Abstract
Objective
To report the clinical characteristics of patients hospitalised with COVID-19 in Southeast Michigan.
Design
Retrospective cohort study.
Setting
Eight hospitals in Southeast Michigan.
Participants
3219 hospitalised patients with a positive SARS-CoV-2 infection by nasopharyngeal PCR test from 13 March 2020 until 29 April 2020.
Main outcomes measures
Outcomes were discharge from the hospital or in-hospital death. Examined predictors included patient demographics, chronic diseases, home medications, mechanical ventilation, in-hospital medications and timeframe of hospital admission. Multivariable logistic regression was conducted to identify risk factors for in-hospital mortality.
Results
During the study period, 3219 (90.4%) patients were discharged or died in the hospital. The median age was 65.2 (IQR 52.6–77.2) years, the median length of stay in the hospital was 6.0 (IQR 3.2–10.1) days, and 51% were female. Hypertension was the most common chronic disease, occurring in 2386 (74.1%) patients. Overall mortality rate was 16.0%. Blacks represented 52.3% of patients and had a mortality rate of 13.5%. Mortality was highest at 18.5% in the prepeak hospital COVID-19 volume, decreasing to 15.3% during the peak period and to 10.8% in the postpeak period. Multivariable regression showed increasing odds of in-hospital death associated with older age (OR 1.04, 95% CI 1.03 to 1.05, p<0.001) for every increase in 1 year of age and being male (OR 1.47, 95% CI 1.21 to 1.81, p<0.001). Certain chronic diseases increased the odds of in-hospital mortality, especially chronic kidney disease. Administration of vitamin C, corticosteroids and therapeutic heparin in the hospital was associated with higher odds of death.
Conclusion
In-hospital mortality was highest in early admissions and improved as our experience in treating patients with COVID-19 increased. Blacks were more likely to get admitted to the hospital and to receive mechanical ventilation, but less likely to die in the hospital than whites.
Keywords: infectious diseases, respiratory infections, epidemiology
Strengths and limitations of this study.
This is the largest study to date to describe the hospitalised patient population with SARS-CoV-2 in Southeast Michigan.
The study population represents a large and diverse metropolitan area using data from the largest healthcare system in the region.
This study relied on data collected from the electronic health record and thus there is risk of missing data points if they were not reported in a structured data element that could be queried.
Although our health system cared for the largest share of patients with SARS-CoV-2 in the region, the patients may not completely represent the entire population of Southeast Michigan.
Due to its retrospective design, results are subject to confounding factors.
COVID-19 was first reported as an outbreak of pneumonia of unknown cause in Wuhan, China in December 2019.1 The virus responsible was subsequently named SARS-CoV-2. The first confirmed case in the USA was reported on 31 January 2020, and the first case in Michigan was reported on 10 March 2020.2 As of 1 June 2020, 57 532 cases have been confirmed in Michigan with 5516 attributed deaths.2 Southeast Michigan has been the epicentre of COVID-19 in the state.2
As the pandemic spread, clinical characteristics of hospitalised patients with COVID-19 were described in the medical literature from around the world, including China,3 Italy,4 New York City,5 Louisiana6 and Michigan.7 These studies indicated that increased age, male sex and presence of chronic medical conditions increase the risk of death during hospitalisation. In this report we aim to describe the clinical characteristics of a large cohort of patients hospitalised with COVID-19 in Southeast Michigan. Understanding the clinical characteristics of hospitalised patients with COVID-19 in the Midwest region of the USA will help to provide a more complete description of this population at a national level. We compared those who did not survive hospitalisation with those who were discharged alive between 13 March 2020 and 29 April 2020. We also report overall mortality rates during the three periods of the COVID-19 surge, before, during and after the peak of COVID-19 hospital volumes.
