Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disorder of the central nervous system wherein, after an initial phase of transient neurological defects, slow neurological deterioration due to progressive neuronal loss ensues. Age is a major determinant of MS progression onset and disability. Over the past years, several mechanisms have been proposed to explain the key drivers of neurodegeneration and disability accumulation in MS. However, the effect of commonly encountered age‐related cerebral vessel disease, namely small vessel disease (SVD), has been largely neglected and constitutes the aim of this review. SVD shares some features with MS, that is, white matter demyelination and brain atrophy, and has been shown to contribute to the neuronal damage seen in vascular cognitive impairment. Several lines of evidence suggest that an interaction between MS and SVD may influence MS‐related neurodegeneration. SVD may contribute to hypoperfusion, reduced vascular reactivity and tissue hypoxia, features seen in MS. Venule and endothelium abnormalities have been documented in MS but the role of arterioles and of other neurovascular unit structures, such as the pericyte, has not been explored. Vascular risk factors (VRF) have recently been associated with faster progression in MS, though the mechanisms are unclear since very few studies have addressed the impact of VRF and SVD on MS imaging and pathology outcomes. Therapeutic agents targeting the microvasculature and the neurovascular unit may impact both SVD and MS and may benefit patients with dual pathology.
Keywords: aging, cerebral small vessel disease, multiple sclerosis, neurodegeneration, vascular comorbidities
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system with neurodegeneration contributing to long‐term disability 112. After a phase of active inflammatory demyelination, translating into transient neurological worsening 37, patients enter into a progressive phase wherein accumulation of neurologic disability is driven by neuronal/axonal loss disproportionate to inflammatory activity 123, 211.
Age is one of the stronger predictors of entry into the progressive phase and accumulation of severe disability 38, 178, 207. This implies that factors known to be associated with aging, such as hypoxia163, mitochondrial dysfunction 43 and iron accumulation 215 may be important contributors to neuronal damage, and by extension, disability in MS 53, 124, 125, 191.
Cerebral small vessel disease (SVD) is another age‐related phenomenon and affects cerebral small cerebral arterioles, capillaries and venules 60, 152. SVD associates with microinfarcts, microbleeds and with periventricular white matter (WM) pathology. Age and vascular risk factors (VRF), such as hypertension, are the most important predictors of SVD 174. SVD associates with neurodegeneration in the elderly 229 and in young adults with VRFs 32, 64 and may contribute to age‐related neurodegenerative pathology seen in MS (Figure 1).
Figure 1.
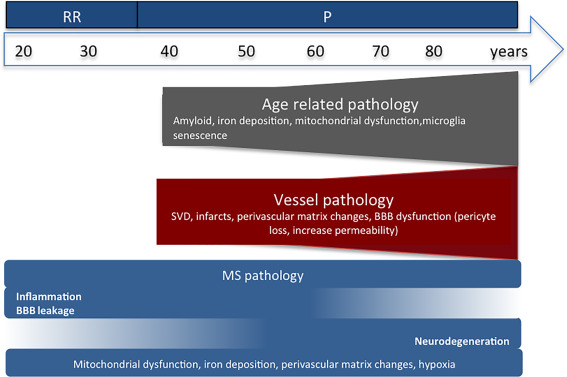
Aging with multiple sclerosis (MS): review outline. BBB, blood–brain barrier; RR, relapsing–remitting MS; P, progressive MS.
There are several reasons why the interaction between MS and age related—SVD warrants further study, including: (i) MS patients have a longer life expectancy 128 reaching 60 years of age and beyond 187 and thus susceptible to the accumulation of vascular comorbidities; (ii) vascular comorbidities contribute to MS progression 129, reduced life expectancy 27, 73, 131, increase load of WM lesions and brain atrophy 97; (iii) in MS focal demyelination occurs in watershed areas 78, where hypoperfusion and tissue hypoxia are features 47, 53, 124, suggesting an important relationship between MS pathology and cerebral arterial perfusion; (iv) the chronic inflammatory milieu in MS may predispose to SVD and (v) drugs targeting the microvasculature may be beneficial not only in SVD but also in MS 33, 135, 148.
Herein, we present a comprehensive review on MS and SVD pathology. Through a critical analysis of clinical, radiographic and pathologic data, we explore the impact of these diseases on the cerebral microvasculature, their overlapping features and the possible additive effect of SVD on MS clinical outcomes and neurodegeneration. In so doing, we hope to shed light onto the striking age‐related accumulation of neurologic disability that characterises the later stages of MS.
CEREBRAL MICROCIRCULATION
The cerebral circulation can be classified according to vessel size into macro‐ and microcirculation 94, 98, 106 as illustrated in Figure 2. Microvessels do not provide sufficient collateral flow to perfuse tissue when a penetrating arteriole or venule is blocked 20. This contributes to deep WM vulnerability to ischaemia because its major blood supply is via long medullary arterioles 24, 217. Communication between the brain parenchyma and microvasculature is mediated through the neurovascular unit (NVU) that includes different structures including the pericytes that act as capillary sphincters and control blood flow 80. The normal microvasculature structural features and functions have been reviewed elsewhere 11, 89, 106, 113, 156, 182, 226 and are summarized in Table 1.
Figure 2.
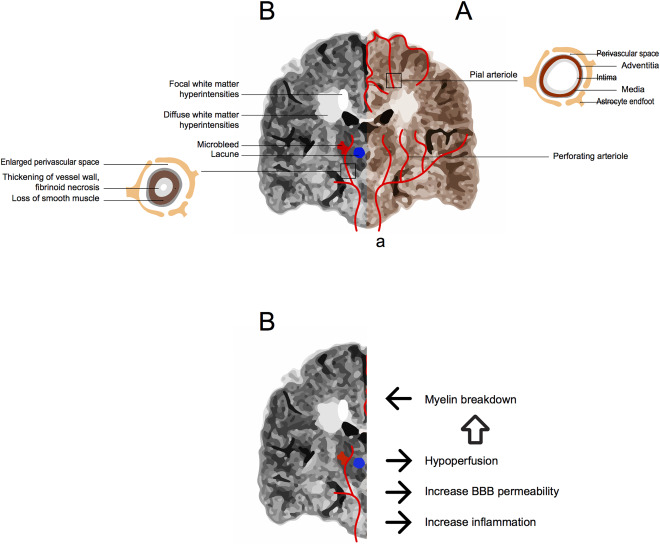
A. Cerebral circulation is divided in macrocirculation (a) that includes the internal carotid and vertebral arterial systems and in microcirculation. The microcirculation is composed of pial and perforating arterioles, capillaries and venules. Pial arterioles give rise to superficial perforating arterioles that course centripetally entering the brain at right angles to its surface, branching and terminating as end arteries. Perforating arterioles also arise from the main arteries branches and irrigate the basal ganglia, deep WM and brainstem. Perforating arterioles lie within the perivascular spaces (PVS), where vessel wall components are in close contact with the astrocyte endfeet, and terminate as end arteries (ie, without shunts) in capillary beds. The capillary plexus drains into venules (not shown). B. Potential interaction between Small Vessel Disease (SVD) and Multiple Sclerosis (MS). Longstanding MS Brain MRI (FLAIR coronal section), showing focal and diffuse periventricular white matter hyperintensities adjacent to the lateral ventricles. A perforating artery affected by SVD is illustrated where enlargement of the perivascular space, thickening of the vessel wall with significant reduction of the vessel lumen is present. These vessel changes are associated with subsequent vessel occlusion leading to lacunes, vessel rupture causing microbleeds, hypoperfusion, blood–brain barrier damage and subsequent myelin break down. This could cause additional white matter damage and neuronal loss in MS.
