Abstract
Ependymomas originate in posterior fossa (PF), supratentorial (ST) or spinal cord (SC) compartments. At present, grading schemes are applied independent of anatomic site. We performed detailed histological examination on 238 World Health Organization grade II and III ependymomas. Among PF ependymomas, the presence of hypercellular areas, necrosis, microvascular proliferation and elevated mitotic rate (all P < 0.01) were significantly associated with worse progression‐free survival (PFS), while extensive ependymal canal formation was not (P = 0.89). Similar to the PF tumors, microvascular proliferation (P = 0.01) and elevated mitotic rate (P = 0.03) were significantly associated with worse PFS in the ST tumors. However, in contrast to PF tumors, extensive ependymal canals (P = 0.03) were associated with worse clinical outcome in ST ependymomas, but hypercellularity (P = 0.57) and necrosis (P = 0.47) were not. On multivariate Cox regression, after adjusting for relevant clinical variables, individual histological factors and a composite histological score remained significant among ST and PF ependymoma. In contrast to both PF and ST ependymoma, histological features were not found to be associated with PFS in SC tumors. Taken together, the clinical relevance of specific histological features in ependymoma appears to be related to the anatomic site of origin and suggests that site‐specific grading criteria be considered in future classification systems.
Keywords: ependymoma, histology, progression‐free survival
Introduction
Ependymomas are primary central nervous system (CNS) tumors that morphologically resemble ependymal cells that line the ventricles. These tumors may originate in the cerebral hemispheres, posterior fossa (PF) or spinal cord (SC) 14. While these tumors constitute only about 3–5% of adult CNS tumors 5, their relative incidence rises among children and young adults, in whom they account for approximately 10% of CNS tumors 13. The age at diagnosis of ependymomas also appears to vary by location of the tumor 14. The current World Health Organization (WHO) 2007 edition guidelines for grading ependymomas from all locations are based on the predominance of histological features that have been well established as markers of aggressive behavior, including tumor cellularity, cytologic anaplasia, mitotic index, microvascular proliferation and tumor necrosis. The influence of clinical factors including age at diagnosis, site of origin and extent of tumor resection has been well established in prior reports 4, 5, 6, 15, 21. Recently, stereotypic genetic alterations that are associated with clinically distinct subgroups of ependymomas have also been identified 8, 10, 19, 22, 23. However, these factors are not considered in the current WHO criteria when grading ependymomas from the different sites of origin. Studies examining the influence of individual histological features and clinical factors on the prognosis of patients with ependymomas originating from these distinct locations are lacking.
In the present study, we report findings following a detailed evaluation of individual histological features, along with the well‐established clinical parameters of age at diagnosis and extent of surgical resection, among a heterogeneous population of pediatric and adult patients with ependymomas originating in the PF, supratentorial (ST) compartment and SC.
Materials and Methods
Cases of ependymomas with tissue sections available for histological review were identified from among patients registered in the database of the Collaborative Ependymoma Research Network (CERN) tissue repository. All demographic data, dates of events, clinical, therapeutic and outcome information were obtained from the CERN database. All patients with a minimum follow‐up of 3 years or recurrence within 3 years of the first diagnosis were considered eligible. Tumors that that did not have a specified site of origin, specimens that had not been obtained from the first surgery, and those that carried a diagnosis of subependymoma or myxopapillary ependymoma were excluded from further analysis. All histopathology slides had been submitted for central review, and those with a confirmed diagnosis of ependymoma were selected.
A predetermined set of qualitative and quantitative histological features were evaluated for each case. These are summarized in Table 1 and representative areas are illustrated in Figure 1. Briefly, the architectural features that were qualitatively assessed as absent, poorly formed or well‐formed included perivascular pseudorosettes, ependymal canals, papillary structures and microvascular proliferation. True microvascular proliferation was defined as per current WHO guidelines as vascular cell hyperplasia with multilayered, mitotically active endothelial cells together with smooth muscle cells/pericytes. The overall tumor cellularity, highest cellularity identified, extent of nuclear anaplasia, highest nuclear anaplasia identified and the presence of tumor necrosis, with or without pseudopalisading of tumor cells, were separately noted. Hypercellularity was defined as the presence of at least one 400× field where the neoplastic cells had sparse to absent gliofibrillary cytoplasmic processes, and the neoplastic nuclei appeared to be in contact with each other. In contrast, low cellularity was defined as areas where each neoplastic nucleus was separated from adjacent neoplastic nuclei by abundant gliofibrillary cytoplasmic processes. The extent of fibrillary areas, perivascular pseudorosettes, ependymal canals, papillary structures, hypercellular areas, areas with extensive nuclear anaplasia, tumor necrosis and microvascular proliferation were also quantitatively evaluated as being absent (0%) or forming <25%, 25–50% and >50% of the tumor tissue examined. The highest mitotic count was determined in 10 consecutive high‐power fields (HPF) in the most active area of the tumor. A cut‐off of <5 vs. ≥5 mitoses per 10 HPF was used to code low vs. high mitotic rate. The extent of invasion was assessed in samples where an interface between the ependymoma and adjacent tissue was present in the submitted slide, noted as absent, focal or extensive. In addition, the extent of areas with overall features consistent with Grade III ependymoma based on the WHO 2007 guidelines was also recorded. All available slides were independently reviewed by two neuropathologists and the histological scores were recorded. Subsequently, slides where the two neuropathologists had assigned different scores to any histological factor were reviewed concurrently, and the consensus score was recorded for further analysis. The slide reviews were conducted without knowledge of clinical or demographic factors, or of patient outcomes. To exclude possible misclassification of supratentorial embryonal tumor with multilayered rosettes (ETMR) 11, immunohistochemical staining using the LIN28A antibody (A177, #3978, Cell Signaling Inc, Boston, MA, USA) was performed on a total of 18 supratentorial ependymomas (10 with high ependymal canal score; eight with low ependymal canal score). Immunohistochemical staining was performed with antigen retrieval using the citrate buffer pH 6.0 and a working antibody dilution of 1:100, with incubation overnight at 4°C.
