Clinical History
A 29‐year‐old man presented with a 1 year history of memory impairment. He had become somnolent and lethargic for 8 months before seeking medical attention. He needed to sleep over 20 hours per day. The physical examination and neurological examination were unremarkable.
Radiology
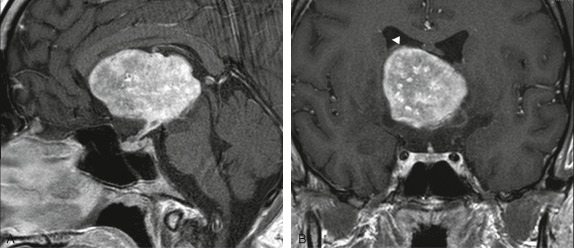
Brain MRI revealed a 5.7x4.3x4.2 cm in the third ventricle with isosignal intensity on T1W, heterogeneous signal on T2W and FLAIR (not shown). The mass showed intense heterogeneous enhancement on T1W after gadolinium injection. Extension to the suprasellar cistern was demonstrated on sagittal T1W after gadolinium injection (Figure 1a). There was also thickening and enhancement of the pituitary stalk, suggestive of involvement or origin of hypothalamus. The coronal T1W after gadolinium injection showed separation of the mass from lateral ventricle (Figure 1b). The inferior wall of the lateral ventricle was displaced upward as well as the septum pellucidum.
Figure 1.
Surgical Treatment and Post Operative Outcome
The patient underwent biopsy and later bifrontal craniotomy for tumor removal. Intraoperatively, the tumor appeared white and had rubbery to hard consistency. It was located in the third ventricle adhering to lamina terminalis and both thalami. There was low tumor vascularization. The tumor was totally resected. Postoperatively, the patient developed transient diabetes insipidus. There was no evidence of tumor recurrence on follow up MRI after 9 months of surgery.
Microscopic Pathology
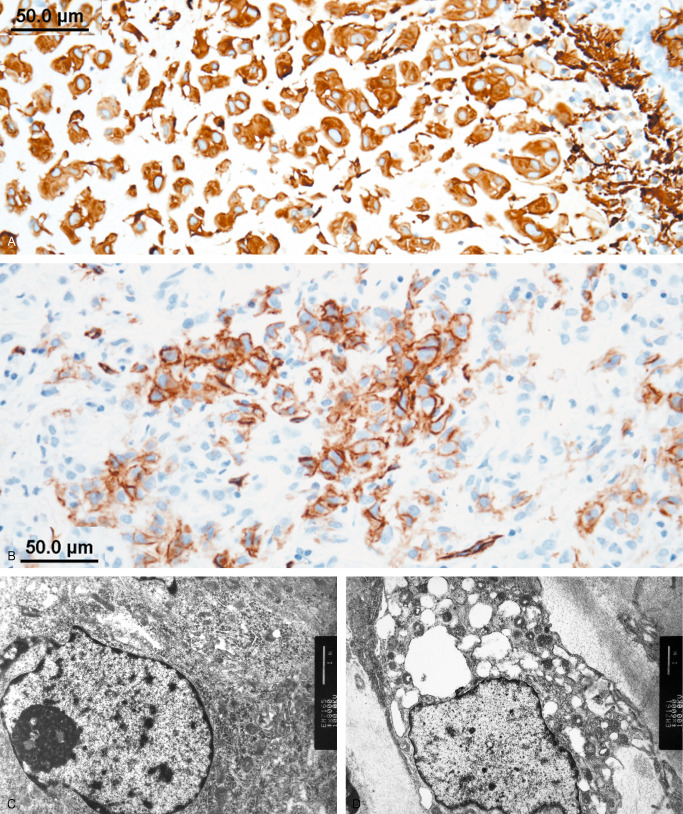
Intraoperative squash preparation showed hypercellular smear with epithelioid tumor nests, non‐cohesive tumor cells, and 3D papillary‐like fragments (Figure 2a). The epithelioid tumor cells contained moderate amount of eosinophilic cytoplasm. They were relatively uniform and possessed round to oval, central or eccentric nuclei, with open chromatin and small nucleoli (Figure 2b). The non‐cohesive tumor cells had delicate cytoplasmic process and show similar nuclear features (Figure 2c). Permanent section revealed a well demarcated mass composed of clusters and cords of epithelioid cells in a myxoid stroma with scattered lymphoplasmacytic infiltration (Figure 2d, Figure 2e). Rosenthal fibers are observed in adjacent brain parenchyma. Mitotic figure, necrosis, and vascular proliferation were not present. Higher magnification showed uniform to mildly variate (Figure 2f) epithelioid tumor cells with moderate to ample amount of eosinophilic cytoplasm in a myxoid stroma. Findings on nuclear detail were similar to those present in squash cytology. Immunohistochemically, the tumor cells were diffusely positive to GFAP (Figure 3a), and vimentin (not shown). Focal positivity to CD34 was noted (Figure 3b). Staining for S‐100 protein and EMA were negative (not shown). Ultrastructural study on specimen retrieved from paraffin‐embedded tissue revealed microvilli (Figure 3c) at the luminal surface, and intermediate junctions (not shown). The cytoplasm contained mitochondria, ribosome, lysosome, and dilated rER. Intermediate filaments (Figure 3d) are noted in the cytoplasm of many tumor cells. What is your diagnosis?