Table 1.
Microcirculation: normal structure and changes with aging, small vessel disease (SVD) and in multiple sclerosis (MS).
| Microcirculation | Structure | Function | Aging | SVD | MS |
|---|---|---|---|---|---|
|
Pial arteries Perforating arteries |
Surrounded by CSF and pia‐arachnoid and glia limitans. Three vessel wall layers: 1. tunica adventitia (mostly collagen and fibroblasts) 2. a tunica media (smooth muscle) 3. a tunica intima (single layer of endothelial cells and internal elastic lamina) |
1. Nutrition 2. Cerebral blood flow regulation 3. Vasophylic neuroblast migration |
1. Tortuousity 2. Thickened walls 3. Loss of elastin and smooth muscle 4. Reduced coverage by pericytes and perivascular nerve plexus |
1. Severe thickening vessel wall 2. Arteriosclerosis/fibrinoid necrosis 3. Enlarged PVS 4. Vascular ectasia 5. Amyloid wall infiltration 6. PVS Hemosiderin deposition |
1. Enlarged PVS 2. Scarce information |
| Capillary | Single layer of endothelial cells |
1. Nutrition 2. Cerebral blood flow regulation 3. Immune response |
1. Increased diameter 2. Basal membrane thickening, reduplication and vacuolization 3. Increased vessel wall hyalinosis and fibrosis 4. Reduced coverage by pericytes |
1. Perivascular rarefaction 2. Increased endothelial permeability |
1. Increase endothelial permeability, with protein leakage, red blood cell extravasation 2. Perivascular inflammation |
| Venules |
Thin‐walled vessels: 1. tunica adventitia (mostly collagen and fibroblasts) 2. a tunica media (thin layer of smooth muscle) 3. a tunica intima (single layer of endothelial cells) 4. No valves |
1. Metabolite clearance 2. Cerebral blood flow regulation |
1. PVS collagenosis | 1. Vessel wall and PVS collagenosis |
1. Thickening of vessel wall, vessel tortuosity 2. Intramural fibrinoid deposition, wall reduplication 3. Iron deposition 4. Enlarged PVS 5. PVS collagenosis 6. Perivascular inflamation 7. Thrombosis |
| Neurovascular Unit |
1. Endothelial cells 2. Pericytes 3. Vascular smooth muscle cells 4. Astrocytes 5. Basal membrane 6. Neurons 7. Perivascular macrophages |
1. Immune response regulation (adaptive and innate immune responses regulation of leukocyte transfer) 2. Transport of substances 3. Blood flow 4. Angiogenesis regulation 5. Coagulation and fibrinolysis |
1. Endothelial cell increased permeability 2. Reduced pericytes |
1. Endothelial cell increased permeability |
1. Changes in endothelial cell tight and adherens junction expression (occludine, claudins, caderins, zonula‐ocludens), with increase permeability 2. Basal membrane/extracellular matrix abnormalities |
DIFFERENTIATING MS FROM SVD
Classical MS features detected by conventional MRI and its pathologic correlates
MS lesions occur in different areas of the CNS and can be detected in vivo using brain magnetic resonance imaging (MRI) and on postmortem examination. Typical conventional MRI and pathology features are summarized in Table 2.
Table 2.
Summary of core brain MRI and pathology features in MS and SVD.
| MS | SVD | |||
|---|---|---|---|---|
| Conventional Brain MRI | Histopathology | Conventional Brain MRI | Histopathology | |
| T2/FLAIR WMH |
Focal lesions Sharply defined ! U‐ or S‐shaped juxtacortical ! Perpendicular to the lateral ventricles (Dawson fingers) ! Corpus callosum ! Optic nerve ! Spinal cord ! Pons periphery |
Myelin loss (all constituents) Different degrees of axonal loss At high venule density and arterial watershed areas Active—rich in macrophages and lymphocytes Inactive—minimal macrophage infiltration |
Focal lesions Watershed regions ! Central pons ! Sparing of the spinal cord ! Less frequent in the corpus callosum ! Sparing U fibres |
Myelin loss Axonal loss |
|
Diffuse WM lesions Periventricular |
Myelin loss with selective reduction of phospholipids |
Diffuse WM lesions Symmetrical Mild periventricular WM |
Selective loss of phospholipids and MAG with PLP preservation Loosening of the fibre network around “tortuous venules.” Minor arteriosclerotic vessel changes |
|
|
Irregular periventricular WM |
Severe myelin loss and reactive gliosis Incomplete infarcts Fibrohyalinotic and arteriosclerotic vessels |
|||
|
Punctate deep WM |
Mild tissue changes surrounding dilated PVS Myelin loss and atrophic neuropil around fibrohyalinotic arterioles |
|||
|
Deep partial confluent/confluent WM |
Axonal loss and astrogliosis. Myelin, axon and oligodendrocyte loss, focal transitions to complete infarcts | |||
| T1 |
Transient hypointensities Widespread WM Persistent hypointensities Periventricular > juxtacortical |
Increase extracellular space due to oedema Tissue loss |
Areas >2 and <15 mm in the perforating arteries territory more in BG, pons (central), internal capsule and corona radiate | Irregular cavitations with scattered fat‐laden macrophages, reactive gliosis and myelin loss |
| SWI |
Diffuse hyposignal BG Cortex |
Perivascular iron deposition R* signal changes do not always correspond to iron deposition Iron in activated macrophages/microglia at the edges of WM lesions Iron precipitation in aggregates typical of microbleeds |
Small rounded hypointensities visualized on brain MRI, 2–10 mm not well visualized on T2 Artheriosclerosis SVD—deep WM, BG and brainstem in arteriosclerosis‐related SVD Cerebral amyloid SVD—cortex, convexity/sulcus hypointensities |
Microscopic bleeds, small lacunes or to hemosiderin containing macrophages in PVS Microscopic and macroscopic bleeds; β‐amyloid vessel wall deposition |
| PVS | Along convexity perforating medullary arteries | Perivascular collagenosis and inflammatory cuffs within these enlarged spaces |
Arteriosclerosis SVD—lenticulostriate arteries entering the BG through the anterior perforated substance Cerebral amyloid SVD—pial and superficial perforating arteries |
Enlarged PVS Perivascular collagenosis Enlarged PVS |
| CEL |
Widespread in WM Different patterns: nodular, punctate, ring or open‐ring |
Macrophage/microglia,lymphocytic infiltrates BBB disruption |
Only in the context of acute stroke | Acute ischemic changes—red hypoxic neurons, inflammatory infiltrate |
!Differentiating features between multiple sclerosis and small vessel disease, WMH, white matter hyperintensities; FLAIR, fluid‐attenuated inversion recovery; PVS, perivascular spaces; SWI, susceptibility weighted imaging; CEL, contrast enhancing lesions; MAG, myelin‐associated glycoprotein; PLP, proteolipid protein.