Methods
This study was conducted at an eight-hospital health system in Southeast Michigan. Southeast Michigan is the metro area of Detroit and is home to 4.5 million people, almost half of the population of the state of Michigan. Patients were included in the study if they tested positive for SARS-CoV-2 infection by nasopharyngeal PCR test and were admitted to one of the eight hospitals between 13 March 2020 and 29 April 2020. Data were collected retrospectively from the electronic health record (EHR) (Epic). Data collected included date of admission and discharge, patient demographics, home medications, common chronic medical conditions, inpatient medications received for treatment of COVID-19, oxygen therapy and status at time of discharge from the hospital. Data were available for all patients during the study period. Patients who were still admitted at the end of the study period were not included in data analysis.
Race and ethnicity were available by self-reported status in the EHR. White patients tend to live in suburban communities, while black patients tend to live in urban and poorer communities. Home medications of interest were assessed based on medication reconciliation by the attending physician at the time of admission. Inpatient medications of interest were obtained from the medication administration record. Chronic medical conditions assessed include diabetes mellitus, hypertension, heart failure, coronary artery disease, chronic kidney disease, obesity (body mass index ≥30), asthma and chronic obstructive pulmonary disease. Documentation of these conditions in the medical history, problem list before admission, problem list during the admission or discharge diagnoses in the EHR was used to evaluate the presence of these conditions. Patients were grouped as living or deceased based on status at the time of discharge from the hospital.
To evaluate the change in risk of mortality during the study, three periods were created: prepeak, peak and postpeak hospital COVID-19 volume. These periods were from 13 March 2020 to 30 March 2020, from 31 March 2020 to 13 April 2020, and from 14 April 2020 to 29 April 2020. Peak was defined as the 2-week period when the maximum number of patients were admitted to the hospital system with a diagnosis of COVID-19.
Based on discharge status, groups were compared using Pearson’s χ2 test for categorical variables and two-sample, unpaired t-test for continuous variables. Multivariate logistic regression was performed with death as the outcome of interest using age, gender and chronic medical conditions and bivariate associations within the data. Four separate models were created and are described in further detail in the online supplemental material. All variables were added to the models a priori. All statistical analyses were performed with Stata V.14.2.
bmjopen-2020-042042supp001.pdf (61.5KB, pdf)
Patient and public involvement
Due to the urgent need to publish data on the current pandemic, patients or the public were not involved in the design, conduct or reporting of this research study.
Results
During the study period 3560 patients were admitted with a diagnosis of COVID-19; 3219 patients (90.4%) were discharged or deceased and 341 patients (9.6%) were still hospitalised at the end of the study period (29 April 2020). The demographic data of the 3219 patients are shown in table 1.
Table 1.
Overall characteristics of patients with COVID-19 and by hospital discharge outcome
| Total discharged patients, N=3219 | Discharged alive, n=2703 | Died in hospital, n=516 | P value* | |
| Demographic characteristics | ||||