Table 1.
Histological features evaluated
| Score | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Infiltration of adjacent brain | Absent | Focal | Extensive | — |
| Pseudorosettes | ||||
| Quality | Absent | Poorly formed | Well formed | |
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| Ependymal canals | ||||
| Quality | Absent | Poorly formed | Well formed | — |
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| Papillary structures | ||||
| Quality | Absent | Poorly formed | Well formed | — |
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| Nuclear Anaplasia | ||||
| Quality | None | Mild | Moderate | Severe |
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| Hypercellular areas | ||||
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| Tumor necrosis | ||||
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| Microvascular proliferation | ||||
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| World Health Organization Grade III features | ||||
| Extent in tissue examined | Absent | <25% | 25–50% | >50% |
| Mitotic rate | ||||
| Per 10 high power fields | — | — | — | — |
Figure 1.
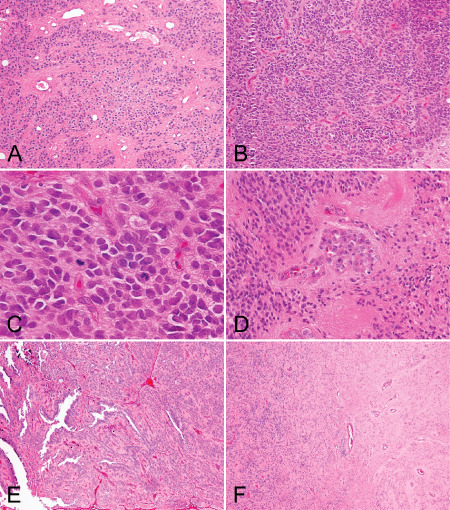
An illustration of selected histological features, including. A. Areas of low cellularity, B. areas of hypercellularity, C. mitoses, D. microvascular proliferation, E. extensive ependymal canals and F. tumor interface with the adjacent brain parenchyma.
The primary end point for statistical analyses was progression‐free survival, which was defined as the time from first surgery to date of first recurrence, last follow‐up or death from any cause. All statistical analyses were performed on Statistica 6.1 software package (Statsoft Inc., Tulsa, OK, USA). Univariate and multivariate Cox regression analyses evaluated the association of individual and multiple covariates with progression‐free survival. The Kaplan–Meier method was used to estimate the time to event functions of progression‐free survival, and the survival curves were compared using the log‐rank test. All statistical tests were two sided, and P ≤ 0.05 was considered statistically significant. The use of the tissue and clinical data for this study was covered under a protocol approved by the M.D. Anderson Institutional Review Board.
Results
A total of 238 ependymomas with sufficient pathologic material and clinical information, and that met the selection criteria, were identified from the CERN database. The clinical characteristics of the study population are summarized in Table 2. The results specific to the three sites of origin of the ependymomas are described here separately.
Table 2.
Patient characteristics
| Posterior fossa (n = 103) | Supratentorial (n = 64) | Spinal cord (n = 71) | |
|---|---|---|---|
| Age at diagnosis, years | |||
| Mean (Range) | 22 (0–72) | 25 (0–65) | 42 (5–71) |
| <18 years | 62 (60%) | 32 (50%) | 6 (8%) |
| Extent of surgical resection | |||
| Gross total | 57 (55%) | 42 (66%) | 52 (73%) |
| Subtotal/Biopsy | 42 (41%) | 22 (34%) | 19 (27%) |
| Unknown | 4 (4%) | 0 (0%) | 0 (0%) |
| Adjuvant therapy | |||
| Radiation alone | 40 (39%) | 16 (25%) | 0 (0%) |
| Chemotherapy alone | 11 (11%) | 6 (9%) | 4 (6%) |
| Radiation & Chemotherapy | 7 (7%) | 17 (26%) | 0 (0%) |
| None/Unknown | 45 (44%) | 25 (39%) | 67 (94%) |
| World Health Organization Grade | |||
| II | 87 (84%) | 54 (84%) | 66 (93%) |
| III | 16 (16%) | 10 (16%) | 5 (7%) |
| Follow‐up, weeks | |||
| Mean (Range) | 210 (4–1252) | 176 (10–1224) | 256 (3–884) |
| Tumor recurrence | |||
| Yes | 48 (47%) | 46 (72%) | 17 (24%) |
| No | 55 (53%) | 18 (28%) | 54 (76%) |
| Death during follow‐up | |||
| After recurrence | 8 (8%) | 21 (33%) | 3 (4%) |
| Without recurrence | 7 (7%) | 3 (5%) | 3 (4%) |
PF ependymomas
Patient population
A total of 103 cases of PF ependymomas were reviewed, with their first surgery between 1986 and 2011. The median age of the patients at the time of initial diagnosis was 22 years (range 0–72). There were 29 patients (28%) below 3 years of age and 41 patients (40%) aged 18 years or older. Median clinical follow‐up from the time of first surgery till first recurrence or last contact was 210 weeks (range 4–1252). Fifty‐seven of these patients received gross total resections and 42 patients received subtotal resections. There were 16 anaplastic ependymomas (Grade III) among these tumors by the WHO 2007 edition guidelines. Additional radiation or chemotherapy information was available for 94 patients, with 40 patients receiving radiation therapy only, 11 receiving chemotherapy only, and seven receiving both. Tumor recurrence occurred among 48 patients (47%) and 40 of these patients (83%) were alive at the time of last follow‐up. Among the recurrences, 26 patients (54%) had received radiation and/or chemotherapy.