WM hyperintensities (bright) lesions (WMH) on T2/fluid‐attenuated inversion recovery (FLAIR) imaging sequences are key features of MS, corresponding to different degrees and types of pathology 51, 69, 102, 111, 114, 142. Myelin constituents are differentially affected in well‐defined WM lesions (plaques), diffusely abnormal WM (DAWM) or areas of normal WM (NAWM) on conventional MRI. Global loss of all myelin constituents is a key feature of plaques, whereas in areas of DAWM, myelin phospholipids and certain proteins such as myelin associated glycoprotein (MAG) are reduced with relative preservation of other constituents such as proteolipid protein (PLP) 114. However, in practice, differentiating histological changes in NAWM from DAWM may be challenging. Lesions also change over time, older patients with longer disease duration having more inactive than active lesions 69.
While no area is typically spared, there are regions of predilection for WM pathology 32, 37, 111, 134. The underlying substrate for this topographic predilection is not fully understood, but some observations suggest that the relationship between MS lesions and the cerebral vessels is important. Persistent T2 lesions are more common in central areas of the brain relative to the peripheral regions, whereas acute, transient contrast enhancing lesions are more evenly distributed throughout the cerebral WM 116. This suggests that additional factors, such as periventricular WM susceptibility to hypoxia, may contribute to persistent tissue damage 66 in some areas. Focal WM lesions occur at sites of high venous density and also in watershed areas of low arterial blood flow 78 further supporting a dynamic interaction between MS pathology and the cerebral circulation.
While cortical and deep gray matter pathology are important features of progressive MS that associate with clinical disability, 51 sensitivity to detect lesions in these areas on conventional MRI is relatively poor. This is particularly true in the cerebral cortex. As gray matter signal change is conspicuously absent in MRI diagnostic criteria, which concentrate on brain WM lesions (periventricular, juxta‐cortical) 15, we have focussed the current review on WM changes classically described radiographically in the disease. That being said, it should be acknowledged that non‐conventional MRI techniques and recent pathology studies demonstrate damage in the cerebral cortex and deep gray matter including demyelination, inflammation and neuronal loss 51. The relationship between these lesions and cerebral vasculature is yet to be explored and therefore is beyond the scope of the current review.
Age‐related small vessel disease
The aging brain
Brain volume and myelin loss occur during “normal aging” 195. At a cellular level, there is astrocyte and microglial hyperactivity, cellular senescence, stem cell exhaustion, altered intercellular communication, genomic instability, mitochondrial dysfunction and free radical generation, loss of proteostasis and dysregulated nutrient sensing 22, 34, 92, 120, 153 to name a few. These changes also affect the cerebral vessels 223 particularly arterioles and also veins 23, 62, 95. Cerebral vasoreactivity is impaired in aged brains and changes in the nitric‐oxide pathways 121, reduction of endothelin‐A and beta‐adrenergic receptors 181 have been reported. This age‐related degeneration of brain vessels may impair local perfusion 89. With aging, there is an increased BBB permeability and BBB‐pericyte injury 63, 141, 149. Other neuronal signalling changes, serotonin, acetylcholine and other vasoactive neurotransmitters may also affect cerebral blood flow control 55. All these factors may predispose the aging brain to increased vulnerability to ischemic/hypoxic injury. Structural age‐related microvascular changes have been summarized in Table 1.
Typical clinical, conventional MRI and pathology features of age‐related SVD
VRF such as hypertension, diabetes, smoking, hypercholesterolemia and obesity, associate with increased risk of cerebrovascular disease in general, even in younger populations 210. Clinical manifestations of arteriolosclerosis‐related SVD are varied, including stroke, cognitive decline, urinary and walking dysfunction 9, 28, 34, 118, 153, 155, 186, 189. SVD lesions can also be asymptomatic 93, 217. Amyloid deposition is also an important cause of age‐related SVD 31, 60, 77, 122. Although this principally affects cortical arterioles, the underlying subcortical WM may be affected and show signs of damage because these arterioles supply blood to this area. It is generally accepted that the spinal cord is relatively spared in SVD, though this is based on relatively scant literature 67, 205. Morphological vascular changes in age‐related SVD are summarized in Table 1 16, 52, 88, 186 and illustrated in Figure 2. Of the microvasculature, arterioles are particularly affected with vessel wall thickening and tortuosity. That being said, thickening of vein vessel walls and perivenous collagenosis have also been described 23, 95. Key imaging‐pathology features SVD are leukoaraiosis 64, 66, 74, defined as WMH on FLAIR/T2 brain MRI images without prominent hypointensity on the T1 images, lacunes 28, 56, 217, microbleeds 41, 74, 90 and enlarged perivascular spaces 119, 217 as summarized in Table 2. Leukoaraiosis occurs in 5‐10% of patients aged 20–40 years 32 and in up to one‐third of people aged 65–84 years 22. Lesion distribution patterns differ according to the different VRF 174. A selective loss of MAG and relative preservation of PLP is a feature of SVD WM abnormalities 12. The role of energy failure in SVD‐related disease is highlighted by the observation that there is a decrease in the number of mitochondria in leukoaraiosis 193.
VRF and SVD also associate with generalized and focal brain atrophy 18, 82, 115, 143, 147, 206 and reduced perfusion in NAWM 200, 213.
Imaging discriminators between MS from SVD
Several WM lesion locations and characteristics segregate more clearly with MS 14, 21, while others more with SVD 4, 13, 15, 16, 21, 44, 71, 107, 166, 199 (Table 2). The distinction between these two disease processes on imaging can be difficult in longstanding and late onset MS 184 where both conditions are more likely to coexist. Comparative quantitative measures have shown more heterogeneous lesions in MS 138, 199. Medial lemniscus T2 hyperintensity in the dorsal pons has been reported more frequently in SVD than in MS patients 79. SWI 100, 109 improves differentiation between MS and vascular lesions based on lesion location, perivascular orientation, and the presence of hypointense (rims around) lesions as well as detection of central veins within WM lesions 136. The exact specificity of central vein sign needs to be assessed in larger studies, particularly taking into account vascular comorbidities. Differentiating MS lesions without a central vein from ischemic lesions still remains difficult, particularly in diffuse WM lesions. Annual rate of SVD‐related lesion volume increase was similar to the rate of MS‐related lesion burden increase in secondary progressive MS observed in natural history studies or the placebo arms of treatment trials 140, 179, 180 and thus not very useful in distinguishing the two disorders. Only small studies using nonconventional imaging techniques have compared MS with SVD 39, 164. In magnetization transfer studies, normal appearing WM seems to be spared in SVD 164 in contrast to MS. Diffusion coefficient measurements 150, as well as magnetic resonance spectroscopy 96, may be useful in the differential diagnosis.