| Age, median (IQR), years | 65.2 (52.6–77.2) | 63.4 (50.7–74.5) | 75.7 (65.3–84.2) | <0.001 |
| Length of stay, median (IQR), days | 6.0 (3.2–10.1) | 5.6 (3.1–9.3) | 8.6 (4.6–13.4) | <0.001 |
| Gender, n (% of group) | 0.019 | |||
| Male | 1576 (49.0) | 1299 (48.1) | 277 (53.4) | |
| Female | 1643 (51.0) | 1404 (51.9) | 239 (46.3) | |
| Race | <0.001 | |||
| White | 1277 (39.7) | 1022 (37.8) | 255 (49.4) | |
| Black | 1713 (53.2) | 1482 (54.8) | 231 (44.7) | |
| Asian | 67 (2.1) | 59 (2.1) | 8 (1.6) | |
| American Indian | 5 (0.2) | 5 (0.2) | 0 (0.0) | |
| Pacific Islander | 2 (0.1) | 2 (0.1) | 0 (0.0) | |
| Other | 155 (4.8) | 133 (4.9) | 22 (4.3) | |
| Ethnicity | 0.253 | |||
| Arab or Middle Eastern | 157 (4.9) | 142 (5.3) | 15 (2.9) | |
| Hispanic or Latino | 82 (2.5) | 69 (2.6) | 13 (2.5) | |
| Non-Hispanic | 2776 (86.2) | 2319 (85.8) | 457 (88.6) | |
| Other | 170 (5.3) | 146 (5.4) | 24 (4.7) | |
| Unavailable | 33 (1.0) | 26 (1.0) | 7 (1.4) | |
| Medical condition | ||||
| Diabetes | 1329 (41.3) | 1073 (39.7) | 256 (49.6) | <0.001 |
| Hypertension | 2386 (74.1) | 1949 (72.1) | 437 (84.7) | <0.001 |
| Heart failure | 609 (18.9) | 440 (16.3) | 169 (32.8) | <0.001 |
| Heart disease | 763 (23.7) | 599 (22.2) | 204 (39.5) | <0.001 |
| Chronic kidney disease | 1299 (40.4) | 929 (34.4) | 300 (58.1) | <0.001 |
| Asthma | 429 (13.3) | 362 (13.4) | 67 (13.0) | 0.803 |
| Chronic obstructive pulmonary disease | 568 (17.6) | 428 (15.8) | 140 (27.1) | <0.001 |
| Obesity (BMI ≥30)† | 1642 (51.0) | 1405 (52.0) | 237 (45.9) | 0.036 |
| Smoking‡ | 133 (4.1) | 115 (4.3) | 18 (3.5) | <0.001 |
| Health insurance payor | <0.001 | |||
| Medicare | 1808 (56.2) | 1393 (51.5) | 415 (80.4) | |
| Medicaid | 460 (14.3) | 429 (15.9) | 31 (6.0) | |
| Commercial | 897 (27.9) | 836 (30.9) | 61 (11.8) | |
| Military | 7 (0.2) | 5 (0.2) | 2 (0.4) | |
| Exchange | 41 (1.3) | 37 (1.4) | 4 (0.8) | |
| Unknown | 6 (0.2) | 3 (0.1) | 3 (0.6) | |
| Home medication | ||||
| Aspirin | 1354 (42.1) | 1054 (39.0) | 300 (58.1) | <0.001 |
| ACE inhibitor | 940 (29.2) | 757 (28.0) | 183 (35.5) | 0.001 |
| Angiotensin receptor blocker | 676 (21.0) | 533 (19.7) | 143 (27.7) | <0.001 |
| Metformin | 688 (21.4) | 565 (20.9) | 123 (23.8) | 0.136 |
| Insulin | 490 (15.2) | 377 (14.0) | 113 (21.9) | <0.001 |
| Warfarin | 230 (7.1) | 173 (6.4) | 57 (11.1) | <0.001 |
| NOAC | 347 (10.8) | 271 (10.0) | 76 (14.7) | 0.002 |
| Inhaled corticosteroid | 472 (14.7) | 367 (13.6) | 105 (20.4) | <0.001 |
| LABA | 318 (9.9) | 240 (8.9) | 78 (15.1) | <0.001 |
| LAMA | 197 (6.1) | 150 (5.6) | 47 (9.1) | 0.002 |
| Hospital medication | ||||
| Hydroxychloroquine | 2496 (77.5) | 2061 (76.3) | 435 (84.3) | <0.001 |
| Azithromycin | 2463 (76.5) | 2046 (75.7) | 417 (80.8) | 0.012 |
| Prophylactic heparin | 2547 (79.1) | 2136 (79.0) | 411 (79.7) | 0.748 |
| Therapeutic heparin | 1257 (39.0) | 916 (33.9) | 341 (67.0) | <0.001 |
| Tocilizumab | 30 (0.9) | 18 (0.7) | 12 (2.3) | <0.001 |
| Remdesivir | 8 (0.2) | 7 (0.3) | 1 (0.2) | 0.785 |
| Systemic corticosteroids | 1631 (50.7) | 1265 (46.8) | 366 (70.9) | <0.001 |
| NOAC | 340 (10.6) | 291 (10.8) | 49 (9.5) | 0.390 |
| Zinc | 1596 (49.6) | 1340 (49.6) | 256 (49.6) | 0.987 |
| Vitamin C | 794 (24.7) | 637 (23.6) | 157 (30.4) | 0.001 |
| Oxygen therapy | ||||
| High-flow oxygen | 848 (26.3) | 534 (19.8) | 314 (60.9) | <0.001 |
| BiPAP | 125 (3.9) | 73 (2.7) | 52 (10.1) | <0.001 |
| CPAP | 93 (2.9) | 59 (2.2) | 34 (6.6) | <0.001 |
| Non-rebreather mask | 867 (26.9) | 537 (19.9) | 330 (64.0) | <0.001 |
| Mechanical ventilation | 571 (17.7) | 255 (9.4) | 316 (61.2) | <0.001 |
*P value for the difference between discharged alive and died in the hospital groups.
