Until quite recently, the autoimmune demyelinating disease multiple sclerosis (MS)1 had been considered a prototypic T cell-mediated autoimmune disease, based largely on analogy to murine models of nervous system autoimmunity. This view changed dramatically with the development of better disease models and the re-examination of the immunopathology of MS, both of which revealed a critical role for B cells and the humoral immune system. Most important, the demonstration that B cell depletion with anti-CD20 monoclonal antibodies is highly effective in treating all forms of MS solidified this new understanding that B cells play a central role in pathogenesis.2,3 It is now recognized that clonally restricted B cells, especially antigen-educated memory B cells and antibody-secreting plasmablasts, circulate between the bloodstream and central nervous system (CNS) and are probably activated in both compartments. These B cells are pro-inflammatory and produce “oligoclonal bands” (seen after electrophoresis through a gel),”4 the characteristic immunoglobulin molecules in the cerebrospinal fluid (CSF) that have long been used in MS diagnosis. It initially seemed likely that B cell-mediated pathology in MS was due to these CNS-derived antibodies, but subsequent work indicated that most appear to be nonpathogenic. The focus then shifted to the presentation of antigens by B-cells to T cells. Indeed, in some laboratory models, antigen presentation by B cells is an obligate requirement for the generation of pathogenic T cells and clinical manifestations of CNS disease.
Both genetic factors and environmental influences, including infection by Epstein-Barr virus, contribute to MS susceptibility. The association between susceptibility to MS and the human leukocyte antigen (HLA)-DR15 is well established and the HLA-DR15 locus, which encodes two heterodimeric proteins, DR2a and DR2b (Panel A of Fig. I), accounts for half of the total genetic risk.5 (There are two different DRB genes at this locus; each encodes a different β chain, and each β chain can combine with the α chain form a heterodimer.) HLA-D proteins (including HLA-DR15), also known as major histocompatibility complex (MHC) class II molecules, are expressed primarily on B cells and myeloid cells and are required for binding and presenting peptide fragments to antigen-specific CD4+ T cells. Collectively, the association between HLA-DR15 and MS, the presence of T cells in MS lesions1 and the demonstration that individual myelin protein-specific CD4+ T cell clones can cause relapsing paralysis and CNS demyelination in animal models, strongly suggest that CD4+ T cells contribute to MS pathology. However, the identification of peptide targets that bind HLA-DR15 and activate pathogenic CD4+ T cells in MS has eluded investigators.
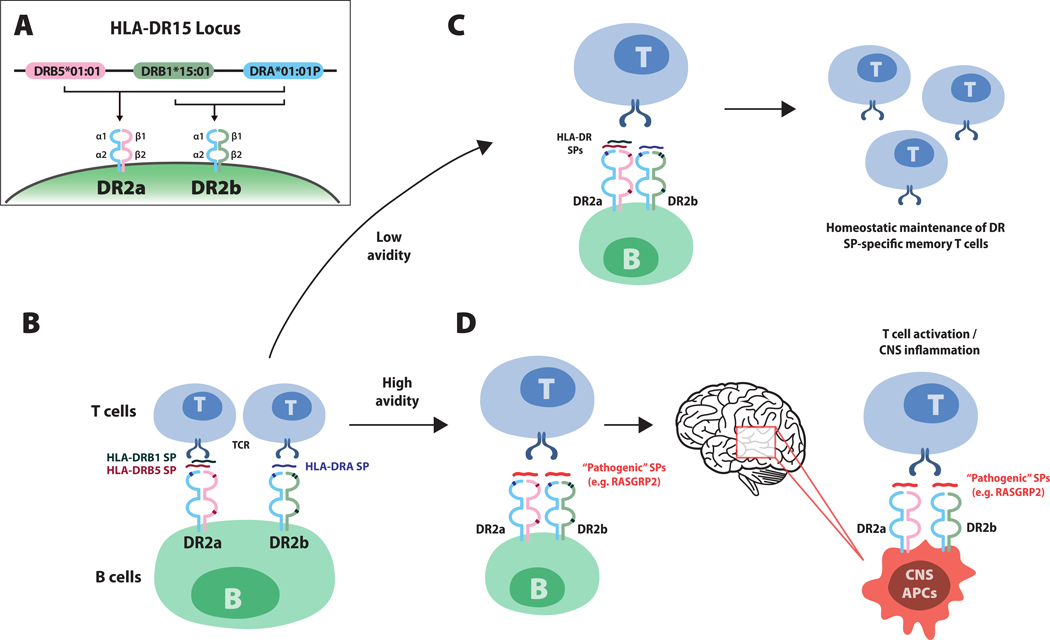
Figure I.