Figure 2.
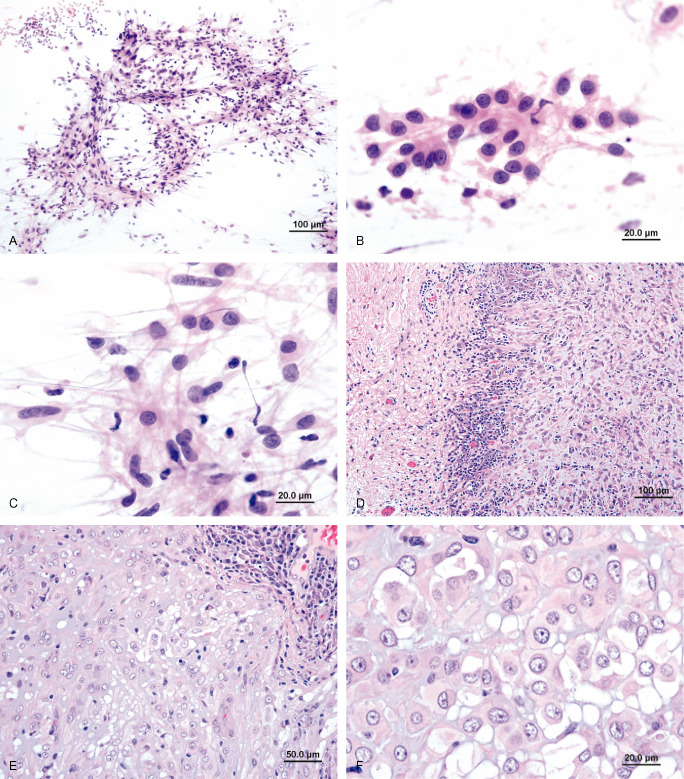
Figure 3.
Final Diagnosis
Chordoid glioma of the third ventricle WHO 2007 grade II.
Discussion
Chordoid glioma is a rare tumor of the third ventricle, firstly proposed as a distinct entity by Brat et al in 1998 1. To our knowledge (September, 2013), 84 cases (including ours) have been reported in the English literature. The tumor predominantly occurs in adult women with a female to male ratio of 1.7 to 1 and the mean age at presentation at 44.8 years old (age range, 5–72 years). Most patients have headache, visual symptoms, and memory disturbances. Other symptoms are lethargy, somnolence, and endocrine disturbance such as hypothyroidism and diabetes insipidus. Symptoms caused by space occupying lesion and obstructive hydrocephalus are also observed in some patients.
Chordoid glioma is mainly located in the third ventricle or nearby structures, hence the name “Chordoid glioma of the third ventricle”. Unusual locations without third ventricular association that have been reported are parieto‐temporal region 3 and thalamic pulvinar area 5. The tumors arising in or involving the third ventricle, especially at the anterior part, are considered in the differential diagnosis for chordoid glioma 4. These tumors include ependymoma, pilocytic astrocytoma, central neurocytoma, choroid plexus papilloma, pituitary adenoma, suprasellar meningioma, and craniopharyngioma. Other lesions such as lymphoma, aneurysm, granular cell tumor of neurohypophysis, histiocytosis, sarcoidosis, and germ cell tumors may also involve the third ventricle. On CT, chordoid glioma is a well circumscribed hyperdensity mass with homogeneous contrast enhancement. On MRI, the tumor is a solid or solid‐cystic mass. It typically shows isosignal intensity on T1, hypersignal intensity on T2, with homogeneous enhancement 6.
