Abstract
Even though ACP is a benign tumor, treatment is challenging because of the tumor's eloquent location. Today, with the exception of surgical intervention and irradiation, further treatment options are limited. However, ongoing molecular research in this field provides insights into the pathways involved in ACP pathogenesis and reveal a plethora of druggable targets. In the next step, appropriate models are essential to identify the most suitable and effective substances for clinical practice. Primary cell cultures in low passages provide a proper and rapid tool for initial drug potency testing. The patient‐derived xenograft (PDX) model accommodates ACP complexity in that it shows respect to the preserved architecture and similar histological appearance to human tumors and therefore provides the most appropriate means for analyzing pharmacological efficacy. Nevertheless, further research is needed to understand in more detail the biological background of ACP pathogenesis, which provides the identification of the best targets in the hierarchy of signaling cascades. ACP models are also important for the continuous testing of new targeting drugs, to establish precision medicine.
Keywords: craniopharyngioma, EGFR, PDX model, primary cell cultures, radioresistance
Introduction
Sufficient in vitro and in vivo models are required to determine the impact of biological processes involved in adamantinomatous craniopharyngioma (ACP) development, progression, and relapses so that an effective adjuvant treatment mode can be developed. Until recently, molecular research based on primary tumor tissue (Figure 1) revealed promising insights into ACP pathogenesis as well as druggable approaches. These descriptive results are summarized in the first section of this article and correlated to histological appearance. However, in order to conduct a detailed analysis of the functional effects of the dysregulated pathways identified and possible therapeutic targets, in vitro and in vivo models established from primary ACP tumor tissue as primary cell cultures and organotypic patient‐derived xenograft (PDX) models are appropriate tools. Here, we will discuss our research efforts at developing appropriate ACP models, as well as the advantages and limits of these models on the path toward precision and prospective personalized treatment.
Figure 1.
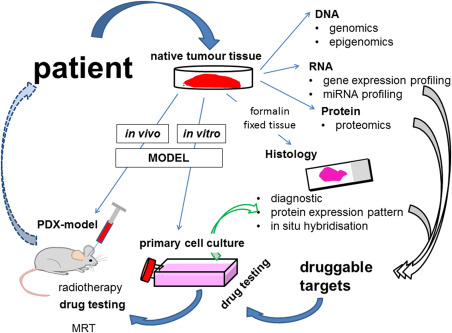
Craniopharyngioma research facets. Based on the most important source of this research, the patient‐derived native tumor tissue obtained during surgical treatment, molecular analyses reveal druggable targets (gray arrows). Both in vitro and in vivo models are indispensable for the functional analysis of identified targets. Primary cell cultures and patient‐derived xenograft (PDX) models were established from native ACP tissue. The efficacy of drugs could be examined using both models. Because of the limits of cell cultures, gene regulatory events based on the inhibition of a specific target should be verified by histology, for example, utilizing double immunofluorescence to analyze the target, as well as the expression of the identified gene (green arrow). In contrast to cell cultures, the PDX model consistently conserves molecular tumor appearances, as seen in the patient. Therefore, this model seems to be most suitable for translating results into humans. Tumor screening using MRI and the development of radiotherapy protocols, adapted for the clinical course, not only allow the further investigation of mechanisms leading to recurrence even after irradiation, but also drug primed enhancement of radiosensitivity. Using these models, our prospective goal is the identification of patient precision adjuvant therapy (broken arrow).