Correlation of histological features with progression‐free survival in PF tumors
Univariate Cox regression analysis was performed to determine significant association between the various histological factors assessed in PF ependymomas and progression‐free survival. The frequencies of individual histological factors are summarized in Table 3, and the significant findings are summarized in Table 4. The increasing extent of anaplastic features, as described by the WHO 2007 guidelines for Grade III ependymomas, was also significantly associated with progression‐free survival (P < 0.01). Tumors that showed focal areas with anaplastic histological features appeared to be significantly associated with worse progression‐free survival than exclusively Grade II tumors, although to a lesser extent than tumors showing predominantly (>50%) Grade III features (P < 0.01, Figure 2). The presence of hypercellular areas of any size, microvascular proliferation and tumor necrosis were scored as 0 (absent) or 1 (present). Based on previously published reports 4, 7, 21, a cut‐off value of <5 mitoses per 10 HPFs was selected for dichotomizing a score of 0 from 1. The individual histological factors that were significantly associated with progression‐free survival included the presence of hypercellular areas of any size [P < 0.01, hazard ratio (HR) = 2.58], presence of true microvascular proliferation (P < 0.01, HR = 2.37), mitotic rate (P < 0.01, HR = 3.26) and tumor necrosis (P = 0.01, HR = 2.15) (Figures 3 and 4). The presence or extent of ependymal canals was not associated with progression‐free survival (P = 0.89; Figure 5). The interface between the tumor and normal tissue was identified in 28 ependymomas, and invasion into the adjacent tissue was noted in 11 of these cases (39%). However, invasion was not significantly associated with outcome (P = 0.51).
Table 3.
Frequencies of individual histological features among ependymomas arising from the three compartments (* = any extent; ∧ = for unequivocal well‐formed histological feature)
| Posterior fossa (n = 103) | Supratentorial (n = 64) | Spinal cord (n = 71) | |
|---|---|---|---|
| Pseudorosettes | |||
| Well‐formed pseudorosettes* | 92 (89%) | 50 (78%) | 61 (86%) |
| >25% of tissue examined ∧ | 82 (80%) | 42 (66%) | 59 (83%) |
| Ependymal canals | |||
| Well‐formed ependymal canals* | 48 (47%) | 11 (17%) | 48 (68%) |
| >25% of tissue examined ∧ | 18 (17%) | 5 (8%) | 31 (44%) |
| Papillary structures | |||
| Well‐formed papillary structures* | 40 (39%) | 10 (16%) | 24 (34%) |
| >25% of tissue examined ∧ | 16 (15%) | 1 (2%) | 12 (17%) |
| Nuclear Anaplasia | |||
| Present | 18 (17%) | 32 (50%) | 12 (17%) |
| >25% of tissue examined ∧ | 9 (9%) | 17 (27%) | 3 (4%) |
| Hypercellular areas present* | 23 (22%) | 22 (34%) | 11 (15%) |
| Tumor necrosis present* | 49 (47%) | 45 (70%) | 17 (24%) |
| Microvascular proliferation present* | 34 (33%) | 42 (66%) | 8 (11%) |
| Mitotic rate | |||
| ≥5 mitoses per 10 high‐power fields | 26 (25%) | 34 (53%) | 7 (10%) |
| <5 mitoses per 10 high‐power fields | 77 (75%) | 30 (47%) | 64 (90%) |
| Extent of World Health Organization Grade III features | |||
| Absent or <25% of tissue examined | 77 (75%) | 35 (55%) | 62 (87%) |
| 25–50% of tissue examined | 10 (10%) | 5 (8%) | 4 (6%) |
| >50% of tissue examined | 16 (15%) | 24 (38%) | 5 (7%) |
Table 4.
