. Epidemiol. Community. 2002;56(10):773–779. doi: 10.1136/jech.56.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G.I., Saravia J., You D., Shrestha B., Jaligama S., Hebert V.Y., Dugas T.R., Cormier S.A. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part. Fibre Toxicol. 2014;11:57. doi: 10.1186/s12989-014-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Liu Y., Purevjav A.O., Yang L. Does subway expansion improve air quality? J. Environ. Econ. Manag. 2019;96:213—–235. [Google Scholar]
- Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit. Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Fang L., Pan H., Zhang K., Kan H., Brook J.R., Sun Q. PM 2.5 in Beijing - temporal pattern and its association with influenza. Environ. Health. 2014;13:102. doi: 10.1186/1476-069X-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Chen F., Liu T., Chen F., Liu S., Yang J. The role of oxidative stress in influenza virus infection. Microb. Infect. 2017;19(12):580–586. doi: 10.1016/j.micinf.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Mannucci P.M., Franchini M. Health effects of ambient air pollution in developing countries. Int. J. Environ. Res. Publ. Health. 2017;14(9):8. doi: 10.3390/ijerph14091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelletti L., Martelletti P. Air pollution and the novel COVID-19 disease: a putative disease risk factor. SN Comprehensive Clinical Medicine. 2020:1–5. doi: 10.1007/s42399-020-00274-4. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitze T., Kosfeld R., Rode J., Wälde K. Proceedings of the National Academy of Sciences. 2020. Face masks considerably reduce COVID-19 cases in Germany. (forthcoming) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadas S.M., Shoukat A., Fitzpatrick M.C., Wells C.R., Sah P., Pandey A., Sachs J.D., Wang Z., Meyers L.A., Singer B.H., et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(16):9122–9126. doi: 10.1073/pnas.2004064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N.Z., Mendelsohn R. Measuring the damages of air pollution in the United States. J. Environ. Econ. Manag. 2007;54(1):14. [Google Scholar]
- Nishiura H., Kobayashi T., Yang Y., Hayashi K., Miyama T., Kinoshita R., Linton N.M., Jung S.-m., Yuan B., Suzuki A., Akhmetzhanov A.R. The rate of underascertainment of novel coronavirus (2019-nCoV) infection: estimation using Japanese passengers data on evacuation flights. J. Clin. Med. 2020;9(2):419. doi: 10.3390/jcm9020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitto A., Amato F., Salmatonidis A., Moreno T., Alastuey A., Reche C., Buonanno G., Benito C., Querol X. Effectiveness of commercial face masks to reduce personal PM exposure. Sci. Total Environ. 2019;650:1582—–1590. doi: 10.1016/j.scitotenv.2018.09.109. [DOI] [PubMed] [Google Scholar]
- Pansini R., Fornacca D. 2020. Initial Evidence of Higher Morbidity and Mortality Due to SARS-CoV-2 in Regions with Lower Air Quality. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico C., Johnson K.R. Institute of Labor Economics (IZA); 2020, May. Deregulation in a Time of Pandemic: Does Pollution Increase Coronavirus Cases or Deaths? IZA Discussion Papers 13231. [Google Scholar]
- Pestel N., Wozny F. 2019. Low Emission Zones for Better Health: Evidence from German Hospitals; p. 12545. IZA Discussion Paper. [Google Scholar]
- Read J.M., Bridgen J.R., Cummings D.A., Ho A., Jewell C.P. 2020. Novel Coronavirus 2019-nCoV: Early Estimation of Epidemiological Parameters and Epidemic Predictions. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya K.M., Noyes A., Kallin R., Peltier R.E. Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. J. Expo. Sci. Environ. Epidemiol. 2017;27(3):352–357. doi: 10.1038/jes.2016.42. [DOI] [PubMed] [Google Scholar]
- Sorek-Hamer M., Just A.C., Kloog I. Satellite remote sensing in epidemiological studies. Curr. Opin. Pediatr. 2016;28(2):228–234. doi: 10.1097/MOP.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H., Schulte B., Kuemmerer B., Richter E., Hoeller T., Fuhrmann C., Bartok E., Dolscheid R., Berger M., Wessendorf L., Eschbach-Bludau M., Kellings A., Schwaiger A., Coenen M., Hoffmann P., Noethen M., Eis-Huebinger A.-M., Exner M., Schmithausen R., Schmid M., Hartmann G. 2020. Infection Fatality Rate of SARS-CoV-2 Infection in a German Community with a Super-spreading Event. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W., Wu X., Geng X., Zhao X., Liu Q., Liu T. The short-term effects of air pollutants on influenza-like illness in Jinan, China. BMC Publ. Health. 2019;19:1319. doi: 10.1186/s12889-019-7607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D.M., Krupnick A. 2018. Using Satellite Data to Fill the Gaps in the US Air Pollution Monitoring Network; pp. 18–21. RFF Working Paper. [Google Scholar]
- Thakur M., Boudewijns E.A., Babu G.R., van Schayck O.C. Biomass use and COVID-19: a novel concern. Environ. Res. 2020;186 doi: 10.1016/j.envres.2020.109586. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. 2020. Links between Air Pollution and COVID-19 in England. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter Z.S., Aunan K., Chowdhury S., Lelieveld J. Covid-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(32):18984–18990. doi: 10.1073/pnas.2006853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 16-24 February 2020. Technical report. [Google Scholar]
- World Bank . World Bank; 2020. Global Economic Prospects. Technical report. [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. medRxiv; 2020. Exposure to Air Pollution and {COVID}-19 Mortality in the {U}nited {S}tates: A Nationwide Cross-Sectional Study. [Google Scholar]
- Yao Y., Pan J., Wang W., Liu Z., Kan H., Meng X., Wang W. 2020. Spatial Correlation of Particulate Matter Pollution and Death Rate of COVID-19. medRxiv. [Google Scholar]
- Yongjian Z., Jingu X., Fengming H., Liqing C. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Mu Q. Air pollution and defensive expenditures: evidence from particulate-filtering facemasks. J. Environ. Econ. Manag. 2018;92:517–536. [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, january-march 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]