Abstract
Thorn‐shaped astrocytes (TsA) are mainly localized in the periventricular white matter of the temporal lobe in a subgroup of aged individuals usually in the context of Alzheimer's disease (AD). Immunohistochemistry of TsA shows 4Rtau deposition, tau phosphorylation at different sites recognized with phosphospecific anti‐tau antibodies Thr181, Ser202, Ser214, Thr231, Ser396, Ser422, and clones AT8 and PHF‐1, and conformational changes revealed with Alz50 and MC‐1 antibodies; TsA are also immunostained with antibodies to active tau kinases MAPK/ERK‐P, SAPK/JNK‐P, p38‐P and GSK‐3β. These findings are common to neurofibrillary tangles in AD. However, TsA are not stained with 3Rtau antibodies, and they are seldom stained or not at all with phosphospecific tauSer262 and with Tau‐C3 antibody, which recognizes the latter tau truncation at aspartic acid 421. Previous studies have shown that tau phosphorylation at Ser262 reduces tau binding to microtubules and increases caspase‐3 activity, whereas tau truncation at aspartic acid 421 is associated with tau ubiquitination, and toxic effects of tau. In this line, ubiquitin is not accumulated in TsA, and in situ end‐labeling of nuclear DNA fragmentation shows absence of degeneration in TsA. These observations support the concept that tau lesions in neurons differ from those seen in TsA in AD.
Keywords: Alzheimer, tau, thorn‐shaped astrocytes, truncation, ubiquitin
Introduction
Hyperphosphorylated tau accumulation in astrocytes, often forming disease‐specific deposits such as tufted astrocytes, astrocytic plaques and small perinuclear globular inclusions, is common in several tauopathies including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and frontotemporal lobar degeneration linked with MAPT mutations 7, 10, 31, 32, 33, 41, 43, 45, 49. Tufted‐like astrocytes and astroglial neurofibrillary tangles (NFTs) may also occur in other neurodegenerative diseases 27. Distinct glial inclusions occur in globular glial tauopathies with characteristic globular inclusions in oligodendrocytes and distinct tau deposition in astrocytes 1. Some of these distinct tauopathies are manifested as frontotemporal degeneration, parkinsonism and motor neuron disease 22. A subset of dementia in the elderly is associated with granular tau immunoreactivity in astrocytic processes 35.
In addition to these particular types, thorn‐shaped astrocytes (TsA) or thorny astrocytes are distinct tau‐positive astrocytes found in several diseases including various tauopathies, and in the aging brain 28, 29. TsA are common in argyrophilic grain disease (AGD), mainly in the white matter of the temporal lobes 18, and in diffuse NFTs with calcification 26. TsA, mainly located in the white matter of the temporal lobe but also scattered in other regions, may occur in cases with Alzheimer's disease (AD)‐related pathology and lack of argyrophilic grains from individuals in their 60s to centenarians 46. Clusters of TsA in the fronto‐temporo‐parietal cortex and subcortical white matter have been reported in a series of cases with primary progressive aphasia and AD pathology 42. TsA are also observed in some cases with dementia with Lewy bodies with associated AD pathology 30. Interestingly, TsA have also been reported in the spinal cord in cervical spondylotic myelopathy 48.
Some aspects of TsA are well identified such as argyrophilia including Gallyas positivity, diffuse or fine granular immunostaining with phosphospecific anti‐tau antibodies, including AT8, predominance of 4Rtau isoforms, lack of ubiquitination, and predominance of bundles of 15‐nm straight tubules mingled with glial filaments 28, 29. For these reasons, TsA have been considered as putative glial counterparts of neuronal pretangles 29. Studies geared to unveil the mechanism leading to hyperphosphorylated tau inclusions in neurons have been conducted in AD and Pick's disease, among other tauopathies, using specific cleavage and conformational anti‐tau antibodies 36, 37, 38, 39, 40. This information has been useful in increasing understanding of the pathogenesis of NFTs, the incorporation of truncated forms produced as a consequence of different sites of cleavage, and the conformational modifications of altered tau. However, little is known about the characteristics of phospho‐tau composition in TsA, including phosphorylation sites, conformational changes and presence or absence of truncated species. This is a crucial aspect if we are to increase understanding of the lack of ubiquitination of TsA in comparison with NFTs.
The present study was designed to unveil the composition of hyperphosphorylated tau in TsA in cases with AD‐related pathology not accompanied by other known tauopathies, using immunohistochemistry with a wide panel of anti‐tau antibodies.
Material and Methods
Cases and general processing
Brain tissue was obtained from the Institute of Neuropathology Brain Bank following the guidelines of Spanish legislation on this matter and of the local ethics committee.