Univariate Cox regression analyses of individual histological features associated with progression‐free survival among ependymomas from the three compartments
| Posterior fossa (n = 103) | Supratentorial (n = 64) | Spinal cord (n = 71) | ||||
|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | |
| Infiltration of adjacent brain | 1.22 | 0.51 | 1.03 | 0.91 | 0.65 | 0.77 |
| Pseudorosettes | ||||||
| Well‐formed pseudorosettes | 1.44 | 0.49 | 1.73 | 0.08 | 0.68 | 0.56 |
| >25% of tissue examined | 0.84 | 0.41 | 1.44 | 0.08 | 0.78 | 0.44 |
| Ependymal canals | ||||||
| Well‐formed ependymal canals | 0.98 | 0.89 | 1.19 | 0.38 | 0.74 | 0.23 |
| >25% of tissue examined | 1.02 | 0.89 | 1.69 | 0.03 | 0.67 | 0.10 |
| Papillary structures | ||||||
| Quality | 1.08 | 0.64 | 0.96 | 0.83 | 1.17 | 0.57 |
| Extent in tissue examined | 1.08 | 0.63 | 0.94 | 0.80 | 1.32 | 0.30 |
| Nuclear anaplasia | ||||||
| Quality | 1.64 | 0.06 | 1.14 | 0.50 | 1.73 | 0.18 |
| Extent in tissue examined | 1.29 | 0.07 | 1.23 | 0.15 | 1.20 | 0.48 |
| Hypercellular areas present | 2.58 | <0.01 | 1.04 | 0.57 | 1.84 | 0.15 |
| Tumor necrosis present | 2.15 | 0.01 | 1.12 | 0.47 | 1.31 | 0.25 |
| Microvascular proliferation present | 2.37 | <0.01 | 2.35 | 0.01 | 1.68 | 0.09 |
| Mitotic rate (<5 vs. ≥5/10 HPF*) | 3.26 | <0.01 | 1.94 | 0.03 | 1.07 | 0.10 |
| World Health Organization Grade | 5.71 | <0.01 | 1.95 | 0.04 | 1.29 | 0.67 |
| Posterior fossa metagene score (n = 52) | 5.03 | <0.01 | – | – | – | – |
*High power fields.
Figure 2.
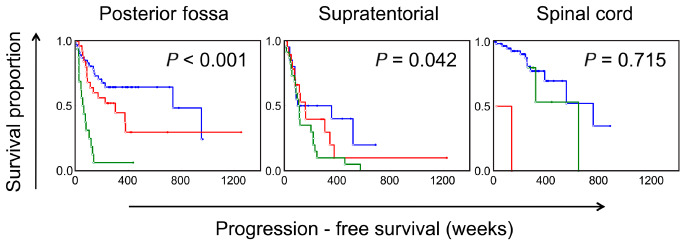
Kaplan–Meier survival curves showing the effect of the extent of World Health Organization (WHO) grade III features on progression‐free survival among ependymomas arising from the three different compartments. Blue = WHO grade III features absent; Red = <50%; Green = >50%. Values based on univariate Cox regression analysis.
Figure 3.
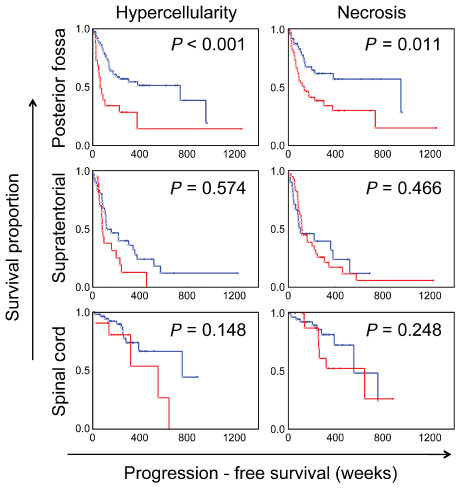
Kaplan–Meier survival curves showing the effects of the presence of hypercellularity and necrosis on progression‐free survival among ependymomas arising from posterior fossa, supratentorial and spinal cord compartments. Blue = absent; Red = present. P‐values based on univariate Cox regression analysis.
Figure 4.
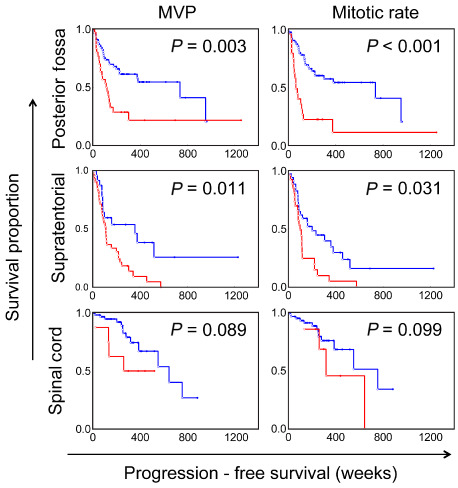
Kaplan–Meier survival curves showing the effects of the presence of microvascular proliferation (MVP) and mitotic rate (at a cut‐off value of ≥5 per 10 high‐power fields) on progression‐free survival among ependymomas arising from posterior fossa, supratentorial and spinal cord compartments. Blue = absent; Red = present. P‐values based on univariate Cox regression analysis.
Figure 5.
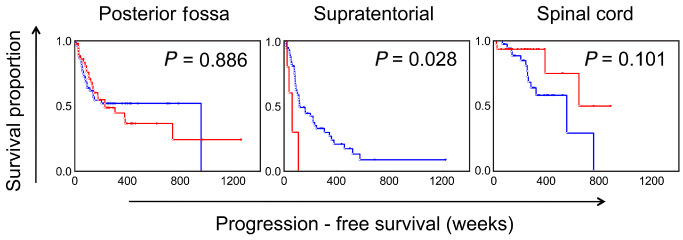
Kaplan–Meier survival curves showing the effect of the extent of ependymal canals on progression‐free survival among ependymomas arising from the posterior fossa, supratentorial and spinal cord compartments. Blue = <25%; Red = >25%. P‐values based on univariate Cox regression analysis.