†BMI data available for 3135 patients.
‡Smoking data available for 2517 patients.
BiPAP, bilevel positive airway pressure; BMI, body mass index (calculated as weight in kilograms divided by height in metres squared); CPAP, continuous positive airway pressure; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; NOAC, non-vitamin K oral anticoagulation.
The overall mortality was 16.0%. Male patients had higher mortality than female patients (17.6% vs 14.5%, respectively). White patients had a mortality of 20.0% and blacks had a mortality of 13.5%. Whites represented 37.8% of patients who survived and 49.4% of those who died, while blacks represented 54.8% of those patients who survived and 44.7% of those who died. For Arab or Middle Eastern patients mortality was 9.5% and for Hispanic patients was 15.8%. The median length of hospital stay was 6.0 days, 5.6 days for patients who were discharged alive and 8.6 days for patients who died in the hospital.
Mortality increased with increasing age, reaching 28.1% for patients 80 years of age and older. The results are shown in table 2.
Table 2.
Overall mortality by age category of discharged patients who were admitted with a COVID-19 diagnosis
| Age category, years | Total patients | Alive at discharge, n (%) | Deceased, n (%) |
| <18 | 8 | 7 (87.5) | 1 (12.5) |
| 19–40 | 295 | 285 (96.6) | 10 (3.4) |
| 41–50 | 370 | 350 (94.6) | 20 (5.4) |
| 51–60 | 554 | 510 (92.1) | 44 (7.9) |
| 61–70 | 737 | 631 (85.6) | 106 (14.4) |
| 71–80 | 621 | 464 (74.7) | 157 (25.3) |
| >80 | 634 | 456 (71.9) | 178 (28.1) |
Comorbid medical conditions were common, with hypertension being the most common, followed by obesity, diabetes and chronic kidney disease. Each of the chronic medical conditions except asthma correlated with increased in-hospital mortality.
There were higher rates of hospital administration of hydroxychloroquine, azithromycin, therapeutic heparin, tocilizumab and systemic corticosteroids in the group of patients who died. Use of remdesivir, prophylactic heparin, zinc and vitamin C did not differ between the two groups.
During hospitalisation, 571 (17.7%) received mechanical ventilation, 125 (3.9%) received bilevel positive airway pressure, and 848 (26.3%) received high-flow oxygen. Black patients had higher rates of receiving mechanical ventilation than whites (19.6% vs 15.2%, respectively). The rates of these oxygen therapies were higher in the group who died in the hospital compared with those who were discharged alive. Specifically, 61.2% of patients who died received mechanical ventilation compared with only 9.4% of those who survived.
Mortality was evaluated in three time periods, prepeak, peak and postpeak hospital COVID-19 volume. During the peak period there were over 800 patients with COVID-19 hospitalised each day. Overall mortality decreased significantly with each successive time period. The results are shown in table 3.
Table 3.
Overall mortality by time of admission of patients who were discharged during the study period (N=3219)
| Timeframe | Total hospital admissions | Discharged alive (%) | Died in the hospital (%) |
| Prepeak (13–30 March 2020) | 1447 | 1180 (81.5) | 267 (18.5) |
| Peak (31 March–13 April 2020) | 1279 | 1083 (84.7) | 196 (15.3) |
| Postpeak (14–29 April 2020) | 493 | 440 (89.2) | 53 (10.8) |
A difference in the use of some treatment medications was noted in the prepeak, peak and postpeak periods. Specifically, hydroxychloroquine use decreased in the postpeak period but was still used in over 60% of patients. Similarly, azithromycin use decreased in the postpeak period to less than 35% compared with over 83% in the prepeak and peak periods. A logistic regression model was used to estimate the OR of death when controlling for age, gender, race, current smoking and chronic medical conditions. In this model, male patients had an increased odds of dying compared with female patients. The odds of dying were 1.04 for every increase in year of age. There was no difference in mortality based on race. The presence of diabetes mellitus, heart failure, obesity and chronic kidney disease resulted in increased odds of death, with chronic kidney disease having the highest effect. Hypertension, coronary artery disease, asthma, chronic obstructive pulmonary disease and current smoking status were not associated with increased odds of dying. The results are shown in figure 1.