Seventeen cases fulfilled the neuropathological criteria selected for this study: AD‐related pathology stages III–V of Braak 3 and TsA at least in the white matter of the temporal lobe. The majority of these cases, those corresponding to stages III and IV, had not suffered from cognitive impairment and the diagnosis was made at the neuropathological study. The five cases with stage V had suffered from cognitive impairment and dementia. None of them had suffered from progressive aphasia. Cases with argyrophilic grains, coiled bodies, tufted astrocytes, astrocytic plaques or tau‐positive globular glial inclusions were excluded. Small vessel disease was a common feature but cases with lacunae and infarcts were excluded. The cases were nine men and eight women, mean age 76 (between 65 and 83 years old). The post‐mortem interval between death and tissue processing was less than 6 h in all cases. One hemisphere was immediately cut in coronal sections, 1‐cm‐thick, and selected areas of the encephalon were rapidly dissected, frozen on metal plates over dry ice, placed in individual air‐tight plastic bags, numbered with water‐resistant ink and stored at −80°C. The other hemisphere was fixed by immersion in 4% buffered formalin for 3 weeks for morphological studies. In addition, samples of the temporal cortex and subcortical white matter, and hippocampus, were fixed in 4% paraformaldehyde for 24 h and then cryoprotected with 30% saccharose for 48 h, frozen in liquid nitrogen, and maintained at −80°C until use.
Neuropathological study and immunohistochemistry
The neuropathological study was carried out, at first, in paraffin sections of the following regions in every case: medulla oblongata; pons; mesencephalon; upper cerebellar vermis; cerebellum and dentate nucleus; anterior hippocampus and parahippocampal gyrus; caudate, putamen, accumbens; frontal cortex area 8; primary visual area and area 19; amygdala; basal nucleus of Meynert; globus pallidus; hypothalamus and mammillary bodies; anterior cingulate cortex; posterior hippocampus; parietal cortex at the level of the splenium; anterior superior and middle temporal gyri; posterior middle and inferior temporal gyri at the level of the geniculate nucleus; posterior cingulate cortex; medial and anterior thalamic nuclei, and subthalamic nucleus; and posterior thalamus.
Dewaxed sections were stained with hematoxylin and eosin, and luxol fast blue/cresyl violet method, or they were processed for immunohistochemistry. The sections were boiled in citrate buffer (20 min) to retrieve tau antigenicity. Endogenous peroxidases were blocked by incubation in 10% methanol‐1% H2O2 solution (15 min) followed by 3% normal horse serum solution. Then the sections were incubated at 4°C overnight with the primary antibodies against glial fibrillary acidic protein (GFAP), β‐amyloid, ubiquitin, mutant ubiquitin (UBB+1) 20, 50, GAP‐associated tyrosine phosphoprotein p62 (p62) C terminus, microglia (CD68), phospho‐tau (AT8 and PHF1), phosphospecific tauThr181, Ser202, Ser214, Thr231, Ser262, Ser396 and Ser422, 3Rtau (3‐repeat isoform), 4Rtau (4‐repeat isoform), conformational tau (Alz50 and MC‐1), tau N‐terminal, tau 421 (Tau‐C3), α‐synuclein, TDP‐43 full length (directed against the full‐length recombinant human TARDBP), TDP‐43 C‐terminal (directed against a synthetic peptide corresponding to C‐terminal, amino acids 350‐414, of human TARDBP), phosphospecific mitogen‐activated protein kinases MAPK‐ERK1 and ERK2 (Thr202/Tyr204: MAPK/ERK1/ERK2‐P), phospho‐p38 kinase (Thr180/Tyr182, p38‐P), phospho‐stress‐activated protein kinase/Jun‐amino‐terminal kinase (Thr183/Tyr185, SAP/JNK‐P) and glycogen synthase kinase‐3β (GSK‐3 βSer9). See Table 1 for details.
Table 1.
Summary of antibodies used, and differential characteristics of the immunostaining of NFTs and TsA in white matter of temporal lobe in AD brains. Abbreviations: IHC = immunohistochemistry; IF = immunofluorescence; TsA = thorn‐shaped astrocytes; NFT = neurofibrillary tangle; AD = Alzheimer's disease
| Primary antibody | Host | Source | Working IHC | Dilution IF | NFTs | TsA |
|---|---|---|---|---|---|---|
| α‐Synuclein | Rabbit polyclonal | Chemicon (USA) | 1:3000 | − | − | |
| 3Rtau | Mouse monoclonal | Millipore (USA) | 1:800 | + | − | |
| 4Rtau | Mouse monoclonal | Millipore (USA) | 1:50 | + | + | |
| A1z50 | Mouse monoclonal | Generous gift of Dr. Peter Davies (USA) | 1:20 | 1:20 | + | + |
| AT8 | Mouse monoclonal | Innogenetics (Belgium) | 1:50 | + | + | |
| CD68 | Mouse monoclonal | Dako (Denmark) | 1:100 | − | − | |
| GFAP | Rabbit polyclonal | Dako (Denmark) | 1:250 | − | + | |
| GSK‐3 | Rabbit polyclonal | Oncogen (USA) | 1:100 | + | + | |
| MC‐1 | Mouse monoclonal | Generous gift of Dr. Peter Davies (USA) | 1:500 | 1:500 | + | + |