Multivariate Cox regression analyses were performed to determine whether grading based on the histological features identified as significant on univariate analysis was independently associated with progression‐free survival in the context of the significant prognostic factors of age at diagnosis as a linear variable, WHO grade, extent of resection, radiation therapy and chemotherapy. On multivariate analysis, all four individual histological factors remained significantly associated with progression‐free survival (Table 5). These factors were combined to obtain a PF histological score that was then dichotomized based on the overall score of 0–1 representing a low score and 2–4 representing a high score. On multivariate analysis, the combined PF histological score (P < 0.01, HR = 1.38) remained independently predictive of progression‐free survival, (Table 6).
Table 5.
Adjusted hazard ratios and P‐values for the histological variables in posterior fossa and supratentorial ependymomas in a multivariate Cox regression analysis model. Hazard ratios and P‐values are adjusted for age at diagnosis, World Health Organization grade, extent of resection, adjuvant chemotherapy and adjuvant radiotherapy
| Posterior fossa (n = 103) | Supratentorial (n = 64) | |||
|---|---|---|---|---|
| HR | P | HR | P | |
| Hypercellular areas present | 2.26 | 0.02 | N/A* | N/A |
| Tumor necrosis present | 1.39 | 0.06 | N/A | N/A |
| Microvascular proliferation present | 1.94 | 0.03 | 1.98 | 0.10 |
| Mitotic rate | 2.61 | <0.01 | 1.06 | 0.04 |
| Extensive ependymal canals | N/A | N/A | 1.72 | 0.04 |
*N/A = not applicable (multivariate analysis not performed because variable was not significant in univariate analysis).
Table 6.
Multivariate Cox regression analyses of the combined histological score and standard clinical parameters of prognosis in posterior fossa and supratentorial ependymomas
| Posterior fossa (n = 103) | Supratentorial (n = 64) | |||
|---|---|---|---|---|
| HR | p | HR | p | |
| Age at diagnosis | 0.99 | 0.39 | 1.00 | 0.93 |
| Extent of resection | 1.00 | 0.55 | 1.00 | 1.00 |
| World Health Organization grade | 4.38 | <0.01 | 0.95 | 0.88 |
| Adjuvant radiotherapy | 1.03 | 0.02 | 1.02 | 0.03 |
| Adjuvant chemotherapy | 0.97 | 0.05 | 0.99 | 0.33 |
| Combined histological score | 1.38 | <0.01 | 1.62 | 0.03 |
| Metagene score (n = 52) | 3.89 | 0.02 | N/A* | N/A |
*N/A = data not available.
Correlation of PF histological features and clinical factors with gene expression profiling
Gene expression analysis data was available for 52 of the analyzed PF ependymomas. On univariate Cox regression analysis, the composite metagene score based on a previously published 10‐gene signature 22 was a significant independent factor related to progression‐free survival (P < 0.01, HR = 5.03). The individual histological factors that had been associated with progression‐free survival in the overall group remained significant in this subgroup as well. On multivariate analysis, in addition to the metagene score, the PF combined histological score remained independently predictive of progression‐free survival (P < 0.01). When the favorable/unfavorable PF histologic score and favorable/unfavorable metagene scores were combined, Kaplan–Meier analyses showed three distinct groups with significant survival differences—one group with both scores being favorable, a second group with one of the two scores being unfavorable and a third group with both scores being unfavorable (Figure 6).
Figure 6.
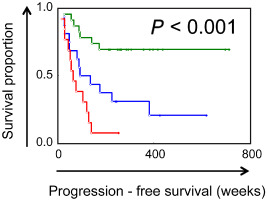
Kaplan–Meier analyses showed three distinct groups of ependymomas with significant differences in progression‐free survival when the histological score was combined with the gene expression‐derived metagene score. Ependymomas with favorable metagene scores and favorable histological scores (green) had significantly longer progression‐free survival vs. tumors with unfavorable metagene scores and unfavorable histological scores (red).
ST ependymomas
Patient population
A total of 64 cases of ST ependymomas were reviewed, with their first surgery between 1986 and 2009. The median age of the patients at the time of initial diagnosis was 25 years (range 0–65). There were six patients (9%) below 3 years of age and 32 patients (50%) were of age 18 years or older. Median clinical follow‐up from the time of first surgery till first recurrence or last contact was 176 weeks (range 10–1224). Forty‐two patients received gross total resections and 22 patients received subtotal resections. There were 10 anaplastic ependymomas (Grade III) by the WHO 2007 guidelines. Additional radiation or chemotherapy information was available for 61 patients, with 16 receiving radiation therapy only, six receiving chemotherapy only and 17 receiving both. Tumor recurrence occurred among 46 patients (72%) and 25 of these patients (54%) were alive at the time of last follow‐up. Among the recurrences, 29 patients (63%) had received radiation and/or chemotherapy.