Figure 1.
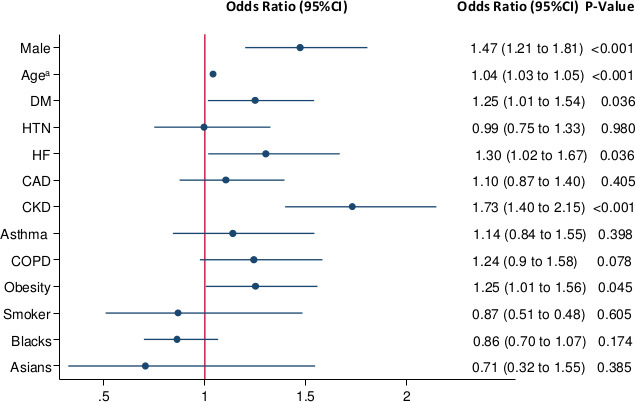
OR of death from logistic regression model when controlling for gender, age, race, current smoking and comorbidities. aFor every increase of 1 year in age. CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension.
A second logistic regression model was used to estimate the OR of death with each of the 10 home medications of interest when controlling for age, gender, smoking and chronic medical conditions. None of the medications was associated with an increase in odds of mortality. Specifically, the OR for ACE inhibitors (ACEi) was 0.93 (CI 0.74 to 1.18, p=0.971) and for angiotensin receptor blockers (ARBs) was 1.00 (CI 0.79 to 1.28, p=0.566). The full results of this model are found in online supplemental table S1.
A third logistic regression model was used to estimate the odds of death when receiving the medications of interest in the hospital when controlling for age, gender, smoking and chronic medical conditions. Administration of systemic corticosteroids, therapeutic heparin and vitamin C was associated with increased odds of dying in the hospital. Administration of zinc and novel oral anticoagulants was associated with decreased odds of dying in the hospital. There was an increase in odds of dying with the administration of tocilizumab, although only 30 patients received this drug during the study period. Similarly, only eight patients received remdesivir. The results are shown in table 4.
Table 4.
OR of death from logistic regression model for in-hospital treatment medications when controlling for age, gender and chronic medical conditions
| Medication | OR of death | CI | P value |
| Hydroxychloroquine | 1.33 | 0.95 to 1.88 | 0.102 |
| Azithromycin | 1.11 | 0.82 to 1.50 | 0.489 |
| Vitamin C | 1.40 | 1.08 to 1.81 | 0.011 |
| Zinc | 0.50 | 0.39 to 0.64 | <0.001 |
| Novel oral anticoagulants | 0.42 | 0.29 to 0.60 | <0.001 |
| Systemic corticosteroids | 2.45 | 1.91 to 3.12 | <0.001 |
| Remdesivir* | 2.22 | 0.18 to 27.5 | 0.535 |
| Tocilizumab† | 2.23 | 0.99 to 5.02 | 0.052 |
| Prophylactic heparin | 0.76 | 0.57 to 1.02 | 0.071 |
| Therapeutic heparin | 3.06 | 2.44 to 3.83 | <0.001 |
*Only 8 patients received remdesivir.
†Only 30 patients received tocilizumab.
Categorical variables were created in the fourth logistic regression model to look for differing levels of effect for hospital-administered hydroxychloroquine, azithromycin and therapeutically dosed heparin at the three different time periods (prepeak, peak and postpeak). All three medications showed significant variation in their associated odds of death across time periods.