| p62 | Guinea pig polyclonal | Progen (Denmark) | 1:100 | + | − | |
| PHF1 | Mouse monoclonal | Generous gift of Dr. Peter Davies (USA) | 1:500 | + | + | |
| Phosphospecific MAP Kinase ERK1/ERK2 (Thr202/Tyr204) | Rabbit polyclonal | Calbiochem (Denmark) | 1:150 | + | + | |
| Phosphospecific p38 (Thr180/Tyr182) | Rabbit polyclonal | Calbiochem (Denmark) | 1:200 | + | + | |
| Phosphospecific SAPK/JNK (Thr183/Tyr185) | Rabbit polyclonal | Cell signaling (USA) | 1:150 | + | + | |
| Phosphospecific tau (Ser202) | Rabbit polyclonal | Calbiochem (Denmark) | 1:100 | + | + | |
| Phosphospecific tau (Ser214) | Rabbit polyclonal | Calbiochem (Denmark) | 1:100 | + | + | |
| Phosphospecific tau (Ser262) | Rabbit polyclonal | Calbiochem (Denmark) | 1:500 | + | − | |
| Phosphospecific tau (Ser396) | Rabbit polyclonal | Calbiochem (Denmark) | 1:100 | + | + | |
| Phosphospecific tau (Ser422) | Rabbit polyclonal | Calbiochem (Denmark) | 1:100 | + | + | |
| Phosphospecific tau (Thr18l) | Rabbit polyclonal | Calbiochem (Denmark) | 1:100 | 1:100 | + | + |
| Phosphospecific tau (Thr231) | Rabbit polyclonal | Calbiochem (Denmark) | 1:500 | + | + | |
| Tau (N‐terminal) | Goat polyclonal | Chemicon (USA) | 1:400 | + | + | |
| Tau‐C3 | Mouse monoclonal | Abcam (UK) | 1:300 | 1:300 | + | − |
| TDP‐43 | Mouse monoclonal | Abnova (USA) | 1:1000 | − | − | |
| TDP‐43 | Rabbit polyclonal | Abcam (UK) | 1:2000 | − | − | |
| UBB+1 | Rabbit polyclonal | Generous gift of Dr. Fred W. van Leeuwen (Maastricht) | 1:200 | + | − | |
| Ubiquitin | Rabbit polyclonal | Dako (Denmark) | 1:200 | + | − | |
| β‐amyloid clone 6F/3D | Mouse monoclonal | Dako (Denmark) | 1:50 | − | − |
Following incubation with the primary antibody, the sections were incubated with EnVision + system peroxidase (Dako, Glostrup, Denmark) for 30 min at room temperature. The peroxidase reaction was visualized with diaminobenzidine and H2O2. Control of the immunostaining included omission of the primary antibody; no signal was obtained following incubation with only the secondary antibody. Sections were slightly counterstained with hematoxylin, dehydrated and cover‐slipped for microscopic observation.
A second set of studies was carried out in every case in cryoprotected, 20‐μm‐thick sections of the temporal cortex and subcortical white matter, and hippocampus, cut with a cryostat processed for free‐floating immunohistochemistry with one of the primary antibodies against phosphospecific tauThr181, Thr231, Ser262, 3Rtau, 4Rtau, and with antibodies AT8, Alz50, MC‐1 and Tau‐C3, and incubated at 4°C overnight. Following incubation with the primary antibody, sections were incubated with EnVision + system peroxidase for 30 min at room temperature. The peroxidase reaction was visualized with diaminobenzidine, NH4NiSO4 and H2O2. The immunoreaction results in a blue‐gray precipitate. See Table 1 for details.
In situ end‐labeling of nuclear DNA fragmentation was carried out using the ApopTag peroxidase labeling kit (Millipore, Billerica, MA, USA), following the instructions provided by the manufacturer. Immediately afterward, the sections were processed for phosphospecific tauThr181 immunohistochemistry.
Double‐labeling immunofluorescence and confocal microscopy
Dewaxed sections, 4‐μm‐thick, of the temporal white matter were stained with a saturated solution of Sudan black B (Merck, Darmstadt, Germany) for 15 min to block the autofluorescence of lipofuscin granules present in cell bodies, and then rinsed in 70% ethanol and washed in distilled water. The sections were boiled in citrate buffer to enhance antigenicity and blocked for 30 min at room temperature with 10% fetal bovine serum diluted in phosphate‐buffered saline (PBS). Cryostat sections, 35‐μm‐thick, were processed in the same way but without boiling in citrate buffer. Then, the sections were incubated at 4°C overnight with combinations of primary antibodies against phospho‐tauThr181, and clones Alz50, MC‐1 or Tau‐C3. After washing, the sections were incubated with Alexa488 or Alexa546 (1:400, Molecular Probes, Eugene, OR, USA) fluorescence secondary antibodies against the corresponding host species. Nuclei were stained with DRAQ5TM (1:2000, Biostatus, Leicestershire, UK). After washing, the sections were mounted in Immuno‐Fluore mounting medium (ICN Biomedicals, Costa Mesa, CA, USA), sealed and dried overnight. Sections were examined with a Leica TCS‐SL confocal microscope (Wetzlar, Germany).
Quantification of TsA
The number of TsA was counted in the temporal white matter in five different fields in every case and by using antibodies AT8, anti‐phospho‐tauThr181, MC‐1, Tau‐C3 and Alz50. As antibody phospho‐tauThr181 stained the largest number of TsA, the representation of the other positive staining was expressed as the percentage of positive astrocytes compared to tauThr181‐positive TsA.