Correlation of histological features with progression‐free survival in ST tumors
The frequencies of individual histological factors are summarized in Table 3 and the significant findings are summarized in Table 4. On univariate Cox regression analysis performed on the various histological factors assessed among ST ependymomas, the presence of WHO grade III features as the predominant histology was significantly associated with worse progression‐free survival (P = 0.04, HR = 1.95; Figure 2). However, unlike PF ependymomas, the presence of focal areas with anaplastic histologic features did not distinguish a prognostically distinct group (P = 0.49). The individual histological factors that were significantly associated with progression‐free survival included microvascular proliferation (P = 0.01, HR = 2.35) and mitotic rate (P = 0.03, HR = 1.94) (Figure 4). In contrast to PF tumors, the presence of well‐formed ependymal canals were significantly associated with worse progression‐free survival (P = 0.03, HR = 1.69; Figure 5). None of the 10 ST ependymomas that had well‐formed ependymal canals were positive for LIN28A by immunohistochemistry, when compared to skeletal muscle, as an established positive control 24 (data not shown). In further contrast to PF ependymomas, the presence of hypercellular areas and tumor necrosis were not associated with progression‐free survival (P = 0.57 and P = 0.47, respectively; Figure 3). The tumor‐normal tissue interface was identified in 32 ependymomas, and invasion into the adjacent tissue was noted in 20 of these cases (62.5%). However, invasion was not significantly associated with outcome (P = 0.91).
Multivariate Cox regression analyses were performed to determine whether each of the histological variables were significant when adjusted for relevant clinical variables. Microvascular proliferation, ependymal canals and mitotic rate variables were each placed in multivariate models together with clinical factors (age, WHO grade, extent of resection, adjuvant chemotherapy and adjuvant radiotherapy). When analyzed in this manner, mitotic rate and ependymoma canal formation were significant after adjusting for clinical factors, while microvascular proliferation was not (Table 5). The combined ST histological score was then dichotomized based on the overall score of 0 vs. >0. On multivariate analysis, the combined ST histological score was significantly associated with progression‐free survival (P = 0.03, HR = 1.62; Table 6). To test whether factors important in PF tumors were similarly important in ST ependymomas, we applied the combined PF histological score, as described above, to ST ependymomas. When ST ependymomas were dichotomized based on the PF score, univariate analysis did not demonstrate any difference in outcomes between the low score and high score groups (P = 0.39).
SC ependymomas
Patient population
A total of 71 cases of SC ependymomas were reviewed, with their first surgery between 1989 and 2008. The median age of the patients at the time of initial diagnosis was 42 years (range 5–71). There were six patients (8.4%) below 18 years of age. Median clinical follow‐up from the time of first surgery till first recurrence or last contact was 256 weeks (range 3–884). Fifty‐two patients received gross total resections and 19 patients received subtotal resections. There were five anaplastic ependymomas (Grade III) by the WHO 2007 guidelines. Additional radiation or chemotherapy information was available for all 71 patients, with four receiving chemotherapy only and the remaining receiving neither radiation nor chemotherapy. Tumor recurrence occurred among 17 patients (23.9%) and 11 of these patients (64.7%) were alive at the time of last follow‐up. Six of the patients with recurrence had received subtotal resections (35.3%) and none had received any additional radiation or chemotherapy.
Correlation of histological features with progression‐free survival in SC tumors
On univariate Cox regression analysis, none of the histological factors examined appeared to be significantly associated with progression‐free survival (Figures 2, 3, 4; Table 4). Indeed, only the presence of microvascular proliferation showed a trend toward being associated with worse outcome (P = 0.09, HR = 1.68). The extent of ependymal canals showed a trend toward being associated with better outcome (P = 0.10, HR = 0.67), opposite of the effect of ependymal canal formation among ST ependymomas, where this feature was associated with worse outcomes (Figure 5). Twenty‐nine cases showed the interface between the tumor and adjacent normal tissue, and invasion into the adjacent tissue was noted among 10 of these (34.5%). However, this feature was not associated with patient outcomes (P = 0.77). Neither the WHO grade (P = 0.67), combined PF histological score (P = 0.95) nor the combined ST histological score (P = 0.14) showed any significant association with outcomes when applied to SC ependymomas.
Discussion
There is a growing recognition of the clinical and histopathological heterogeneity in ependymomas originating in various age groups and from different compartments of the CNS, and that these may differ in clinical behavior, response to therapy and patient outcomes 1, 3, 4, 6. Studies evaluating ependymomas across age groups and from different compartments are challenged by the overall rarity of these tumors and the length of clinical follow‐up required. Several studies have described the importance of age at diagnosis, extent of surgical resection, histological grade, radiation therapy and chemotherapy, as significant predictors of outcome in patients with ependymomas 1, 3, 4, 6, 7, 9, 15, 16, 17, 18. Because this tumor predominantly affects the pediatric population, obtaining a less homogenous group of these tumors in numbers which is sufficient to evaluate the influence of various clinical and histological parameters across age groups is even more challenging. In the present study, the age at diagnosis did not show any association with progression‐free survival among PF or ST ependymomas. The relatively low representation of pediatric patients in our study population may help explain these findings, since we were able to include only 29 PF ependymoma patients (28%) and six ST ependymoma patients (9%) below the age of 3 years. The relatively small percentage of pediatric patients was also insufficient for a more detailed age‐specific analysis of the significance of various site‐specific histological features. Future studies on larger populations may help in further clarifying the age‐ and site‐specific significance of these histological features.