MS AND SVD: EVIDENCE FOR A POSSIBLE INTERACTION
Most of the current literature has concentrated on differentiating MS white matter change from that associated with SVD (Figure S1). In the previous section, we compared the two disorders and identified distinctive clinical and imaging features. Nevertheless, DAWM changes and brain atrophy are common in both disorders, particularly in older patients. SVD may coexist in longstanding MS and be responsible for additional brain damage and clinical disability. In this section, we will summarise the direct and indirect evidence of a possible interaction between MS and SVD. MS predominantly affects the veins and venules while SVD predominantly affects the arterioles. Despite this, factors such as aging, VRFs and chronic inflammation could predispose to microvasculature damage, including in arterioles, that leads to hypoperfusion and tissue hypoxia that contributes to the extent and distribution of MS‐related pathology.
“MS vascular theory” and microcirculation morphological changes in MS
Several vascular changes in the MS brain have been described over the years and a comprehensive review of the history of these observations has recently been published 160. Early pathology studies traced MS lesions to draining CNS veins and capillaries, surrounded by perivascular inflammatory cell infiltrates 2, 48, 170. Structural microvasculature changes reported in MS are summarized in Table 1 and include thickening of vessel walls and sometimes vessel thrombosis 1, 26, 70, 158, 212. The latter findings led to disappointing trials using anticoagulants and hyperbaric oxygen as a treatment for the disease 159 that were followed by reduced interest in the role of vessels in MS pathology 160. During the last decade, the hypothesis of venous insufficiency in MS patients was raised given the claim that venous blood flow alterations were more prevalent in MS patients 224, an observation not replicated in larger, more controlled studies 204. Recently using new MRI techniques, many MS lesions have been demonstrated in vivo to centre around veins of RR and PPMS 101, 105, 196 further supporting postmortem observations that the inflammatory process dominantly involves the veins and venules in MS. However, it is somehow surprising that the arterioles remain intact in longstanding MS and the current published literature does not rule out that structural or functional arteriole changes play a role in MS pathology.
In MS, enlargement of the PVS, particularly at the brain convexity, associates with brain atrophy 99 and perivascular protein changes 70, 139, 208 further pointing to a possible relationship between vascular pathology and MS disease severity. Structural abnormalities of PVS adjacent to venules have been mentioned above, but it is not clear if PVS adjacent to pial and penetrating arterioles are spared. Meningeal inflammation spreads into convexity PVS 85, but it is not always clear if this PVS inflammation occurs exclusively around veins or also surrounds penetrating arterioles, since this information is not always specified. The temporal profile of these structural vascular and perivascular changes in MS, in the context of age‐related changes, requires further study. Even if not directly related to MS, arteriole changes are predictable as part of aging and increased VRF, though they have not been systematically quantified so far.
NVU disintegration is increasingly recognised as a contributor to neurodegeneration 229 and has been described in MS not only in active demyelinating lesions but also in NAWM 3, 101, 154 (Table 1). NVU dysfunction can lead to clotting and fibrinolytic pathway abnormalities 11, may impair repair mechanisms such as angiogenesis in chronic MS 72, 83 and potentially affect CBF regulation.
Indirect evidence of vascular dysfunction in MS
Hemodynamic changes in MS
Several studies have found that patients with MS have reduced cerebral perfusion 45, 76, 162. Cerebral blood flow is reduced in nonenhancing WM lesions 188, cortical and subcortical gray matter 108 associating with higher disability scores and T2 lesion load 151. Chronic MS plaques are more prevalent in WM regions with lower relative perfusion 84 and hypoperfusion associates with T1 hypointensities 146. The observed hypoperfusion in MS appears to be a primary phenomenon and not merely a consequence of neuronal death 176, taking into account the fact that reduced cortical and deep gray matter cerebral blood flow is present in all disease course subtypes 59 even in the absence of corresponding volume loss 50, 151. However, large longitudinal studies are still needed to confirm that hypoperfusion precedes neurodegeneration. Reduced cerebrovascular reactivity has also been reported in MS and impaired dilator capacity of cerebral arterioles to vasomotor stimulation has been proposed as a possible contributor to MS hemodynamic changes 132.
Hypoxia in MS
MS inflammation and associated NVU dysfunction 3 may lead to tissue hypoxia 45, 110. In the animal model, experimental autoimmune encephalitis (EAE), acute tissue hypoxia develops rapidly in response to inflammation, triggers enlargement of vessel lumen and increased vessel number and is related to neurological deficits 47. Reducing tissue hypoxia may be an underestimated therapeutic target since it may reduce demyelination in animal models 53. There has been an increased interest in the relationship between angiogenesis and MS 72, 117, 177, but few studies have investigated the relationship between tissue hypoxia and structural microvasculature abnormalities in MS 47. MS WM lesions and classic ischaemic WM disease share some histopathological changes 49, 110. Using vascular distance maps, larger MS lesions tend to be further from vessels 104. Recently, it has been shown that MS lesions tend to accumulate not only in areas of high venule density but also in watershed areas 78 where hypoxia due to low arterial perfusion may contribute to and/or amplify mitochondrial dysfunction, previously documented in MS 125. It is not clear if only arteriolar and venule damage could explain these findings or if they relate to downstream changes at the capillary level, including pericyte and astrocyte dysfunction 49, 80. Indeed, pericytes when exposed to hypoxia and ischemia constrict and die 80. However, abnormalities at the capillary level, particularly pericyte injury, have been relatively neglected in MS and warrant investigation. Additionally, the link between vessel fibrin deposition and tissue hypoxia/ischemia needs to be clarified. Differences between acute focal demyelinating lesions and diffuse WM changes may associate to different vascular changes, the former to perivenular inflammation and the later with arteriolar changes.
MS and VRF
Epidemiological studies
Studies have shown that MS patients have a greater prevalence of VRF, such as smoking and increased body mass index, compared to the general population while other VRF, such as hypertension, diabetes and hyperlipidaemia do not differ significantly between groups 130. However, the presence of vascular comorbidity (diabetes, hypertension, hypercholesterolemia, heart disease and peripheral vascular disease) 197 associates with an increased risk of more severe MS‐related disability 46, 129, 131. Tobacco smoking has also been associated with increased disability and faster progression of clinical disability in MS in some 81 but not in all studies 144. Studies on other nonclassical VRF have been less explored 192. Higher homocysteine, also associated with SVD 91, has been found in MS when compared to healthy controls in most studies 198 and this could be due to vitamin B12 deficiency 167, 168. The presence of VRF also affects disability (eg, gait impairment) and increases mortality in people without MS. It is not clear if the effect on MS outcome is due to the additive effect of non‐MS pathology or due to worse MS pathology.
MRI studies on the effect of VRF in MS
Few MS imaging studies have taken into account the effect of VRF on lesion distribution and size and brain atrophy (Table S1) 81, 219, 228. North American MS patients with one or more vascular risk factor(s) have an increased lesion burden and more brain atrophy 97 compared to MS patients without VRF. However, how SVD contributes to these more severe imaging features is not clear. It is possible that increased T2 lesion load in MS patients with VRF is due to additive periventricular WM vascular lesions, lacunes and microbleeds, as a mere association of two disorders (Figure 2). Similarly, vascular risk factor associated atrophy could simply be additive to that in MS patients 115.