Characterization of ACP Biology Exemplified by Histological Appearance
Histology provides a snap shot view of the tumor's appearance and the status of biological networks at the time of fixation, which takes place directly after surgical excision. The first description of ACP dates to 1904, when Jacob Erdheim, a Viennese pathologist described this lesion in detail 7. He noted that ACP originates in the sellar region, in close association to the pituitary gland, the hypothalamus and optic chiasm, and can cause severe and life threatening symptoms in this eloquent brain region. His drawings impressively depict, (nearly identical with modern photomicrographs) the typical components of ACP, for example, the palisading tumor cells providing at the margin of tumor mass adjacent to brain tissue, the loosely composed stellate reticulum cells as well as solid epithelial cells configured in a whorl‐like shape. Thanks to ongoing technical innovations in pathology in the domain of molecular analysis (Figure 1) 5, 8, 13, 20, the development of specific antibodies and new visualization modes, our understanding of ACP pathogenesis made a great leap forward. Important resultant knowledge of the activated pathways involved and their subcellular mapping are summarized in greater detail below. A crucial finding for our understanding of the underlying cause in ACP pathology was made in 2002. Sekine et al identified the genetic alteration of the beta‐catenin encoding gene CTNNB1, preventing its cytoplasmic degradation 21. The stabilization of beta‐catenin (Figure 2A) mimics the activation of the Wnt pathway and plays a critical role in development and tissue homeostasis 19. The subcellular analysis of beta‐catenin using immunohistochemistry showed a distinct, circumscribed cytoplasmic and nuclear accumulation in whorl‐like cell clusters 3, 4, 15, 21 revealing increased expression of Lef1 22, which facilitates the upregulation of Wnt target genes 12, 13. However, some ACP show single beta‐catenin immunoreactive cells distributed randomly all over the tumor instead of clustered beta‐catenin accumulating cells. Interestingly, most accumulating cells within the tumor bulk seem to be involved in bud formation and therefore lead to the peculiar finger shaped morphology of these tumors, constituting micro outgrows into the adjacent brain tissue 10. Careful examination of this whorl‐configured beta‐catenin accumulating cells using double immunohistochemistry, indicates stimulation of the EGFR and the SHH pathway in addition to the activated Wnt signaling 1, 8, 11. Furthermore, these clusters exhibit, in comparison to the surrounding tumor cells, an altered differentiation pattern exemplified by a distinct cytokeratin profile as well as downregulated cell adhesion components, for example, claudin1 and EpCAM (epithelial cell adhesion molecule) 3, 24, 26. The cell cycle inhibitor p21WAF/CIP‐1 is upregulated and the proliferation marker Ki67 is not detectable, implicating a quiescent state. The latter is a hallmark of tumor stem cells, which was proven by the expression of stem cell markers CD44 and CD133 14. This indicates that the spheroid and divergent‐differentiated beta‐catenin accumulating clusters may constitute a tumor stem cell niche. This assumption is strengthened by the fact that effects of a spatial milieu, including occurrence of paracrine acting signaling molecules [e.g., BMP4 12, FGF, SHH 1], are locally restricted by adjacent cells expressing sealing cell‐cell contacts (claudin‐1, Figure 2B).
Figure 2.
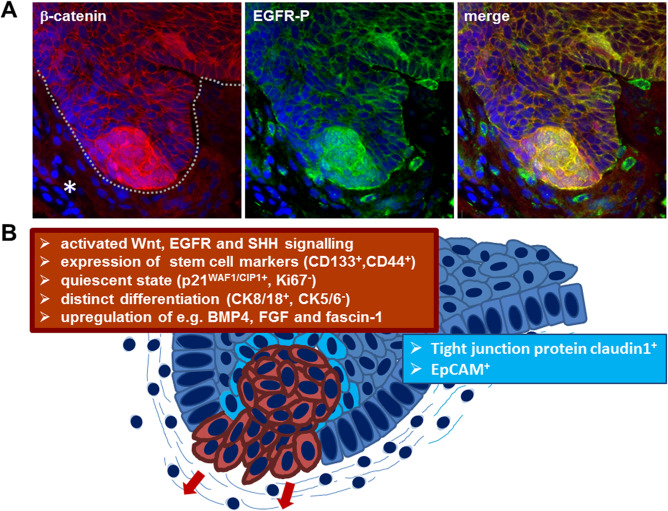
Subcellular characterization of ACP based on immunohistochemistry. Double immunohistochemistry (A) showing budding ACP tumor (line) into adjacent brain tissue (asterisk) with beta‐catenin accumulating cell clusters (red) at the tip of the finger‐shaped tumor protrusion. Detection of activated EGFR (EGFR‐P, green) reveals co‐localization within the beta‐ catenin accumulating cells (yellow) in the merged view, demonstrating co‐activation of Wnt signaling and EGFR signaling pathway within the same cells at the invasion front. Solid ACP areas reveal a different marker expression (B). The beta‐catenin accumulating cells are marked in red with the corresponding marker profile summarized in the red box. Cells surrounding the mostly whirl‐shaped beta‐catenin enriched cells (light blue) express enhanced levels of claudin‐1, involved in tight junction formation and the epithelial cell adhesion molecule EpCAM.