For comparative purposes, the same semiquantitative approach was carried out in the CA1 area of the neighboring hippocampus.
Results
Immunohistochemical characteristics of TsA
TsA were stained with antibody AT8 and with antibodies against 4Rtau but were negative with antibodies against 3Rtau, a feature that was in contrast with the staining of neurons with tangles in the sections which were labeled with anti‐3Rtau and anti‐4Rtau, in addition to AT8 (Figure 1). As a general screening of TsA distribution, the study of paraffin sections representative of several brain regions in every case showed that TsA were mainly found in the periventricular and deep white matter of the temporal lobe, and hilus of the hippocampus. Other areas commonly affected were the entorhinal cortex and deep regions of the temporal cortex, amygdala, inferior part of the putamen and accumbens, hypothalamus and Meynert nucleus, and subpial regions of the bulb. TsA were seldom observed in the putamen, and only rarely in other parts of the encephalon in the present series. The number and distribution of TsA were variable from one case to another and were not related with the stages of NFT degeneration following Braak classification.
Figure 1.
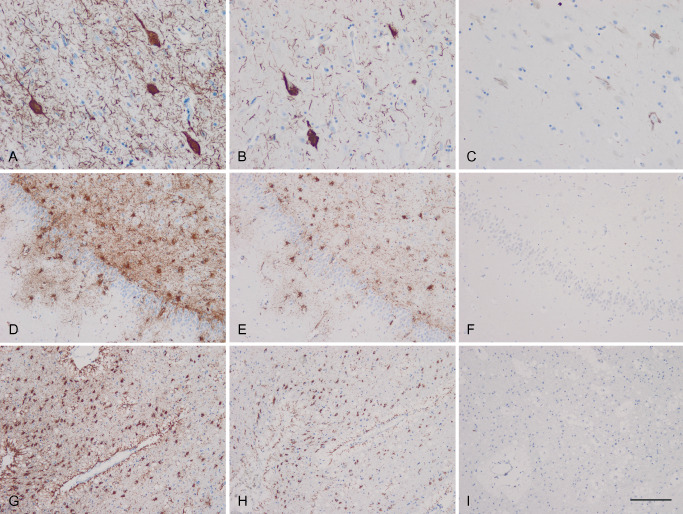
Temporal cortex (A–C), hippocampus (D–F) and periventricular temporal white matter (G–I) of a representative case of the present series showing neurofibrillary tangles and threads in the cortex, and phospho‐tau‐immunoreactive thorn‐shaped astrocytes (TsA) in the white matter of the hippocampus and periventricular white matter as seen with antibody AT8 (A, D, G). TsA are immunoreactive with anti‐4Rtau (E, H) but not with anti‐3Rtau (F, I) antibodies, in contrast to neurofibrillary tangles and threads, which are immunoreactive to 4Rtau (B) and 3Rtau (C). Paraffin sections slightly counterstained with hematoxylin, bar = 100 μm.
In both paraffin and cryostat sections, TsA were stained with phosphospecific anti‐tau antibodies Thr181, Ser202, Ser214, Ser396 and Ser422. TsA were also recognized with PHF1, and Alz50, tau N‐terminal and, less frequently, with MC‐1 antibodies. All these antibodies also stained NFTs and dystrophic neurites of senile plaques in the cerebral cortex of the same sections (Figure 2).
Figure 2.

Immunohistochemistry of the temporal white with thorn‐shaped astrocytes (TsA) using cryostat sections processed free‐floating with antibodies Thr181 (A, B), AT8 (C, D), Alz50 (E, F), MC‐1 (G, H) and Thr231 (I, J). Photographs of the CA1 area of the same sections stained with Thr231 (K) and MC‐1 (L) are included for comparative purposes. TsA are stained with antibodies Thr181, AT8, Alz50, MC‐1 and Thr231. Similar staining is observed in tangles of the cerebral cortex in the same cases. The peroxidase reaction was visualized in blue because of the use of NH4NiSO 4 enhancement. A, C, E, G, I, L, bar in I = 50 μm; B, D, F, H, J, bar in J = 20 μm.
However, in addition to be negative to 3Rtau, TsA were not stained, except for few astrocytes exhibiting scarce small cytoplasmic dots at their periphery, with the antibody Tau‐C3 directed against tau cleaved at aspartic acid 421, and with antibody Ser262. This was in striking contrast to NFTs and dystrophic neuritis, which were strongly immunostained in the same tissue sections (Figure 3).
Figure 3.

Immunohistochemistry of the temporal white matter with thorn‐shaped astrocytes (TsA) using cryostat sections processed free‐floating with antibodies 3Rtau (A, B), Ser262 (D, E) and Tau‐C3 (G, H). Photographs of the CA1 area of the same sections stained with 3Rtau (C), Ser262 (F) and Tau‐C3 (I) are included for comparative purposes. TsA are barely stained or not at all with 3Rtau, Ser262 and Tau‐C3 seen as small dots at the periphery of astrocytes. In contrast, tangles in the CA1 area of the neighboring hippocampus are strongly stained with 3Rtau, Ser262 and Tau‐C3. The peroxidase reaction was visualized in blue because of the use of NH4NiSO 4 enhancement. A, C, D, F, G, I, bar in G = 50 μm; B, E, H, bar in H = 20 μm.