When controlling for other factors, use of hydroxychloroquine was associated with an increase in mortality when given in the prepeak period (OR 2.36, CI 1.39 to 4.00, p=0.018), but non-significant changes in mortality in the other two time periods. When controlling for other factors, use of azithromycin was not associated with significant differences in mortality over the three time periods. When controlling for other factors, use of therapeutically dosed heparin was associated with an increase in mortality when given in the prepeak (OR 3.97, CI 2.90 to 5.44, p<0.001) and peak (OR 3.38, CI 2.47 to 4.61, p<0.001) timeframes, but no significant difference in the postpeak timeframe. The full results of this model are found in online supplemental table S2.
Discussion
This study describes the clinical characteristics of patients who were hospitalised with COVID-19 in the largest health system in Southeast Michigan. Similar to other studies,3–5 8 9 we showed that age and male gender are risk factors for increasing mortality, with similar ORs of death. Mortality reached 28% in patients 80 years of age and older, and the risk of death was elevated (61.2%) in patients who received mechanical ventilation.
In-hospital COVID-19 mortality was 16%, which is lower than mortality rates reported in the New York City area.5 9 This difference could be explained in several ways. First, the COVID-19 peak occurred earlier in New York City than Michigan, which gave our hospitals and providers more lead time to prepare. Second, the number of patients admitted during the peak in New York City was greater than that seen in Southeast Michigan, causing comparatively less stress on hospitals in our area. Lastly, during the peak in Southeast Michigan, a small number of patients were redirected to other hospital systems after presentation to the emergency centres. This ‘load balancing’ resulted in these patients not being admitted and therefore analysed, which is a limitation of our study.
Mortality risk was highest in the first 2 weeks of the pandemic and subsequently decreased during the peak and postpeak timeframes. This likely reflects improvement in the care provided to patients with COVID-19 as hospitals and providers learnt from the earlier cases. Hospital guidelines for care of patients with COVID-19 were updated frequently and communicated broadly as outside studies and internal findings became available. Changes instituted including prone positioning, delayed mechanical ventilation and broader use of anticoagulation.
Blacks represented over half of the admitted patients with a COVID-19 diagnosis, although they only represent 17.4% of the population served by our health system. This is consistent with the Centers for Disease Control and Prevention (CDC) reports showing over-representation of blacks in hospitalised patients with COVID-19.10 Blacks in our study population had a lower mortality rate than whites (13.5% vs 20%), although this difference was not statistically significant when controlling for other factors. This is not consistent with other reports showing higher COVID-19 mortality in non-hospitalised and hospitalised blacks in the USA.11 12 In Michigan, 41.3% of COVID-19-related deaths are blacks, although they only represent 13.8% of the state population.13 Another study of hospitalised patients with COVID-19 in the state of Louisiana similarly reported lower in-hospital mortality in blacks compared with whites (21.6% vs 30.1%).6 In our study hospitalised blacks were younger on average than whites, with a mean age of 61.8 vs 70.5 years. Further evaluation of the data showed 26.7% of blacks in the study were 50 years of age or younger compared with 12.5% of whites, while only 11.6% of blacks were over the age of 80 years compared with 30.4% of whites. This difference in age distribution is significant; the model did control for age, so this difference in age cannot entirely explain the lower rate of mortality in blacks.
Comorbid conditions were common in our patient population. Specifically, rates of hypertension, diabetes and chronic kidney disease were much higher than previously reported by the CDC and similar studies in the USA.5 6 9 10 This could be explained by many factors including the possibility that our patient population has more chronic diseases compared with other areas in the USA. Comorbid conditions that were associated with an increased risk of death were chronic kidney disease, heart failure, diabetes and obesity, which is similar to other studies. Interestingly, hypertension was not associated with worsening in-hospital survival as reported by other studies.14 15
Concerns exist that ACEi and ARBs could increase the risk of death in patients with COVID-19.16 Although our study was not designed to answer this question, we found that use of these medications was not associated with an increased OR of death. This was consistent with other retrospective studies.17
The use of specific medications during the hospital stay was associated with increased odds of death, especially systemic steroids. This may reflect provider overuse of corticosteroids in the sickest patients with COVID-19 when other proven therapies were lacking. Hydroxychloroquine use was associated with an overall higher death rate but did not reach statistical significance. When broken down by study periods, however, there was an increase in odds of dying when hydroxychloroquine was administered during the prepeak period, but no significant change in odds of dying in the postpeak period. This likely reflects a more judicious and evidence-based approach to COVID-19 treatment later in the study period as knowledge evolved. A similar pattern was seen with azithromycin.