Primary Cell Culture Model
Cell cultures are needed to elucidate the functional impact of the activated pathways (e.g., Wnt and EGFR signaling) or the decreased expression of cell adhesion molecules in tumor growth/migration. Since no cell lines are available for this rare tumor entity, primary cell cultures using native human tumor tissue provided by neurosurgical collaborators were prepared according to Ulfarsson et al 27. Prior to processing the ACP tissue for cell culture, representative parts of the tumor need to be analyzed histologically using frozen section in order to review the tumor content. Only tissue pieces with a substantial amount of vital, solid tumor cells are sufficient for primary cell cultures. The adhesively growing tumor cells at first exhibit distinct cell–cell contacts. Furthermore, epithelial differentiation of cultured ACP cells is reflected by the distinct expression of pan‐cytokeratin markers, for example, KL1 10.
The influence of targeted molecules on cell viability (MTT Assay), induction of cell death (flow cytometry of annexin V/propiduim iodide stained cells), proliferation (BrdU Assay), migration potency (Boydenchamber‐, woundhealing Assay) and the ability of colony formation, as well as the effects on gene and protein expression could be analyzed in more detail using primary cell cultures. These functional studies provide insights into the biological impact and effectivity of affected targets, as discussed below.
Using primary cell cultures, it was demonstrated that the downregulation of beta‐catenin with short interfering RNA (siRNA) inhibits migratory capacity, supporting in reverse a role of Wnt‐signaling activation in tumor growth/migration. Additionally, the migratory potential of primary ACP tumor cells was found to be decreased after fascin‐1 and enhanced after claudin‐1 siRNA mediated knockdown 10, 24. These in vitro findings support the increased motility of beta‐catenin accumulating cells forming tumor protrusions. Furthermore, this is consistent with results obtained by double immunohistochemistry, which revealed an upregulation of fascin‐1 and downregulation of claudin‐1 (Figure 2B) in these cells.
In regard to potential druggable targets, research was focused on EGFR signaling as an enhancer of tumor growth and stem cell formation, inhibitor of apoptosis and promoter of radiation resistance 2, 6, 16. All these processes may play a role in ACP recurrence. Utilizing cell migration assays, the impact of activated EGFR signaling in facilitating tumor cell migration, was confirmed in primary cell cultures and associated with enhanced fascin‐1 expression 10. Notably, treatment with the EGFR inhibitor Gefitinib impeded cell migration as well as the expression of fascin‐1 11. Therefore, EGFR emerges as a potential target for drug based therapy for the prevention of tumor outgrowth. Another important aspect that is relevant for ACP therapy is EGFR‐mediated inhibition of apoptosis and radiation sensitivity 2. Clinical data documents recurrence after radiotherapy in ACP patients 17. In vitro experiments support this notion, as radiation induced cell death is lower in primary ACP tumor cells where EGFR signaling 23 is activated. This effect is maintained by the EGFR‐induced upregulation of the anti‐apoptotic protein survivin. Notably, the inhibition of EGFR signaling significantly increases irradiation susceptibility and reveals that EGFR inhibitor potency varies considerably. CUDC‐100 targeting the EGFR was shown to be much more effective than the EGFR inhibitor Gefitinib 23.
Because of the absence of alternative in vitro solutions, primary cell cultures are a good tool for functional analyses. Nevertheless, it should be realized that this model also has limits. In order to perform various experiments a considerable number of cells are needed, which makes splitting indispensable. With increasing passages, however, epithelial phenotype and the tumor‐associated mutation are lost. The latter phenomenon is also found in other primary cultures 18. Furthermore, a cell culture is an artificial 2D model lacking a tumor‐specific extracellular matrix. Therefore, it is unable to reflect the complexity of 3D tumor circuits, which would be expected to maintain characteristic, cellular appearances as described previously (Figure 2B). For this reason, results should be considered with caution and need to be validated in an additional setting. One possibility is checking back gene‐regulatory events in human formalin fixed tissue using double immunohistochemistry (Figure 1, green arrow). Another method of proving the functional impact of the treatment is the usage of an in vivo model reproducing ACP characteristics.