TsA were not stained with anti‐ubiquitin antibodies or with the antibody against mutant ubiquitin (UBB+1), and seldom with anti‐p62 antibodies.
Double‐labeling immunofluorescence and confocal microscopy with phosphospecific anti‐tauThr181 and Alz50 or MC‐1 showed partial colocalization of the different forms of tau in TsA. Many tauThr181‐positive astrocytes were not stained with Alz50 and MC‐1. Moreover, within the same cell, subcellular localization of Alz50 and MC‐1 did not always correlate with tauThr181 immunoreactivity, thus indicating that only a part of phosphorylated tau at Thr181 was recognized by the conformational antibodies Alz50 and MC‐1. In line with observations with light microscopy, Tau‐C3 was only seen in few TsA, mainly as small dots located at the periphery of scanty astrocytic processes (Figure 4).
Figure 4.

Double‐labeling and confocal microscopy of thorn‐shaped astrocytes (TsA) stained with antibodies Alz50 (A, green), MC‐1 (D, green), Tau‐C3 (G, green) and phosphospecific anti‐tauThr181 (B, E, H, red), showing variable partial colocalization of Alz50 and MC‐1 with phospho‐tau in some TsA but not in others (C, F, merged: yellow). Tau‐C3 antibodies recognize small dots at the peripheral processes of a few TsA, but the cytoplasm was largely devoid of Tau‐C3 immunostaining (I, merged). Nuclei are stained DRAQ5TM and visualized in blue. A–F, bar = 10 μm; G–I, bar = 20 μm.
Semiquantitative studies in paraffin and cryostat sections showed that Alz50 immunoreactivity was present in about 50% of phospho‐tauThr181‐positive astrocytes, whereas MC‐1 was seen in less than 20% of TsA in paraffin sections but in about 60% of TsA in cryoprotected sections processed free‐floating.
A majority of TsA were stained with antibodies against different tau kinases including p38‐P, SAPK/JNK‐P, MAPK/ERK‐P and GSK‐3‐P. The immunoreaction was characterized by fine granular deposits in the cytoplasm (data not shown) as previously observed in TsA in AGD 18.
A summary of the comparative immunostaining characteristics of TsA compared with NFTs in the same sections is shown in Table 1.
Abnormal inclusions of α‐synuclein, β‐amyloid and TDP‐43 were not seen in astrocytes containing hyperphosphorylated tau.
Sections stained with the method of in situ end‐labeling of nuclear DNA fragmentation and immunohistochemistry to tauThr181 did not show TsA with fragmented DNA (data not shown).
Discussion
Tau pathology in astrocytes has been reproduced in transgenic mice expressing human tau protein driven by the GFAP promoter 21. But the characteristics of tau in these astrocytes are not fully known. Tau protein nitrated at Tyr18, as revealed with the antibody Tau‐nY18, is found in reactive astrocytes associated with β‐amyloid plaques, in addition to NFTs and dystrophic neurites of β‐amyloid plaques 44. However, reactive astrocytes around plaques clearly differ from TsA on the basis of their morphology and complex hyperphosphorylated tau pathology.
TsA contain 4Rtau phosphorylated at Thr181, Ser202, Ser214, Thr231, Ser396 and Ser422; tau is phosphorylated at Ser396‐404 and at Ser202‐Thr205, as revealed with PHF‐1 and AT8 antibodies, respectively. Thr181, PHF‐1 and AT8 stain the vast majority of, if not all, TsA. In addition, altered conformation occurs in a percentage of TsA as seen with Alz50 and MC‐1 antibodies that recognize tau conformational changes including amino acids 5‐15 and 312‐322, respectively. The fact that not all tau inclusions in TsA are stained with Alz50 and MC‐1 antibodies supports the concept that conformational modifications occur after tau hyperphosphorylation. TsA are also stained with antibodies recognizing active tau kinases MAPK/ERK‐P, SAPK/JNK‐P, p38‐P and GSK‐3β, thus suggesting that these kinases are involved in tau phosphorylation in TsA, as already reported in neurons in AD, AGD and other tauopathies 11, 12, 14, 15, 16, 17. Taken together, the present observations agree with the idea that tau hyperphosphorylation precedes altered tau conformation in TsA as it occurs in NFT.
A major difference of TsA when compared with NFTs in AD is the absence of 3Rtau immunoreactivity, indicating, as already reported, that tau in TsA is composed of 4Rtau. Another important difference is the lack of positivity of TsA with the phosphospecific antibody Ser262, although this antibody strongly stains NFTs in the CA1 region in the same cases. Tau phosphorylation at Ser 262 decreases binding of tau to microtubules 2, 8, 47. Interestingly, tufted astrocytes in PSP, astrocytic plaques in CBD and tau‐containing astrocytes in AGD are not immunostained with the anti‐tau phosphospecific Ser262 antibody 13.