The current WHO 2007 guidelines recommend grading all ependymomas in a uniform manner, based on the predominant morphology, including hypercellularity, cytologic anaplasia, tumor necrosis, microvascular proliferation and mitotic rate. This grading scheme has an association with patient outcomes, even within pediatric and adult patient populations 15, 21. However, the histological variability and intratumoral heterogeneity seen within these tumors makes evaluating the effect of individual histological features across age groups and from different intracranial compartments difficult. In keeping with the recommendation to grade based on the predominant histological features, we found the increasing extent of areas with WHO grade III features to be associated with worse outcomes among PF ependymomas. Tumors with predominantly WHO grade III features were associated with the worst progression‐free survival, and the presence of focal areas with WHO grade III features also appeared to be associated with worse outcome than tumors that exhibited WHO grade II features in their entirety. The significance of the increasing extent of WHO grade III features in ependymomas from the PF and ST ependymomas in comparison to the various individual histological factors is illustrated in Table 4. While intratumoral heterogeneity and restricted tumor sampling may account for this finding, this does suggest that evaluation of qualitative criteria for their presence or absence may be less influenced by such variations during the grading of ependymomas.
Among PF ependymomas, tumor cell density and mitotic count were significantly associated with progression‐free survival. In previous studies, mitotic count has also been established as a significant histological factor associated with clinical outcome among adults with infratentorial ependymomas 7. In our patient cohort, hypercellular areas, microvascular proliferation, tumor necrosis and mitotic index were all significantly associated with progression‐free survival in PF tumors. These were combined into a single score and on multivariate analysis including clinical factors as well as histological features; we found the combined histological score to be significantly associated with progression‐free survival after adjusting for relevant clinical variables. This PF‐specific combined histological score was not similarly predictive among ST and SC ependymomas, raising the possibility that distinct histological features may be correlated to patient outcomes among ependymomas, depending on the anatomic site of origin.
Molecularly and cytogenetically distinct subgroups have been identified within PF ependymomas, associated with clinically distinct behavior 2, 12, 22, 23. Among our cases, within the subgroup of tumors with gene expression data available, we found the composite histological score and metagene score to be independent predictors of progression‐free survival. Combining the gene expression‐derived metagene score with the histological score resulted in identification of subgroups of PF ependymomas that had highly significant differences in survival indicating the significance of both gene expression and histology based clinical stratification of ependymomas (Figure 6). Given the observation that frequently heterogeneous areas associated with higher grade ependymoma are found within the same regions of the tumor 6, 21, our finding also suggests that these may indeed be part of the same biological process that drives both, anaplastic histological appearance and aggressive clinical behavior. The gain of chromosomal region 1q25 has also been identified as being prognostically related to disease progression, particularly among pediatric patients 10. The additive effect of genetic profiling, clinical factors and histological features to help refine outcome prediction needs further exploration in future studies.
ST ependymomas have been recognized as having distinct clinical behavior from PF ependymomas among children and adults1, 6, 16, as well as being associated with distinct genetic and epigenetic changes 6, 19, 20. On a histological evaluation, the presence of tumor cells infiltrating the adjacent brain has been reported as significantly associated with progression‐free survival 6. However, we did not find this association in our study population. In our study, we did find the mitotic rate to be significantly associated with progression‐free survival. This is consistent with prior reports that describe increasing proliferation index as significantly associated with progression‐free survival among adults 15. Interestingly, we found the presence of extensive well‐formed ependymal canals as associated with worse outcome, which was an unexpected finding. The possibility of these representing misdiagnosed cases of ETMR was excluded by the finding of negative immunohistochemical staining for LIN28A, as described for ETMR 11. Similar to PF tumors, microvascular proliferation and mitotic rate were associated with outcome. However, in contrast to PF tumors, hypercellularity and tumor necrosis were not significantly associated with patient outcome (Table 4), suggesting that these features may not be relevant to grading in ST ependymoma.
Among the SC ependymomas examined, none of the histological features evaluated appeared to be significantly predictive of patient outcome. SC ependymomas have been shown to have favorable survival among older adults 1. This is reflected in our patient group as well, with SC tumors having a lower rate of recurrence than tumors arising in the other two compartments. Further, the composite PF and ST histological scores, which combined individual histologic factors prognostic for the specific anatomic site, were not significantly associated with outcome among the SC ependymomas. Whether this is a reflection of SC tumors presenting clinically at an earlier stage, the high proportion of tumors that receive gross total resection, or whether this is related to true differences in the biology of SC ependymomas, requires further exploration.
Overall, our data align with previously described factors that have been associated with progression‐free survival among distinct pediatric and adult study populations. The histological evaluation of ependymomas appears to remain a significant independent predictor of progression‐free survival among intracranial ependymomas. However, there appear to be distinctions in the histological features associated with an increased risk of progression‐free survival among tumors from these distinct compartments. In brief, extensive ependymal canals appear to be a significant feature only among ST ependymomas, tumor necrosis and hypercellularity are significant among PF ependymomas, and mitotic rate and microvascular proliferation are significant for both compartments. Interestingly, none of these features are associated with progression‐free survival among SC ependymomas. Site‐specific histologic features in ependymomas require further study. However, the differences found in our cohort suggest that schemas for optimal clinically relevant grading of ependymomas may need to include consideration of the individual histological features associated with each site of origin.
Acknowledgments
The authors gratefully acknowledge support from the CERN Foundation. We also thank Alicia Ledoux, Susan Cweren and Heather Pena for histology services.