Model illustrating how human leukocyte antigen (HLA)-DR15-associated self peptides (SPs) on B cells influence the autoreactive T cell repertoire in multiple sclerosis (MS). Panel A shows the MS-associated HLA-DR15 locus. HLA-D proteins, which include three isotypes (-DQ, -DP and -DR), are major histocompatibility complex (MHC) class II molecules composed of alpha chain and beta chain heterodimers. MHC class II molecules, which are expressed antigen-presenting cells (e.g. B cells, monocytes and central nervous system [CNS] microglia), are required for the presentation of processed peptide fragments of foreign antigens or autoantigens to CD4+ T cells. The DR15 haplotype contains one alpha chain gene, DRA*01:01P, and two beta chain genes, DRB5*01:01 and DRB1*15:01 (Panel A). Pairing of the alpha chain with the beta chain encoded by DRB5*01:01 or the beta chain encoded by DRB1*15:01, respectively creates DR2a and DR2b heterodimers. A recent study by Wang et al.8 showed that small peptide fragments derived from DR2a and DR2b proteins themselves bind intact DR2a and DR2b molecules on B cells and serve as antigens for presentation to T cells (Panel B). Small peptides derived from the beta chains DRB5 (red) and DRB1 (green), bind to DR2a and small peptides from the alpha chain, DRA (blue), bound to DR2b. Panel C shows that HLA small peptides, which represent a large proportion of peptides binding DR2a and DR2b molecules on peripheral memory B cells in patients with multiple sclerosis, exhibit low avidity T cell receptor (TCR) engagement, supporting homeostatic (non-pathogenic) maintenance of HLA DR small peptide–specific memory T cells. Panel D shows how pathogenic small peptides, for example, peptides derived from RASGRP2, which is expressed by both B cells and neurons in the cortical gray matter, may replace DR2a and DR2b small peptides, leading to high avidity TCR engagement that would then promote the activation of pathogenic-peptide CD4+ T cells, which then traffic to the CNS. Antigen-presenting cells in the CNS that express DR2a and DR2b may present the corresponding pathogenic peptide expressed in the CNS (for example, RASGRP2) to CD4+ T cells, eliciting inflammatory responses and tissue damage.
In 1995, Wucherpfennig and Strominger6 demonstrated that some HLA-DR15-restricted myelin protein-specific T cell clones from MS patients also recognized (in addition to myelin) structurally similar peptides from the Epstein-Barr virus and other viruses, suggesting these ‘molecular mimics’ might trigger pathogenic T cells. It was a surprise when Jelcic et al.7 observed in 2018 that memory B cells from HLA-DR15+ MS patients could activate CD4+ T cells in the absence of exogenous proteins, and identified a peptide of RAS guanyl releasing protein-2 (RASGRP2), an intracellular protein, that was responsible for this T cell auto-proliferation. RASGRP2 is also expressed in cortical gray matter neurons, leading to the possibility that presentation of RASGRP2 by HLA-DR15 molecules expressed on B cells might stimulate CD4+ T cells that then target RASGRP2 in the brain.