In contrast to NFTs in AD and other tauopathies, TsA do not form astrocytic tangles, are not ubiquitinated, do not express mutant ubiquitin (UBB+1), and are only very weakly, and rarely, stained with anti‐p62 antibodies.
Truncation at aspartic acid 421, as detected by antibody Tau‐C3, is an alteration unique to neuronal lesions occurring in NFTs in AD 36, and in NFTs in other tauopathies 25. However, TsA are basically negative, excepting scattered dots in few TsA, with Tau‐C3 antibodies, thus indicating that truncated tau at aspartic acid 421 seldom occurs in TsA. Lack of Tau C‐3 immunoreactivity has also been noted in lesions associated with glia in PSP and CBD 25.
Previous studies have shown that tau truncation occurs after tau phosphorylation in AD and Pick bodies, and in transgenic mice 23, 38, 39, that it precedes the formation of NFTs in tau transgenic mice 6, and that it is needed to fully develop the cascade of NFT formation including recruitment of full‐length tau 34, 51, 52. Along these lines, the scarcity of tau truncation may likely underlie the absence of tangle formation in TsA.
A strong association has been found between ubiquitin deposition and truncation at aspartic acid 421 in AD 24. Again, the almost total absence of tau truncation at aspartic acid 421 in TsA argues in favor of their lack of ubiquitin deposition.
Finally, tau cleavage products induce neurodegeneration in a variety of cell models 4, 5, 9, 19, 53. In this line, no evidence of apoptotic cell death has been observed in TsA using double‐labeling with in situ end‐labeling of nuclear DNA fragmentation, thereby suggesting that the lack of tau truncation protects TsA from degeneration in spite of tau hyperphosphorylation.
In summary, morphological and biochemical characteristics of TsA have commonalities with NFTs, but the lack of phosphorylation at Ser262 and the almost total absence of truncation at aspartic acid 421 clearly differentiate TsA from NFTs, and they seem to play a crucial role in the relative resistance to degeneration of TsA.
Financial Disclosure and Conflict of Interests
None.
Acknowledgments
This study was funded by the Seventh Framework Programme of the European Commission, grant agreement 278486: DEVELAGE. We thank B. Torrejón‐Escribano for the confocal microscopy studies and T. Yohannan for editorial help. This paper is dedicated to Dr. Laia Acarín.
References
- 1. Ahmed Z, Doherty KM, Silveira‐Moriyama L, Bandopadhyay R, Lashley T, Mamais A et al (2011) Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4‐repeat tauopathies. Acta Neuropathol 122:415–428. [DOI] [PubMed] [Google Scholar]
- 2. Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF‐like immunoreactivity and microtubule binding. Neuron 11:153–163. [DOI] [PubMed] [Google Scholar]
- 3. Braak H, Braak E (1999) Temporal sequence of Alzheimer's disease‐related pathology. In: Cerebral Cortex Vol. 14, Neurodegenerative and Age‐Related Changes in Structure and Function of Cerebral Cortex, Peters A, Morrison JH (eds), pp. 475–512. Kluwer Academic/Plenum Publishers: New York, Boston, Dordrecht, London, Moscow. [Google Scholar]
- 4. Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM et al (1998) Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci 18:7061–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG et al (2001) Pro‐apoptotic effects of tau cleavage product generated by caspase‐3. Neurobiol Dis 8:162–172. [DOI] [PubMed] [Google Scholar]
- 6. de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires‐Jones TL, Hyman BT (2010) Caspase activation precedes and leads to tangles. Nature 464:1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration‐tau (FTLD‐tau). J Mol Neurosci 45:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt‐Ulms G, Meyer HE et al (1995) Microtubule‐associated protein/microtubule affinity‐regulating kinase (p110mark). A novel protein kinase that regulates tau‐microtubule interactions and dynamic instability by phosphorylation at the Alzheimer‐specific site serine 262. J Biol Chem 270:7679–7688. [DOI] [PubMed] [Google Scholar]
- 9. Fasulo L, Ugolini G, Visintin M, Bradbury A, Brancolini C, Verzillo V et al (2000) The neuronal microtubule‐associated protein tau is a substrate for caspase‐3 and an effector of apoptosis. J Neurochem 75:624–633. [DOI] [PubMed] [Google Scholar]
- 10. Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396. [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrer I (2004) Stress kinases involved in tau phosphorylation in Alzheimer's disease, tauopathies and APP transgenic mice. Neurotox Res 6:469–475. [DOI] [PubMed] [Google Scholar]
- 12. Ferrer I, Barrachina M, Puig B (2002) Glycogen synthase kinase‐3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 104:583–591. [DOI] [PubMed] [Google Scholar]