MS and cerebrovascular disease
Epidemiological studies
MS patients have reduced life expectancy that is estimated to be between 7 and 14 years compared to individuals without the disease 178. Though increased mortality can be directly related to MS, cardiovascular diseases are also important contributors 73. MS is associated with an increased rate of ischemic stroke, myocardial infarction and heart failure in the first years after diagnosis 35, 36. The reported cardiovascular disease excessive risk early after MS diagnosis might be due to surveillance bias and later in the disease course may relate to venous disorders in progressive MS, suggesting that immobilization may be a predisposing factor 173. The heterogeneity among studies on the incidence and prevalence of cardiovascular disorders in MS makes it difficult to fully understand the epidemiology of vascular comorbidity in MS 127. There is scarce information of global atherosclerosis burden in MS patients assessed clinically or with vascular imaging, though an increase in subclinical markers of atherosclerosis has been reported in a small group of MS patients particularly in those with reduced physical activity 161.
SVD imaging and pathology correlates in MS
As previously mentioned, MS is associated with haemodynamic changes. These could be related to MS but the contribution of additional factors, such as concomitant SVD, has not been evaluated. Though this may not be a significant concern in young MS patients with no VRF, it is somehow surprising that the potential presence of SVD has not been considered in older patients since SVD dramatically increases with age. Most of the imaging studies have excluded patients with previous symptomatic cerebrovascular disease (eg, stroke and ischemic heart disease) and recording of VRF has not been documented systematically.
After an extensive search (supplementary figure 1) for publications assessing SVD imaging characteristics in MS only two MRI studies with conflicting results assessed the prevalence of microbleeds in MS patients 58, 227. An imaging pathological study aiming to track iron in two MS brains disclosed iron precipitation in aggregates typical of microbleeds. Indeed, both cases were older than 60 years, and one had significant VRF and evidence of severe atherosclerosis at autopsy 10. An older pathology study also reported perivenular hemosiderin deposition related to MS plaques in 21 out of 70 MS cases, of which four had coexistent cardiovascular disease 1. There are no publications quantifying concomitant arteriolar SVD in MS. Differentiating venous collagenosis associated with leukoaraiosis from MS‐associated venous abnormalities may be challenging due to the lack of specific markers. In longstanding MS, basal ganglia T1 hypointensities along with diffuse WM changes have been reported, but no information regarding vascular comorbidities was provided 165. Since no imaging‐pathological correlation had been performed, the findings could be related to MS and/or SVD pathology.
The chronic inflammatory milieu in MS could contribute to cerebral small vessel damage and vascular damage may impact brain inflammatory response
Of interest is whether there is an interaction between MS and SVD pathology, and if there is in what direction this lies, since the chronic inflammatory milieu of MS could exacerbate SVD 207 and VRF could exacerbate MS pathology. Rheumatoid arthritis, a systemic inflammatory disorder, is associated with an increased risk of cardiovascular disease 8 and accelerated atherosclerosis 225. Inflammation can per se be deleterious to the vessel wall and is thought to be an important risk for systemic atherosclerosis 75, 216. The relationship between inflammation and SVD has not been sufficiently explored, but the following data suggest that it deserves more attention: (i) systemic inflammation measured by interleukin‐6 is associated with SVD 137; (ii) genes associated with inflammatory pathways are upregulated in SVD brains 171, 209 and (iii) prominent inflammatory infiltrates are found in some amyloid angiopathy subtypes 7. In MS, perivascular inflammation may increase cerebral vessel vulnerability to vascular risk factor‐related damage, thus contributing to increase of SVD.
MS and SVD may share common genetic and/or environmental factors
It is possible that common genetic factors simultaneously affect MS and vascular disease phenotypes 214. Apolipoprotein E, an important atherosclerosis risk factor 218 does not seem to affect MS clinical course in humans 25 though it may impact the inflammatory response in MS animal models 185. Mitochondrial genetic variants have been implicated in both MS and leukoaraiosis 194. Underlying variants of fibrinolytic systems can also produce an effect on MS inflammation 57 and also on cerebral ischemia 221.
Environmental factors may also potentially trigger and/or contribute to both MS and atherosclerosis pathology. Chlamydia pneumoniae infection has been associated with both disorders 40, but its role in their pathogenesis has not been demonstrated. Sodium chloride intake strongly correlates with hypertension 190 and has been associated with increased clinical and radiological MS activity 61 possibly secondary to the induction of Th17 lymphocytes as demonstrated in EAE 103. Smoking, another risk factor shared by both disorders 19, 133, associates with endothelial and BBB disruption 133, 145 and may cause brain damage through multiple pathways 30. The deleterious effect of smoking in cerebral vessels could explain increased lesion load in MS smokers when compared to non‐smokers (Table S1), but this not been investigated.
Potential interaction between MS and arteriosclerosis therapies
Current MS treatments, such as fingolimod 148, may have an antiatherogenic effect. Also alpha‐beta1 integrin (VLA‐4) blockade has been shown to be effective in reducing CNS inflammation in MS 169 but may also reduce neointimal growth following vascular damage 17, since this integrin is involved in vascular remodelling and atherosclerosis 87. Fumarates may have a cardioprotective effect 6 and may improve CNS response to hypoxia 220. On the other hand, statins, known to delay atheroma plaque progression and prevent ischemic cardiovascular events, may delay MS progression 33. The potential benefit and risks of aspirin in MS have been recently reviewed 203. Interestingly, a 1961 publication a trial comparing prednisolone, placebo and calcium aspirin, reported no deterioration in patients on aspirin, whereas there was clear clinical worsening in the other two groups 135. Moreover, antihypertensive drugs with a protective effect on cerebrovascular disease, such as amiloride 183, may exert a neuroprotective effect in progressive MS 5. Finally, biotin, an important cofactor for many mitochondrial enzymes that protects against hypoxia associated energy failure may reverse disability in progressive MS 201. These effects may relate to a pluripotent mechanism of these drugs that interfere with different pathophysiological cascades but could reduce the effect of one pathology, on the other hand. Either way to develop individualized treatments, comorbidities, such as SVD, should be taken into account and the influence of therapeutic interventions in these comorbidities should not be overlooked.
SVD AS A POSSIBLE CONTRIBUTOR TO NEURODEGENERATION IN MS
Older MS patients: less inflammation, more age‐related disorders
Although the pathological hallmark of MS is the presence of multifocal areas of demyelination with relative axonal preservation, imaging and pathological studies have shown that neuronal/axonal injury can occur early and associate with active inflammation and demyelination 65, 202 However, age and disease duration affect the inflammatory response in MS lesional and nonlesional WM and GM, all of which seem to decrease in older patients with a longer disease duration 67, 68. In these patients, neurodegeneration is related, in part, to not only on‐going low‐grade inflammation but also several mechanisms including increased energy deficiency, oxidative injury, hypoxia and exhaustion of functional reserve capacity 124. Age‐related pathology, such as Alzheimer's and vascular disease, may amplify all of these mechanisms and thus contribute to increased neuronal damage 67.
“Second hit” hypothesis: could SVD contribute to hypoperfusion and brain atrophy in progressive MS?