References
- 1. Amirian ES, Armstrong TS, Gilbert MR, Scheurer ME (2012) Predictors of survival among older adults with ependymoma. J Neurooncol 107:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carter M, Nicholson J, Ross F, Crolla J, Allibone R, Balaji V et al (2002) Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer 86:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ernestus RI, Schroder R, Stutzer H, Klug N (1996) Prognostic relevance of localization and grading in intracranial ependymomas of childhood. Childs Nerv Syst 12:522–526. [DOI] [PubMed] [Google Scholar]
- 4. Figarella‐Branger D, Civatte M, Bouvier‐Labit C, Gouvernet J, Gambarelli D, Gentet JC et al (2000) Prognostic factors in intracranial ependymomas in children. J Neurosurg 93:605–613. [DOI] [PubMed] [Google Scholar]
- 5. Gilbert MR, Ruda R, Soffietti R (2010) Ependymomas in adults. Curr Neurol Neurosci Rep 10:240–247. [DOI] [PubMed] [Google Scholar]
- 6. Godfraind C, Kaczmarska JM, Kocak M, Dalton J, Wright KD, Sanford RA et al (2012) Distinct disease‐risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol 124:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guyotat J, Metellus P, Giorgi R, Barrie M, Jouvet A, Fevre‐Montange M et al (2009) Infratentorial ependymomas: prognostic factors and outcome analysis in a multi‐center retrospective series of 106 adult patients. Acta Neurochir (Wien) 151:947–960. [DOI] [PubMed] [Google Scholar]
- 8. Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB et al (2010) Cross‐species genomics matches driver mutations and cell compartments to model ependymoma. Nature 466:632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawabata Y, Takahashi JA, Arakawa Y, Hashimoto N (2005) Long‐term outcome in patients harboring intracranial ependymoma. J Neurosurg 103:31–37. [DOI] [PubMed] [Google Scholar]
- 10. Kilday JP, Mitra B, Domerg C, Ward J, Andreiuolo F, Osteso‐Ibanez T et al (2012) Copy number gain of 1q25 predicts poor progression‐free survival for pediatric intracranial ependymomas and enables patient risk stratification: a prospective European clinical trial cohort analysis on behalf of the Children's Cancer Leukaemia Group (CCLG), Societe Francaise d'Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP). Clin Cancer Res 18:2001–2011. [DOI] [PubMed] [Google Scholar]
- 11. Korshunov A, Ryzhova M, Jones DT, van Northcott PA, Sluis P, Volckmann R et al (2012) LIN28A immunoreactivity is a potent diagnostic marker of embryonal tumor with multilayered rosettes (ETMR). Acta Neuropathol 124:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korshunov A, Witt H, Hielscher T, Benner A, Remke M, Ryzhova M et al (2010) Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol 28:3182–3190. [DOI] [PubMed] [Google Scholar]
- 13. McGuire CS, Sainani KL, Fisher PG (2009) Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer 52:65–69. [DOI] [PubMed] [Google Scholar]
- 14. McGuire CS, Sainani KL, Fisher PG (2009) Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg 110:725–729. [DOI] [PubMed] [Google Scholar]
- 15. Metellus P, Figarella‐Branger D, Guyotat J, Barrie M, Giorgi R, Jouvet A, Chinot O (2008) Supratentorial ependymomas: prognostic factors and outcome analysis in a retrospective series of 46 adult patients. Cancer 113:175–185. [DOI] [PubMed] [Google Scholar]
- 16. Metellus P, Guyotat J, Chinot O, Durand A, Barrie M, Giorgi R et al (2010) Adult intracranial WHO grade II ependymomas: long‐term outcome and prognostic factor analysis in a series of 114 patients. Neuro Oncol 12:976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oya N, Shibamoto Y, Nagata Y, Negoro Y, Hiraoka M (2002) Postoperative radiotherapy for intracranial ependymoma: analysis of prognostic factors and patterns of failure. J Neurooncol 56:87–94. [DOI] [PubMed] [Google Scholar]
- 18. Paulino AC, Wen BC (2000) The significance of radiotherapy treatment duration in intracranial ependymoma. Int J Radiat Oncol Biol Phys 47:585–589. [DOI] [PubMed] [Google Scholar]
- 19. Rogers HA, Kilday JP, Mayne C, Ward J, Adamowicz‐Brice M, Schwalbe EC et al (2012) Supratentorial and spinal pediatric ependymomas display a hypermethylated phenotype which includes the loss of tumor suppressor genes involved in the control of cell growth and death. Acta Neuropathol 123:711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider D, Monoranu CM, Huang B, Rutkowski S, Gerber NU, Krauss J et al (2009) Pediatric supratentorial ependymomas show more frequent deletions on chromosome 9 than infratentorial ependymomas: a microsatellite analysis. Cancer Genet Cytogenet 191:90–96. [DOI] [PubMed] [Google Scholar]
- 21. Tihan T, Zhou T, Holmes E, Burger PC, Ozuysal S, Rushing EJ (2008) The prognostic value of histological grading of posterior fossa ependymomas in children: a Children's Oncology Group study and a review of prognostic factors. Mod Pathol 21:165–177. [DOI] [PubMed] [Google Scholar]
- 22. Wani K, Armstrong TS, Vera‐Bolanos E, Raghunathan A, Ellison D, Gilbertson R et al (2012) A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol 123:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R et al (2011) Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu H, Shyh‐Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS et al (2011) The Lin28/let‐7 axis regulates glucose metabolism. Cell 147:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