PDX Model
Stache et al developed a protocol for the orthotopic implantation of native patient‐derived ACP tumor tissue into the hemispheres of nude mice (PDX model), leading to an almost 100% tumor induction 25. Furthermore, engrafted tumors could be monitored using a 7 Tesla small‐animal MRI system (Figure 3A). The PDX models revealed important advantages: (1) The induced tumors concordantly reflect the human counterpart (Figure 3B) and preserve 3D circuits of the primary tissue as depicted in Figure 2; (2) Tumor growth and its effects on adjacent brain tissue could be analyzed in more detail. The latter is of significance, since histological analysis of the tumor's microenvironment in human samples is restricted, as excision of the surrounding brain tissue during surgery is avoided in order to keep surgically induced adverse effects in this sensitive region to a minimum.
Figure 3.
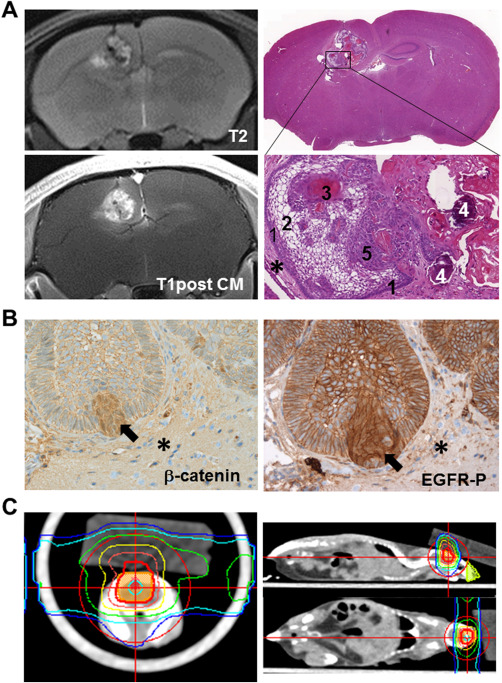
PDX model. (A) Intracranial lesion of transplanted patient‐derived ACP tissue is detectable on T2‐weigted and after contrast media administration T1‐weigted MR images. Hematoxylin & Eosin staining reveal histological appearance of a tumor transplanted into the mouse brain (asterisk), showing typical histological hallmarks in magnification, for example, palisading cells (1), stellate reticulum cells (2), wet keratin (3), calcification (4) and solid cells (5) of ACP. (B) Xenotransplanted tumors also exhibit digitate tumor protrusions into adjacent brain tissue (asterisk) with beta‐catenin accumulating cells (arrow) also showing EGFR activation (EGFR‐P) with nuclear localization (arrow). (C) In order to evaluate the impact of radiation therapy on ACP tissue, PDX model irradiation planning was performed in accordance with the standard clinical approach. Colored isodose lines correspond to the dose levels shown in the coronal, sagittal and axial view (adapted from Hartmann et al).
By using the PDX model, cell culture results were confirmed in regard to tumor growth. In vivo, the finger‐shaped tumor protrusions invading the surrounding brain tissue are also formed by cells showing beta‐catenin accumulations and an activated EGFR (Figure 3B), as seen in humans (Figure 2A). Therefore, these cells can be considered as the driving force of tumor invasion 25. Because of the similar complexity of tumor circuit to human appearance and molecular homology‐like EGFR activation, the expression of the stem cell marker CD133, the upregulation of p21WAF/CIP‐1 and the downregulation of claudin‐1 in beta‐catenin accumulating cells, the PDX model emerges as the gold standard for ongoing in vivo studies. It could be utilized to elucidate recurrence after irradiation and to analyze drug‐based therapeutic intervention, as well as a combination of the two. A protocol for conformal high‐precision brain tumor irradiation using clinical LINAC was developed to confirm in vitro data concerning the impact of EGFR signaling in mediating radiation resistance in the future 9. To achieve the best comparability of the PDX model with the situation found in humans, the treatment planning (Figure 3C) was adapted to clinical procedures. CT and MRI images were employed to demarcate the target volume and the organ at risk. Furthermore, analysis of DNA damage induced immediately after irradiation showed a high level of precision in accordance with the treatment plan. Only the targeted tumor area was affected after fractionated irradiation 9. The PDX model and the protocols developed for monitoring and treatment pave the way for testing the efficacy of substances in order to identify the best curative strategy and, as a result, improve patient outcome.