In a recent publication from the same group, Wang et al.8 characterized the array of endogenously processed self-peptides that bind DR2a and DR2b molecules on B cells and monocytes, a myeloid subpopulation of antigen-presenting cells. While the ‘immunopeptidomes’ derived from B cells and monocytes revealed some overlap, there were also distinct differences. Up to half of the self-peptides eluted from DR2a and DR2b on B cells were derived from DR2a and DR2b molecules themselves. Most striking, DR2a preferentially presented self-peptides from DRβ chains (encoded by DRB5*01:01 and DRB1*15:01) and DR2b preferentially presented peptides from the DRα chain (encoded by DRA*01:01P). Thus, B cell expression of DR2a and DR2b may have dual roles in MS, serving as both antigen-presenting molecules and a source of peptide epitopes. Memory CD4+ T cells derived from CSF responded to self-peptides in association with either DR2a or DR2b molecules and, in some cases, also cross-reacted with myelin basic protein, supporting molecular mimicry. T cell cross-reactivity between HLA-DR self-peptides and RASGRP2 was even more common, and one T cell clone that recognized RASGRP2 also reacted to Epstein-Barr virus and Akkermansia, a commensal gut bacterium associated with MS pathogenesis. An antigen hierarchy was observed, where RASGRP2 was the strongest agonist, Epstein-Barr virus and Akkermansia were modest and HLA-DR self-peptides were weak agonists. These data suggest that B cell DR2a- and DR2b-bound self-peptides together help to shape the autoreactive repertoire of CD4+ T cells in MS.
The results obtained by Wang et al. raise several questions. The dominant self-peptides bound to HLA-DR molecules on B cells were not detected in MS brain tissue. Does the absence of HLA-DR self-peptides on antigen-presenting cells of the CNS provide greater opportunity for other, possibly pathogenic, peptides to bind to these class II molecules and activate T cells in the nervous system? Is RASGRP2, expressed in cortical neurons as well as B cells, the long sought-after “initiating” MS target antigen? In its early phases, MS is considered primarily a demyelinating disease, while neuronal loss (neurodegeneration) becomes predominant as disease progresses. Alternatively, perhaps RASGRP is a secondary CNS target, akin to the ubiquitous intracellular proteins that are presumably exposed during initial damage and recognized by antibodies in the CSF (those that make up the oligoclonal bands mentioned earlier).
These new data have several implications for understanding immunity, autoimmunity and MS. The repertoire of antigens presented by B cells is not limited to proteins recognized by their surface antibody idiotype, as previously thought, but includes a rich array of endogenous peptides. In addition, the repertoire of peptides presented exclusively by B cells, and not by other antigen-presenting cells, is also large. It is quite remarkable that a substantial proportion of these peptides presented by class II HLA molecules on B cells appear to be fragments of the class II molecules themselves. Further, some of these class II derived self-peptides are a source of potential molecular mimicry with viral, bacterial, or cell surface autoantigens, possibly triggering, sustaining or regulating T-cell mediated autoimmunity. Finally, it is worth highlighting that the genomic architecture of the MS-associated HLA-DRB1*15 haplotype is unique because of the presence of the “additional” DRB allele (DRB5*01:01), which is absent on most other common HLA class II haplotypes in humans. Perhaps this feature helps to explain its genetic association with MS risk, by serving as a source of peptides that are cross-reactive with encephalitogenic antigens. The novel observations by Wang et al. are certain to stimulate efforts to identify the HLA-DR-bound peptides on different classes of antigen-presenting cells that participate in MS and other autoimmune diseases, and renew interest in advancing approaches to manipulate the expression of DR-associated peptides for therapeutic benefit.
Footnotes
Disclosure
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Scott S. Zamvil, UCSF Weill Institute for Neurosciences, Department of Neurology, San Francisco, California Program in Immunology, University of California, San Francisco, California.
Stephen L. Hauser, UCSF Weill Institute for Neurosciences, Department of Neurology, San Francisco, California
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. The New England journal of medicine 2018;378:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 2017;376:221–34. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N Engl J Med 2020;383:546–57. [DOI] [PubMed] [Google Scholar]
- 4.Brandle SM, Obermeier B, Senel M, et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proceedings of the National Academy of Sciences of the United States of America 2016;113:7864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019;365(6460):eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 1995;80:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelcic I, Al Nimer F, Wang J, et al. Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell 2018;175:85–100 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Jelcic I, Muhlenbruch L, et al. HLA-DR15 Molecules Jointly Shape an Autoreactive T Cell Repertoire in Multiple Sclerosis. Cell 2020;183(5):1264–1281.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]