Clinical History
A previously healthy 20‐year‐old man with progressive hearing loss for 3 years presented with tetraparesis with sphincter impairment. There is no history of fever, headache or vision loss. Neurologic examination demonstrated global spastic tetraparesis, more severe in lower limbs, with evident pyramidal signs (clonus tetrahyperreflexia and bilateral Babinski sign) and severe bilateral hearing loss. Evaluation of sensibility and coordination was not reliable because of motor deficits and hearing loss. Magnetic resonance imaging (MRI) and computed tomography (CT) scans show widespread thickening of the dura (Figure 1A). Cultures and CSF testing did not reveal any fungal or viral causes.
Figure 1.
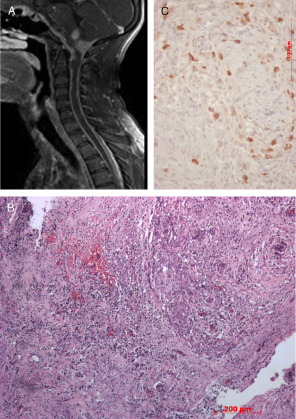
The patient was submitted to posterior fossa decompression with partial resection of the expansive lesion at the cranio‐cervical level.
Histopathologic Findings
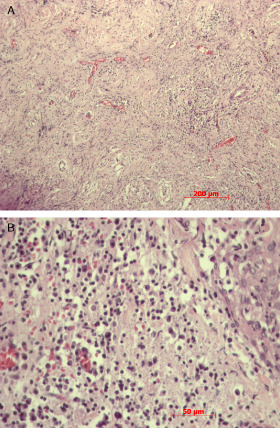
Histopathologic examination of the surgical specimen showed a densely inflamed tissue rich in lymphocytes (CD3+ T‐cells and CD20+ B‐cells), plasma cells and histiocytes (Figures 1C and 2A,B) intermingled with thick collagen bands. Sometimes these cells appeared to form granulomas, but were negative for acid fast bacilli and fungi. Histiocytes were positive for CD68 and negative for CD1A. Reactive meningothelial cells simulating giant cell were positive for the anti‐EMA antibody. Immunoreaction for IgG4 and Alk were negative. The relative density of CD138 cells, corresponding to plasma cells, does not allow the diagnosis of plasmocytoma or IgG4‐related disease (Figure 1B). What is your diagnosis?
Figure 2.
Diagnosis
Idiopathic hypertrophic pachymeningitis (IHP).
Discussion
IHP consists of an inflammatory process with fibrosis and consequent thickening of dura mater, sometimes with pseudotumoral lesions, without secondary cause. According to the site of involvement, it can be classified into cranial, spinal or craniospinal 1, 4. IPH can only be diagnosed after exclusion of infection, neoplasia and other inflammatory diseases, including autoimmune diseases and sarcoidosis. In the present case, histopathological findings favored the diagnosis of IHP as other causes of pachymeningitis were excluded. Inflammatory pseudotumor, which has also been considered as a differential diagnosis, was discarded by the immunohistochemical results.
Clinical features include headache, cranial nerve impairment, paraparesis or tetraparesis, ataxia, hydrocephalus, papilledema, hemorrhage or secondary vascular occlusions 5. Clinical presentation with hearing loss and later tetraparesis suggests cranial‐caudal involvement. Cranial localization is the most common, responsible for about 79% of cases, followed by spinal cord (15%) and finally craniospinal (5.5%) 1. Our patient presented severe and extensive craniospinal involvement, including nerve roots.
CSF analysis reveals high protein levels generally proportional to the extent of involvement, as observed in this case, and mild pleocytosis. The high cell count and low glucose levels could be related to a spinal fluid collection in a confined space as the spinal fluid did not circulate in subarachnoid space.
IHP may mimic lymphoplasmacytic meningioma. There are reports of IHP that were initially diagnosed as lymphocytic meningioma and only after histopathologic revision, the correct diagnosis was established, indicating that the differential diagnosis in some cases may be difficult 3.
MRI is essential for the diagnosis. T1‐weighted images show isointense meningeal thickening with contrast enhancement and T2‐weighted images demonstrate hypointense lesions 4.
Pathologic examination generally reveals chronic inflammatory infiltrate with lymphocytes and plasma cells, sometimes with participation of histiocytes, eosinophils and granulocytes. Less commonly (10%), granulomas, necrosis and vasculitis are also seen 1, 3.
Response to treatment is closely related to early diagnosis and the extension of involvement. The treatment consists of surgery (decompression and removal of as much meninges affected as possible) and drugs such as prednisone, azathioprine, cyclophosphamide and methotrexate 1, 2, 4. The scheme most commonly used is steroids (prednisone 1 mg/kg/day). When no response is observed, immunosuppressors such as azathioprine and cyclophosphamide can be used 1, 4. For refractory cases, there are some studies demonstrating benefits with methylprednisolone 1 g/day for 3 days associated with methotrexate 12.5 mg/week as maintenance 2. In addition to clinical response, other examinations used to follow response to treatment are erythrocyte sedimentation rate, C‐reactive protein and MRI 1, 4. The treatment should be continued for at least 1 year followed by slow withdrawal of medications, but in the case of worsening of the symptoms, it should be returned to the previous dose 1, 2, 4.
In the present case, surgical decompression was performed followed by methylprednisolone. However, there was no response, possibly because of the advanced stage of the disease, with severe neurologic impairment, at presentation. The patient developed dysautonomia just after surgery and then refractory sepsis and death.
References
- 1. Arismendi‐Morillo GJ, Gonzalez M, Molina‐Viloria OM, Cardozo JJ (2004) Idiopathic hypertrophic pachymeningitis: a diagnostic dilemma. Rev Neurol 39:830–834. [PubMed] [Google Scholar]
- 2. Bosman T, Simonin C, Launay D, Caron S, Destée A, Defebvre L (2008) Idiopathic hypertrophic cranial pachymeningitis treated by oral methotrexate: a case report and review of literature. Rheumatol Int 28:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hosler MR, Turbin RE, Cho ES, Wolansky LJ, Frohman LP (2007) Idiopathic hypertrophic pachymeningitis mimicking lymphoplasmacyte‐rich meningioma. J Neuroophthalmol 27:95–98. [DOI] [PubMed] [Google Scholar]
- 4. Rojana‐udomsart A, Pulkes T, Viranuwatti K, Laothamatas J, Phudhichareonrat S, Witoonpanich R (2008) Idiopathic hypertrophic cranial pachymeningitis. J Clin Neurosci 15:465–469. [DOI] [PubMed] [Google Scholar]
- 5. Vargas‐Bellina V, Saavedra‐Pastor H, Alvarado‐Rosales M, Porras‐Carrión M, Cjuno‐Pinto R, Gonzales‐Quispe I, Alban‐Zapata G (2009) Idiopathic hypertrophic pachymeningitis: a case report. Rev Neurol 48:300–303. [PubMed] [Google Scholar]