As previously mentioned, VRF 18, 29 and SVD 206 associate with brain atrophy and cognitive impairment. The exact relationship between SVD and brain damage is incompletely understood 152, 172, 217, but factors such as hypoperfusion/ischemia due to reduced vessel lumen size, impairment of perivascular lymphatic drainage, BBB dysfunction 215 and subclinical inflammation may lead to oligodendrocyte damage and loss of myelin causing WM lesions and neuronal loss 86, 152, 172, 217. NVU dysfunction leading to neuronal‐vascular uncoupling has been implicated in perpetuating tissue damage in ischemia 80. The presence of SVD in MS patients may represent the extra hit that hinders compensatory mechanisms. In this case, vascular dysfunction and hypoperfusion with consequent chronic hypoxia could contribute to neuronal death leading to slow neurological deterioration independent of relapses. Cerebrovascular disease has been shown to contribute to neuronal damage in neurodegenerative diseases such as Alzheimer disease 54, 229 and vascular cognitive impairment 157 promoting cycles of chronic hypoperfusion, pericyte and astrocyte dysfunction with BBB permeability changes, oxidative stress, inflammation and mitochondrial impairment 43, 124, 193. This “vasculo‐neuronal‐inflammatory” model of neurodegenerative diseases, centred in NVU dysfunction, could be applied to MS 229. In MS energy deficiency and tissue hypoxia due to mitochondrial dysfunction leads to ionic imbalance and axonal degeneration and this could be potentiated by concomitant SVD, in particular in watershed areas, where MS lesions tend to accumulate 124. Not only detailed mapping of MS lesions related to arterial and venous blood supply needs to be investigated 124, but also characterization and quantification of SVD, including scoring of arterial vessel wall changes, microbleeds, microinfarcts and its relationship to energy failure in MS is warranted.
Oligodendrocyte regeneration mechanisms can also be impaired due to dual pathology affecting ventricular–subventricular zone‐derived progenitor cells 126, since this area is frequently affected by SVD and MS. These mechanisms may particularly cause or potentiate hypoperfusion and brain damage in MS 49 not only affecting focal lesion topography 78 but also diffuse abnormal WM lesions, where there are imaging and pathologic similarities to SVD. Additionally, chronic inflammation in MS may predispose to microvasculature and NVU damage leading to abnormalities in fibrinolytic pathways 11, 57, impairing angiogenesis 117 and repair after ischemic injury and thus perpetuating neuronal injury.
Could SVD potentiate tissue damage related to acute inflammation in MS?
The effect of the interaction between SVD and MS on acute inflammation and subsequent neuronal damage has not been sufficiently explored but may contribute to the age‐related decline of recovery after a relapse 42. Vessel integrity is essential for many steps of the immune response, including leukocyte priming, activation and migration 222. The effect of cerebral age‐related SVD on each of these steps is not well characterized. Taking into account the previously described structural vascular changes, it is expected that the immune responses will be compromised to some extent. Cerebral SVD‐related BBB dysfunction has been shown to associate with endothelial cell and monocyte/macrophage activation 175, which could contribute to inflammation‐related neuronal damage in MS. As previously mentioned, there is tissue hypoxia associated with inflammation in MS 45, 53, 110, 117 and it is plausible that, if present in MS patients, SVD impairs compensatory mechanisms to acute inflammation‐related hypoxia, potentiating tissue damage.
CONCLUSIONS AND DIRECTIONS FOR FUTURE RESEARCH
Understanding the contribution of SVD to brain damage in MS patients and in particular its role in the neurodegenerative process is a high priority. Some common imaging and pathological features may be markers of SVD and MS interaction: diffuse periventricular WM lesions and enlargement of perivascular spaces. The challenge lies in identifying specific biomarkers that differentiate these two pathologies and ensuring that identified MS‐specific features have arisen from cohorts where VRF have been excluded. VRF and comorbidities are associated with faster MS progression and increased lesion load through unclear mechanisms. Future imaging research on brain volumetrics and WM lesions should take into account VRF and comorbidities in MS patients.
In MS, there is hypoperfusion and reduced vascular reactivity. Venule and endothelium abnormalities have been described but the contribution of arterioles and the NVU to these hemodynamic changes is still to be explored. Human imaging‐pathological studies would allow to better dissecting of the interplay between MS and age related vascular changes/SVD, MS animal models should be set up to look at the direct effect of vascular comorbidities on MS pathology and at potential common MS and SVD pathogenic pathways. Understanding the impact of SVD in MS is important in planning treatment trials, particularly in older progressive patients and may lead to better neuroprotective therapies in the future.
CONFLICTS OF INTEREST
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site:
Figure S1. SVD and MS interaction review. Search strategy: arterioscleros*, basal, basal ganglia hemorrhage, bleed*, brain*, cereb*, cerebral small vessel diseases, crani*disease*,disseminated, ganglia, haemorrhag*, hemorrhag*, intra‐crani*, intracrani*, intracranial, intracranial arteriosclerosis, intracranial hemorrhage, hypertensive, lacun*, leukoaraios*, leukoaraiosis, micro‐bleed*, microangiopath*, microbleed, ms, multiple, multiple sclerosis, multiple sclerosis, chronic progressive, multiple sclerosis, relapsing‐remitting, scleros*, small, stroke*, stroke, lacunar, vessel.
Table S1. Brain MRI studies on the impact of vascular risk factors on MS brain lesions.
ACKNOWLEDGMENTS
We acknowledge Cairns library (Oxford University), particularly Neal Thurley, for his support in the literature review. We are further indebted to Ricardo França for graphical support in Figure 2.