Prospective Outlook
In the future, patient‐specific in vitro and in vivo models derived from native ACP tissue obtained in the course of surgical intervention will provide promising tools for the evaluation of the best patient‐tailored treatment.
Ethical Approval
A declaration of consent for the patient is available for all specimens, approved by the local ethics committee of the Friedrich‐Alexander‐Universität Erlangen‐Nürnberg. Procedures were conducted in accordance to the Declaration of Helsinki. All experiments performed at Friedrich‐Alexander University Erlangen‐Nürnberg were authorized by the local government (Regierung von Unterfranken, Germany) in accordance with the animal protection act.
Conflicts of Interest
The authors have no conflict of interest to declare.
Acknowledgments
ACP research necessitates the close collaboration and networking of clinicians (neurosurgeons, to provide tumor tissue; endocrinologists and oncologists, as well as radiologists, to provide clinical data) with scientists. The basis for the aforementioned models is native ACP tissue, which requires the intraoperative collection of a resected tumor in a special medium to keep tumor cells alive, in addition to rapid transport to the laboratory for further research processing (Figure 1). We therefore greatly appreciate the support of the neurosurgeons, Michael Buchfelder, University Hospital Erlangen, Germany; Jörg Flitsch, University Hospital Hamburg Eppendorf, Hamburg, Germany; Rudolf Fahlbusch, International Neuroscience Institute Hannover, Hannover, Germany as well as Thomas Czech and Harald Stefanits, University of Vienna, Vienna, Austria who make our research possible. These studies were funded by the German Research Foundation DFG (NP‐DFG_BU 2878/2‐1), the Internationale Stiftung Neurobionik and the Dr. Robert Pfleger‐Stiftung.
References
- 1. Andoniadou CL, Gaston‐Massuet C, Reddy R, Schneider RP, Blasco MA, Le Tissier P et al (2012) Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol 124:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL (2013) Nuclear EGFR as a molecular target in cancer. Radiother Oncol 108:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 3. Buslei R, Holsken A, Hofmann B, Kreutzer J, Siebzehnrubl F, Hans V et al (2007) Nuclear beta‐ catenin accumulation associates with epithelial morphogenesis in craniopharyngiomas. Acta Neuropathol 113:585–590. [DOI] [PubMed] [Google Scholar]
- 4. Buslei R, Nolde M, Hofmann B, Meissner S, Eyupoglu IY, Siebzehnrubl F et al (2005) Common mutations of beta‐catenin in adamantinomatous craniopharyngiomas but not in other tumors originating from the sellar region. Acta Neuropathol (Berl) 109:589–597. [DOI] [PubMed] [Google Scholar]
- 5. Campanini ML, Colli LM, Paixao BM, Cabral TP, Amaral FC, Machado HR et al (2010) CTNNB1 gene mutations, pituitary transcription factors, and MicroRNA expression involvement in the pathogenesis of adamantinomatous craniopharyngiomas. Hormones Cancer 1:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dittmann K, Mayer C, Rodemann HP (2010) Nuclear EGFR as novel therapeutic target: insights into nuclear translocation and function. Strahlenther Onkol 186:1–6. [DOI] [PubMed] [Google Scholar]
- 7. Erdheim J (1904) Über Hypophysenganggeschwulste und Hirmcholesteatome. Sitzungsb Kais Akad Wissen Math Naturw Klin 113:537–726. [Google Scholar]
- 8. Gump JM, Donson AM, Birks DK, Amani VM, Rao KK, Griesinger AM et al (2015) Identification of targets for rational pharmacological therapy in childhood craniopharyngioma. Acta Neuropathol Commun 3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartmann J, Wolfelschneider J, Stache C, Buslei R, Derer A, Schwarz M et al (2016) Novel technique for high‐precision stereotactic irradiation of mouse brains. Strahlenther Onkol 192:806–814. [DOI] [PubMed] [Google Scholar]
- 10. Holsken A, Buchfelder M, Fahlbusch R, Blumcke I, Buslei R (2010) Tumor cell migration in adamantinomatous craniopharyngiomas is promoted by activated Wnt‐signalling. Acta Neuropathol 119:631–639. [DOI] [PubMed] [Google Scholar]