- 13. Ferrer I, Barrachina M, Puig B (2002) Anti‐tau phospho‐specific Ser262 antibody recognizes a variety of abnormal hyper‐phosphorylated tau deposits in tauopathies including Pick bodies and argyrophilic grains. Acta Neuropathol 104:658–664. [DOI] [PubMed] [Google Scholar]
- 14. Ferrer I, Barrachina M, Tolnay M, Rey MJ, Vidal N, Carmona M et al (2003) Phosphorylated protein kinases associated with neuronal and glial tau deposits in argyrophilic grain disease. Brain Pathol 13:62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrer I, Blanco R, Carmona M, Puig B (2001) Phosphorylated mitogen‐activated protein kinase (MAPK/ERK‐P), protein kinase of 38 kDa (p38‐P), stress‐activated protein kinase (SAPK/JNK‐P), and calcium/calmodulin‐dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm 108:1397–1415. [DOI] [PubMed] [Google Scholar]
- 16. Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B et al (2001) Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol 11:144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrer I, Gomez‐Isla T, Puig B, Freixes M, Ribé E, Dalfó E, Avila J (2005) Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies. Curr Alzheimer Res 2:3–18. [DOI] [PubMed] [Google Scholar]
- 18. Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 146:1640–1651. [DOI] [PubMed] [Google Scholar]
- 19. Filipcik P, Cente M, Krajciova G, Vanicky I, Novak M (2009) Cortical and hippocampal neurons from truncated tau transgenic rat express multiple markers of neurodegeneration. Cell Mol Neurobiol 29:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer DF, De Vos RA, Van DR, De Vrij FM, Proper EA, Sonnemans MA et al (2003) Disease‐specific accumulation of mutant ubiquitin as a marker for proteasomal dysfunction in the brain. FASEB J 17:2014–2024. [DOI] [PubMed] [Google Scholar]
- 21. Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ (2005) Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci 25:3539–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu YJ, Nishihira Y, Kuroda S, Toyoshima Y, Ishihara T, Shinozaki M et al (2010) Sporadic four‐repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol 120:21–32. [DOI] [PubMed] [Google Scholar]
- 23. García‐Sierra F, Ghoshal N, Quinn B, Berry R, Binder LI (2003) Conformational changes and truncation of tau protein during tangle evolution in Alzheimer's disease. J Alzheimers Dis 5:65–77. [DOI] [PubMed] [Google Scholar]
- 24. García‐Sierra F, Jarero‐Basulto JJ, Kristofikova Z, Majer E, Binder LI, Ripova D (2012) Ubiquitin is associated with early truncation of tau protein at aspartic acid(421) during the maturation of neurofibrillary tangles in Alzheimer's disease. Brain Pathol 22:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guillozet‐Bongaarts AL, Glajch KE, Libson EG, Cahill ME, Bigio E, Berry RW, Binder LI (2007) Phosphorylation and cleavage of tau in non‐AD tauopathies. Acta Neuropathol 113:513–520. [DOI] [PubMed] [Google Scholar]
- 26. Hashimoto N, Takeuchi T, Ishihara R, Ukai K, Kobayashi H, Iwata K et al (2003) Glial fibrillary tangles in diffuse neurofibrillary tangles with calcification. Acta Neuropathol 106:150–156. [DOI] [PubMed] [Google Scholar]
- 27. Hishikawa N, Nashizume Y, Yoshida M, Niwa J, Tanaka F, Sobue G (2005) Tuft‐shaped astrocytes in Lewy body diseases. Acta Neuropathol 109:373–380. [DOI] [PubMed] [Google Scholar]
- 28. Ikeda K, Akiyama H, Arai T, Nishimura T (1998) Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging 19:S85–S91. [DOI] [PubMed] [Google Scholar]
- 29. Ikeda K, Akiyama H, Kondo H, Haga C, Tanno E, Tokuda T, Ikeda S (1995) Thorn‐shaped astrocytes: possibly secondarily induced tau‐positive glial fibrillary tangles. Acta Neuropathol 90:620–625. [DOI] [PubMed] [Google Scholar]
- 30. Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R et al (2003) Dementia with Lewy bodies from the perspective of a tauopathy. Acta Neuropathol 105:265–270. [DOI] [PubMed] [Google Scholar]
- 31. Ito K, Arai K, Yoshiyama Y, Kashiwado K, Sakakibara Y, Hattori T (2008) Astrocytic tau pathology positively correlates with neurofibrillary tangle density in progressive supranuclear palsy. Acta Neuropathol 115:623–628. [DOI] [PubMed] [Google Scholar]
- 32. Iwasaki Y, Yoshida M, Hattori M, Goto A, Aiba I, Hashizume Y, Sobue G (2004) Distribution of tuft‐shaped astrocytes in the cerebral cortex in progressive supranuclear palsy. Acta Neuropathol 108:399–405. [DOI] [PubMed] [Google Scholar]
- 33. Katsuse O, Iseki E, Arai T, Akiyama H, Togo T, Uchikado H et al (2003) 4‐repeat tauopathy sharing pathological and biochemical features of corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 106:251–260. [DOI] [PubMed] [Google Scholar]