REFERENCES
- 1. Adams CW (1988) Perivascular iron deposition and other vascular damage in multiple sclerosis. J Neurol Neurosurg Psychiatry 51:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams CW, Abdulla YH, Torres EM, Poston RN (1987) Periventricular lesions in multiple sclerosis: their perivenous origin and relationship to granular ependymitis. Neuropathol Appl Neurobiol 13:141–152. [DOI] [PubMed] [Google Scholar]
- 3. Alexander JS, Zivadinov R, Maghzi A‐H, Ganta VC, Harris MK, Minagar A (2011) Multiple sclerosis and cerebral endothelial dysfunction: mechanisms. Pathophysiology 18:3–12. [DOI] [PubMed] [Google Scholar]
- 4. Aliaga ES, Barkhof F (2014) MRI mimics of multiple sclerosis. Handb Clin Neurol 122:291–316. [DOI] [PubMed] [Google Scholar]
- 5. Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI et al (2013) Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. Brain 136:106–115. [DOI] [PubMed] [Google Scholar]
- 6. Ashrafian H, Czibik G, Bellahcene M, Aksentijević D, Smith AC, Mitchell SJ et al (2012) Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab 15:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Auriel E, Charidimou A, Gurol ME, Ni J, Van Etten ES, Martinez‐Ramirez S et al (2016) Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy‐related inflammation. JAMA Neurol 73:197–202. [DOI] [PubMed] [Google Scholar]
- 8. Aviña‐Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Rheum 59:1690–1697. [DOI] [PubMed] [Google Scholar]
- 9. Baezner H, Blahak C, Poggesi a, Pantoni L, Inzitari D, Chabriat H et al (2008) Association of gait and balance disorders with age‐related white matter changes: the LADIS study. Neurology 70:935–942. [DOI] [PubMed] [Google Scholar]
- 10. Bagnato F, Hametner S, Yao B, Van Gelderen P, Merkle H, Cantor FK et al (2011) Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 134:3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bardehle S, Rafalski VA, Akassoglou K (2015) Breaking boundaries‐coagulation and fibrinolysis at the neurovascular interface. Front Cell Neurosci 9:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barker R, Wellington D, Esiri MM, Love S (2013) Assessing white matter ischemic damage in dementia patients by measurement of myelin proteins. J Cereb Blood Flow Metab 33:1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barkhof F (1996) Role of MR imaging in the diagnosis of MS. Adv MRI Contrast 4:31–38. [Google Scholar]
- 14. Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH et al (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120:2059–2069. [DOI] [PubMed] [Google Scholar]
- 15. Barkhof F, Rocca M, Francis G, Van Waesberghe JH, Uitdehaag BMJ, Hommes OR et al (2003) Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon β1a. Ann Neurol 53:718–724. [DOI] [PubMed] [Google Scholar]
- 16. Barkhof F, Scheltens P (2002) Imaging of white matter lesions. Cerebrovasc Dis 13(Suppl. 2):21–30. [DOI] [PubMed] [Google Scholar]
- 17. Barringhaus KG, Phillips JW, Thatte JS, Sanders JM, Czarnik AC, Bennett DK et al (2004) Alpha4beta1 integrin (VLA‐4) blockade attenuates both early and late leukocyte recruitment and neointimal growth following carotid injury in apolipoprotein E (−/−) mice. J Vasc Res 41:252–260. [DOI] [PubMed] [Google Scholar]
- 18. Beauchet O, Celle S, Roche F, Bartha R, Montero‐Odasso M, Allali G, Annweiler C (2013) Blood pressure levels and brain volume reduction. J Hypertens 31:1502–1516. [DOI] [PubMed] [Google Scholar]
- 19. Belbasis L, Bellou V, Evangelou E, Ioannidis JP. a, Tzoulaki I (2015) Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta‐analyses. Lancet Neurol 14:263–273. [DOI] [PubMed] [Google Scholar]
- 20. Blinder P, Tsai PS, Kaufhold JP, Knoutsen PM, Suhl H, Klienfeld D (2013) The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci 16:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bot JCJ, Barkhof F, Lycklama à Nijeholt G, van Schaardenburg D, Voskuyl AE, Ader HJ et al (2002) Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology 223:46–56. [DOI] [PubMed] [Google Scholar]
- 22. Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH et al (1994) Cerebral white matter lesions, vascular risk factors, and cognitive function in a population‐based study: the Rotterdam study. Neurology 44:1246–1252. [DOI] [PubMed] [Google Scholar]
- 23. Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA (2002) Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci 204:159–163. [DOI] [PubMed] [Google Scholar]
- 24. Brown WR, Thore CR (2011) Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 37:56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burwick RM, Ramsay PP, Haines JL, Hauser SL, Oksenberg JR, Pericak‐Vance MA et al (2006) APOE epsilon variation in multiple sclerosis susceptibility and disease severity: some answers. Neurology 66:1373–1383. [DOI] [PubMed] [Google Scholar]
- 26. Beggs CB (2013) Venous hemodynamics in neurological disorders: an analytical review with hydrodynamic analysis. BMC Med 11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Capkun G, Dahlke F, Lahoz R, Nordstrom B, Tilson HH, Cutter G et al (2015) Mortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: an observational study using the US Department of Defense administrative claims database. Mult Scler Relat Disord 4:546–554. [DOI] [PubMed] [Google Scholar]
- 28. Caplan LR (2015) Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke 17:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Celle S, Annweiler C, Pichot V, Bartha R, Barthelemy J‐C, Roche F, Beauchet O (2012) Association between ambulatory 24‐hour blood pressure levels and brain volume reduction: a cross‐sectional elderly population‐based study. Hypertension 60:1324–1331. [DOI] [PubMed] [Google Scholar]
- 30. Chang RC‐C, Ho Y‐S, Wong S, Gentleman SM, Ng H‐K (2014) Neuropathology of cigarette smoking. Acta Neuropathol 127:53–69. [DOI] [PubMed] [Google Scholar]
- 31. Charidimou A, Hong YT, Jäger HR, Fox Z, Aigbirhio FI, Fryer TD et al (2015) White matter perivascular spaces on magnetic resonance imaging. Stroke 46:1707–1709. [DOI] [PubMed] [Google Scholar]
- 32. Charil A, Yousry T. a, Rovaris M, Barkhof F, De Stefano N, Fazekas F et al (2006) MRI and the diagnosis of multiple sclerosis: expanding the concept of ‘no better explanation’. Lancet Neurol 5:841–852. [DOI] [PubMed] [Google Scholar]
- 33. Chataway J, Schuerer N, Alsanousi A, Chan D, MacManus D, Hunter K et al (2014) Effect of high‐dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS‐STAT): a randomised, placebo‐controlled, phase 2 trial. Lancet 383:2213–2221. [DOI] [PubMed] [Google Scholar]
- 34. Chen R‐L, Balami JS, Esiri MM, Chen L‐K, Buchan AM (2015) Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol 6:256–265. [DOI] [PubMed] [Google Scholar]
- 35. Christiansen CF (2012) Risk of vascular disease in patients with multiple sclerosis: a review. Neurol Res 34:746–753. [DOI] [PubMed] [Google Scholar]
- 36. Christiansen CF, Christensen S, Farkas DK, Miret M, Sørensen HT, Pedersen L (2010) Risk of arterial cardiovascular diseases in patients with multiple sclerosis: a population‐based cohort study. Neuroepidemiology 35:267–274. [DOI] [PubMed] [Google Scholar]
- 37. Compston A, Coles A (2002) Multiple sclerosis. Lancet 359:1221–1231. [DOI] [PubMed] [Google Scholar]
- 38. Confavreux C, Vukusic S (2006) Age at disability milestones in multiple sclerosis. Brain 129:595–605. [DOI] [PubMed] [Google Scholar]
- 39. Confort‐Gouny S, Vion‐Dury J, Nicoli F, Dano P, Donnet A, Grazziani N et al (1993) A multiparametric data analysis showing the potential of localized proton MR spectroscopy of the brain in the metabolic characterization of neurological diseases. J Neurol Sci 118:123–133. [DOI] [PubMed] [Google Scholar]