The finding of increased mortality with therapeutically dosed heparin may be explained by several factors. In the prepeak and peak periods, therapeutic heparin use was primarily limited to patients with confirmed deep vein thromboses and pulmonary emboli. The OR of death with the therapeutic use of heparin was 5.73 and 2.76 in the prepeak and peak periods, respectively. This likely reflects that these patients were sicker and would be expected to have higher mortality risk. As new data on thrombotic risk in patients with COVID-19 emerged over time, local guidelines shifted at the end of the peak period to include the use of therapeutic heparin in patients with elevated oxygen requirements and elevated D-dimer levels, even in the absence of venous thromboembolism. The use of therapeutic heparin in the postpeak period likely reflects use in a broader range of patients, contributing to the decrease in mortality in that period.
Strengths of the study include being the largest report of hospitalised patients with COVID-19 in Southeast Michigan, and we included diverse population from the largest health system in the Detroit metropolitan area.
Limitations
Our study has several limitations. First, this is a retrospective study with data collected from the EHR. Because of this, there is a risk of missing data points if they were not reported in a structured data element that can be queried. Second, although our health system cared for the largest share of patients with COVID-19 in the area, patients may not completely represent the entire population of Southeast Michigan. Third, as stated above, a few patients were transferred to other health systems during the peak period and their outcomes are not included in this analysis.
Conclusion
We reported the characteristics of the largest cohort of hospitalised patients with COVID-19 in Southeast Michigan. As the coronavirus pandemic continues to progress across the USA, understanding of the medical comorbidities and sociodemographic factors associated with hospitalisation and mortality will aid in identifying populations at elevated risk. In this cohort, comorbid conditions were more common than the national average. Black patients were more likely to get admitted to the hospital and to receive mechanical ventilation, but less likely to die in the hospital than whites. The reported significant improvement in survival during the three study periods is novel and needs to be evaluated further in similar studies.
Supplementary Material
Footnotes
Twitter: @aoleszko_md
Contributors: EM and AO contributed to the design of the study, data interpretation, drafting the article, critical revision and final approval of the manuscript. DL contributed to the design of the study, data analysis and interpretation, drafting the article, critical revision and final approval of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data available per reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by Beaumont IRB (study ID: 2020-161).
References
- 1.Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA 2020;323:709-710. 10.1001/jama.2020.1097 [DOI] [PubMed] [Google Scholar]
- 2.Michigan.gov, C.M.D . Available: https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173-,00.html [Accessed 6 Jan 2020].
- 3.Xie J, Tong Z, Guan X, et al. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open 2020;3): :e205619. 10.1001/jamanetworkopen.2020.5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020;323:2052. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price-Haywood EG, et al. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open 2020;3): :e2012270. 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395): :1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966–1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:458–64. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancy CW. COVID-19 and African Americans. JAMA 2020;323:1891. 10.1001/jama.2020.6548 [DOI] [PubMed] [Google Scholar]
- 12.New York state department of health. COVID-19 fatalities. Available: https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n [Accessed 1 Jun 2020].
- 13.lab, A.R . The color of coronavirus. Available: https://www.apmresearchlab.org/covid/deaths-by-race#reporting [Accessed 16 Jun 2020].
- 14.Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 16.Sommerstein R, Kochen MM, Messerli FH, et al. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme Inhibitors/Angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc 2020;9): :e016509. 10.1161/JAHA.120.016509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackey K, et al. Risks and impact of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers on SARS-CoV-2 infection in adults. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042042supp001.pdf (61.5KB, pdf)
Data Availability Statement
Data available per reasonable request.