Histologically, chordoid glioma is composed of clusters, and cords of relatively uniform epithelioid cells in variable mucinous background. Mitosis is rare. Lymphoplasmacytic infiltrates with numerous Russel bodies are present within the lesion and adjacent brain parenchyma. Reactive astrocytes and Rosenthal fibers are also noted in the adjacent non‐neoplastic brain tissue 2.
Histologic differential diagnoses for chordoid glioma are chordoid meningioma, myxoid chondrosarcoma, and chordoma 10. Immunohistochemically, strong diffuse reactivity for GFAP distinguish chordoid glioma from its mimics. The other antibodies that reported to be positive in chordoid glioma are vimentin, S‐100 protein, EMA, D2‐40, CD34 and CD99 6, 7, 9. Rare positivity to neurofilament protein, synaptophysin and p53 are also noted 6. The tumor shows immunohistochemical reactivity to EGFR without amplification of associate proto‐oncogene 8.
The ependymal nature of chordoid glioma has been suggested by findings on electron microscopy and positivity to EMA, D2‐40, and CD99 7, 9. Ultrastructurally, the tumor exhibits features of ependymal differentiation which include intermediate filaments, intercellular lumina apical microvilli, hemidesmosomes, and basal lamina 2.
Surgical treatment is the primary option for chordoid glioma, but its common location either in or nearby third ventricle usually precludes gross total resection without complications. Common postoperative complications, in descending order, include diabetes insipidus, cognitive dysfunction, pulmonary embolus, and hypothalamic dysfunction. Since there are a limit number of cases, the role of radiotherapy and radiosurgery in subtotal or partially resected tumors is uncertain. There is no report of the use of chemotherapy in chordoid glioma 6.
References
- 1. Brat DJ, Scheithauer BW, Staugaitis SM, Cortez SC, Brecher K, Burger PC (1998) Third ventricular chordoid glioma: a distinct clinicopathologic entity. J Neuropathol Exp Neurol 57:283–290. [DOI] [PubMed] [Google Scholar]
- 2. Brat DJ, Scheithauer BW (2007) Chordoid glioma of the third ventricle. In: WHO classification of tumour of the central nervous system , Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, pp. 90–91.
- 3. Goyal R, Vashishta RK, Singhi S, Gill M (2007) Extraventricular unusual glioma in a child with extensive myxoid change resembling chordoid glioma. J Clin Pathol 60:1294–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grad S, Pasquier B, Gay E, Kremer S, Remy C, Le Bas JF (2002) Chordoid glioma of the third ventricle: CT and MRI, including the perfusion data. Neuroradiology 44:842–846. [DOI] [PubMed] [Google Scholar]
- 5. Kim JW, Kim JH, Choe G, Kim CY (2010) Chordoid glioma: a case report of unusual location and neuroradiological characteristics. J Korean Neurosurg Soc 48:62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu WP, Cheng JX, Yi XC, Zhen HN, Fei Z, Li Q, Zhang X (2011) Chordoid glioma: a case report and literature review. Neurologist 17:52–56. [DOI] [PubMed] [Google Scholar]
- 7. Ni HC, Piao YS, Lu DH, Fu YJ, Ma XL, Zhang XJ (2013) Chordoid glioma of the third ventricle: four cases including one case with papillary features. Neuropathology 33:134–139. [DOI] [PubMed] [Google Scholar]
- 8. Reifenberger G, Weber T, Weber RG, Wolter M, Brandis A, Kuchelmeister K, Pilz P, Reusche E, Lichter P, Wiestler OD (1999) Chordoid glioma of the third ventricle: immunohistochemical and molecular genetic characterization of a novel tumor entity. Brain Pathol 9:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romero‐Rojas AE, Díaz‐Pérez JA, Ariza‐Serrano LM (2012) CD99 is expressed in chordoid glioma and suggests ependymal origin. Virchows Arch 460:119–122. [DOI] [PubMed] [Google Scholar]
- 10. Sangoi AR, Dulai MS, Beck AH, Brat DJ, Vogel H (2009) Distinguishing chordoid meningiomas from their histologic mimics: an immunohistochemical evaluation. Am J Surg Pathol 33:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]