- 34. Kovacech B, Novak M (2010) Tau truncation is a productive posttranslational modification of neurofibrillary degeneration in Alzheimer's disease. Curr Alzheimer Res 7:708–716. [DOI] [PubMed] [Google Scholar]
- 35. Kovacs GG, Molnár K, Lászlo L, Ströbel T, Botond G, Hönigschnabl S et al (2011) A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 122:205–222. [DOI] [PubMed] [Google Scholar]
- 36. Luna‐Muñoz J, Chávez‐Macías L, García‐Sierra F, Mena R (2007) Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho‐dependent tau epitopes in Alzheimer's disease. J Alzheimers Dis 12:365–375. [DOI] [PubMed] [Google Scholar]
- 37. Luna‐Muñoz J, García‐Sierra F, Falcón V, Menéndez I, Chávez‐Macías L, Mena R (2005) Regional conformational change involving phosphorylation of tau protein at the Thr231 precedes the structural change detected by Alz‐50 antibody in Alzheimer's disease. J Alzheimers Dis 8:29–41. [DOI] [PubMed] [Google Scholar]
- 38. Mena R, Luna‐Muñoz J (2009) Stages of pathological tau‐protein processing in Alzheimer's disease: from soluble aggregations to polymerization into insoluble tau‐PHFs. In: Current Hypothesis and Research Milestones in Alzheimer's Disease, Maccione R, Perry G (eds), pp. 79–91. Springer: Heidelberg. [Google Scholar]
- 39. Mondragón‐Rodríguez S, Basurto‐Islas G, Santa‐María I, Mena R, Binder LI, Avila J et al (2008a) Cleavage and conformational changes of tau protein follow phosphorylation during Alzheimer's disease. Int J Exp Pathol 89:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mondragón‐Rodríguez S, Mena R, Binder LI, Smith MA, Perry G, García‐Sierra F (2008b) Conformational changes and cleavage of tau in Pick bodies parallel the early processing of tau found in Alzheimer pathology. Neuropathol Appl Neurobiol 34:62–75. [DOI] [PubMed] [Google Scholar]
- 41. Muñoz DG, Ferrer I (2008) Neuropathology of hereditary forms of frontotemporal dementia and parkinsonism. In: Handbook of Clinical Neurology: Dementias, Duyckaerts C, Litvan I (eds), pp. 393–414. Elsevier: Edinburgh, London, New York, Oxford, Philadelphia, St. Louis, Sydney, Toronto. [DOI] [PubMed] [Google Scholar]
- 42. Munoz DG, Woulfe J, Kertesz A (2007) Argyrophilic thorny astrocyte clusters in association with Alzheimer's disease pathology in possible primary progressive aphasia. Acta Neuropathol 114:347–357. [DOI] [PubMed] [Google Scholar]
- 43. Nishimura M, Namba Y, Ikeda K, Oda M (1992) Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy. Neurosci Lett 143:35–38. [DOI] [PubMed] [Google Scholar]
- 44. Reyes JF, Reynolds MR, Horowitz PM, Fu Y, Guillozet‐Bongaarts AL, Berry R, Binder LI (2008) A possible link between astrocyte activation and tau nitration in Alzheimer's disease. Neurobiol Dis 31:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakai K, Piao YS, Kikugawa K, Ohara S, Hasegawa M, Takano H et al (2006) Corticobasal degeneration with focal, massive tau accumulation in the subcortical white matter astrocytes. Acta Neuropathol 112:34134–34138. [DOI] [PubMed] [Google Scholar]
- 46. Schultz C, Ghebremedhin E, Del Tredici K, Rüb U, Braak H (2004) High prevalence of thorn‐shaped astrocytes in the aged human medial temporal lobe. Neurobiol Aging 25:397–405. [DOI] [PubMed] [Google Scholar]
- 47. Sengupta A, Kabat J, Novak M, Wu Q, Grundke‐Iqbal I, Iqbal K (1998) Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys 357:299–309. [DOI] [PubMed] [Google Scholar]
- 48. Shimizu H, Kakita A, Takahashi H (2008) Spinal cord tau pathology in cervical spondylotic myelopathy. Acta Neuropathol 115:185–192. [DOI] [PubMed] [Google Scholar]
- 49. Togo T, Dickson DW (2002) Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol 104:398–402. [DOI] [PubMed] [Google Scholar]
- 50. van Leeuwen FW, de Kleijn DP, van den Hurk HH, Neubauer A, Sonnemans MA, Sluijs JA et al (1998) Frameshift mutants of beta‐amyloid precursor protein and ubiquitin‐B in Alzheimer's and Down patients. Science 279:242–247. [DOI] [PubMed] [Google Scholar]
- 51. Wang YP, Biernat J, Pickhardt M, Mandelkow E, Mandelkow EM (2007) Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer‐like aggregation of full‐length tau in a neuronal cell model. Proc Natl Acad Sci USA 104:10252–10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Garg S, Mandelkow EM, Mandelkow E (2010) Proteolytic processing of tau. Biochem Soc Trans 38:955–961. [DOI] [PubMed] [Google Scholar]
- 53. Zilkova M, Zilka N, Kovac A, Kovacech B, Skrabana R, Skrabanova M, Novak M (2011) Hyperphosphorylated truncated protein tau induces caspase‐3 independent apoptosis‐like pathway in the Alzheimer's disease cellular model. J Alzheimers Dis 23:161–169. [DOI] [PubMed] [Google Scholar]