- 11. Holsken A, Gebhardt M, Buchfelder M, Fahlbusch R, Blumcke I, Buslei R (2011) EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clin Cancer Res 17:4367–4377. [DOI] [PubMed] [Google Scholar]
- 12. Holsken A, Kreutzer J, Hofmann BM, Hans V, Oppel F, Buchfelder M et al (2009) Target gene activation of the Wnt signaling pathway in nuclear beta‐catenin accumulating cells of adamantinomatous craniopharyngiomas. Brain Pathol 19:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holsken A, Sill M, Merkle J, Schweizer L, Buchfelder M, Flitsch J et al (2016) Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commun 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holsken A, Stache C, Schlaffer SM, Flitsch J, Fahlbusch R, Buchfelder M et al (2014) Adamantinomatous craniopharyngiomas express tumor stem cell markers in cells with activated Wnt signaling: further evidence for the existence of a tumor stem cell niche?. Pituitary 17:546–556. [DOI] [PubMed] [Google Scholar]
- 15. Kato K, Nakatani Y, Kanno H, Inayama Y, Ijiri R, Nagahara N et al (2004) Possible linkage between specific histological structures and aberrant reactivation of the Wnt pathway in adamantinomatous craniopharyngioma. J Pathol 203:814–821. [DOI] [PubMed] [Google Scholar]
- 16. Ma L, Zhang G, Miao XB, Deng XB, Wu Y, Liu Y et al (2013) Cancer stem‐like cell properties are regulated by EGFR/AKT/beta‐catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma. FEBS J 280:2027–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muller HL (2014) Craniopharyngioma. Endocr Rev 35:513–543. [DOI] [PubMed] [Google Scholar]
- 18. Piaskowski S, Bienkowski M, Stoczynska‐Fidelus E, Stawski R, Sieruta M, Szybka M et al (2011) Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br J Cancer 104:968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434:843–850. [DOI] [PubMed] [Google Scholar]
- 20. Samis J, Vanin EF, Sredni ST, de Bonaldo Mde F, Costa FF, Tomita T et al (2016) Extensive miRNA expression analysis in craniopharyngiomas. Child's Nervous Syst 32:1617–1624. [DOI] [PubMed] [Google Scholar]
- 21. Sekine S, Shibata T, Kokubu A, Morishita Y, Noguchi M, Nakanishi Y et al (2002) Craniopharyngiomas of adamantinomatous type harbor beta‐catenin gene mutations. Am J Pathol 161:1997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sekine S, Takata T, Shibata T, Mori M, Morishita Y, Noguchi M et al (2004) Expression of enamel proteins and LEF1 in adamantinomatous craniopharyngioma: evidence for its odontogenic epithelial differentiation. Histopathology 45:573–579. [DOI] [PubMed] [Google Scholar]
- 23. Stache C, Bils C, Fahlbusch R, Flitsch J, Buchfelder M, Stefanits H et al (2016) Drug priming enhances radiosensitivity of adamantinomatous craniopharyngioma via downregulation of survivin. Neurosurg Focus 41:E14. [DOI] [PubMed] [Google Scholar]
- 24. Stache C, Holsken A, Fahlbusch R, Flitsch J, Schlaffer SM, Buchfelder M et al (2014) Tight junction protein claudin‐1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro‐Oncology 16:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stache C, Holsken A, Schlaffer SM, Hess A, Metzler M, Frey B et al (2015) Insights into the infiltrative behavior of adamantinomatous craniopharyngioma in a new xenotransplant mouse model. Brain Pathol 25:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thimsen V, Holsken A, Buchfelder M, Flitsch J, Fahlbusch R, Stefanits H et al (2016) EpCAM (CD326) is differentially expressed in craniopharyngioma subtypes and Rathke's cleft cysts. Sci Rep 6:29731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ulfarsson E, Karstrom A, Yin S, Girnita A, Vasilcanu D, Thoren M et al (2005) Expression and growth dependency of the insulin‐like growth factor I receptor in craniopharyngioma cells: a novel therapeutic approach. Clin Cancer Res 11:4674–4680. [DOI] [PubMed] [Google Scholar]