- 40. Contini C, Seraceni S, Cultrera R, Castellazzi M, Granieri E, Fainardi E (2010) Chlamydophila pneumoniae infection and its role in neurological disorders. Interdiscip Perspect Infect Dis 2010:273573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cordonnier C, Al‐Shahi Salman R, Wardlaw J (2007) Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 130:1988–2003. [DOI] [PubMed] [Google Scholar]
- 42. Cossburn M, Ingram G, Hirst C, Ben‐Shlomo Y, Pickersgill TP, Robertson NP (2012) Age at onset as a determinant of presenting phenotype and initial relapse recovery in multiple sclerosis. 18:45–54. [DOI] [PubMed] [Google Scholar]
- 43. Currais A Ageing and inflammation—a central role for mitochondria in brain health and disease. Ageing Res Rev 21:30–42. [DOI] [PubMed] [Google Scholar]
- 44. DU, SS , Uhlenbrock D, Sehlen S (1989) The value of T1‐weighted images in the differentiation between MS, white matter lesions, and subcortical arteriosclerotic encephalopathy (SAE). Neuroradiology 31:203–212. [DOI] [PubMed] [Google Scholar]
- 45. D'haeseleer M, Hostenbach S, Peeters I, Sankari S, El Nagels G, De Keyser J et al (2015) Cerebral hypoperfusion: a new pathophysiologic concept in multiple sclerosis? J Cereb Blood Flow Metab 35:1406–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dagan A, Gringouz I, Kliers I, Segal G (2016) Disability progression in multiple sclerosis is affected by the emergence of comorbid arterial hypertension. J Clin Neurol 12:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Davies AL, Desai RA, Bloomfield PS, McIntosh PR, Chapple KJ, Linington C et al (2013) Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann Neurol 74:815–825. [DOI] [PubMed] [Google Scholar]
- 48. Dawson JW (1916) The histology of disseminated sclerosis. Trans R Soc Edinburgh 50:517–740. [Google Scholar]
- 49. De Keyser J, Steen C, Mostert JP, Koch MW (2008) Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab 28:1645–1651. [DOI] [PubMed] [Google Scholar]
- 50. Debernard L, Melzer TR, Van Stockum S, Graham C, Wheeler‐Kingshott CA, Dalrymple‐Alford JC et al (2014) Reduced grey matter perfusion without volume loss in early relapsing‐remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 85:544–551. [DOI] [PubMed] [Google Scholar]
- 51. DeLuca GC, Yates RL, Beale H, Morrow SA (2015) Cognitive impairment in multiple sclerosis: clinical, radiologic and pathologic insights. Brain Pathol 25:79–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage C, Kalaria RN (2012) Staging and natural history of cerebrovascular pathology in dementia. Neurology 78:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Desai RA, Davies AL, Tachrount M, Kasti M, Laulund F, Golay X, Smith KJ (2016) Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann Neurol 79:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF (2015) Vascular dysfunction in the pathogenesis of Alzheimer's disease—a review of endothelium‐mediated mechanisms and ensuing vicious circles. Neurobiol Dis 82:593–606. [DOI] [PubMed] [Google Scholar]
- 55. Drake CT, Iadecola C (2007) The role of neuronal signaling in controlling cerebral blood flow. Brain Lang 102:141–152. [DOI] [PubMed] [Google Scholar]
- 56. Duering M, Csanadi E, Gesierich B, Jouvent E, Hervé D, Seiler S et al (2013) Incident lacunes preferentially localize to the edge of white matter hyperintensities: insights into the pathophysiology of cerebral small vessel disease. Brain 136:2717–2726. [DOI] [PubMed] [Google Scholar]
- 57. East E, Baker D, Pryce G, Lijnen HR, Cuzner ML, Gverić D (2005) A role for the plasminogen activator system in inflammation and neurodegeneration in the central nervous system during experimental allergic encephalomyelitis. Am J Pathol 167:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eisele P, Alonso A, Griebe M, Szabo K, Hennerici MG, Gass A (2016) Investigation of cerebral microbleeds in multiple sclerosis as a potential marker of blood–brain barrier dysfunction. Mult Scler Relat Disord 7:61–64. [DOI] [PubMed] [Google Scholar]
- 59. Elsankari S, Balédent O, van Pesch V, Sindic C, de Broqueville Q, Duprez T (2013) Concomitant analysis of arterial, venous, and CSF flows using phase‐contrast MRI: a quantitative comparison between MS patients and healthy controls. J Cereb Blood Flow Metab 33:1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Esiri M, Chance S, Joachim C, Warden D, Smallwood A, Sloan C et al (2015) Cerebral amyloid angiopathy, subcortical white matter disease and dementia: literature review and study in OPTIMA. Brain Pathol 25:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J (2015) Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 86:26–31. [DOI] [PubMed] [Google Scholar]
- 62. Farkas E, de Vos RaI, Donka G, Jansen Steur EN, Mihály A, Luiten PGM (2006) Age‐related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathol 111:150–157. [DOI] [PubMed] [Google Scholar]
- 63. Farrall AJ, Wardlaw JM (2009) Blood‐brain barrier: ageing and microvascular disease—systematic review and meta‐analysis. Neurobiol Aging 30:337–352. [DOI] [PubMed] [Google Scholar]
- 64. Fazekas F, Schmidt R, Kleinert R, Kapeller P, Roob G, Flooh E (1998) The spectrum of age‐associated brain abnormalities: their measurement and histopathological correlates. J Neural Transm Suppl 53:31–39. [DOI] [PubMed] [Google Scholar]
- 65. Ferguson B, Matyszak MK, Esiri MM, Perry VH (1997) Axonal damage in acute multiple sclerosis lesions. Brain 120:393–399. [DOI] [PubMed] [Google Scholar]
- 66. Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R et al (2006) White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 37:1391–1398. [DOI] [PubMed] [Google Scholar]
- 67. Fieschi C, Gottlieb A, De Carolis V (1970) Ischaemic lacunae in the spinal cord of arteriosclerotic subjects. J Neurol Neurosurg Psychiatry 33:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frischer JM, Bramow S, Dal‐Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M et al (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132:1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I et al (2015) Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 78:710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gay D, Esiri M (1991) Blood–brain barrier damage in acute multiple sclerosis plaques. An immunocytological study. Brain 114: 557–572. [DOI] [PubMed] [Google Scholar]
- 71. Gelfand JM (2014) Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. Handb Clin Neurol 122:269–290. [DOI] [PubMed] [Google Scholar]
- 72. Girolamo F, Coppola C, Ribatti D, Trojano M (2014) Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goodin DS, Corwin M, Kaufman D, Golub H, Reshef S, Rametta MJ et al, (2014) Causes of death among commercially insured multiple sclerosis patients in the United States. PLoS ONE 9:e105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gouw Aa, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ (2011) Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 82:126–135. [DOI] [PubMed] [Google Scholar]
- 75. Gregersen I, Holm S, Dahl TB, Halvorsen B, Aukrust P (2016) A focus on inflammation as a major risk factor for atherosclerotic cardiovascular diseases. Expert Rev Cardiovasc Ther 14:391–403. [DOI] [PubMed] [Google Scholar]
- 76. Haeseleer M, Cambron M, Vanopdenbosch L, De Keyser J (2011) Vascular aspects of multiple sclerosis. Lancet Neurol 10:657–666. [DOI] [PubMed] [Google Scholar]
- 77. Haglund M, Englund E (2002) Cerebral amyloid angiopathy, white matter lesions and Alzheimer encephalopathy—a histopathological assessment. Dement Geriatr Cogn Disord 14:161–166. [DOI] [PubMed] [Google Scholar]
- 78. Haider L, Zrzavy T, Hametner S, Hoftberger R, Bagnato F, Grabner G et al (2016) The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hakky M, Erbay SH, Brewer E, Midle J, French R, Erbay SH (2013) T2 hyperintensity of medial lemniscus: higher threshold application to roi measurements is more accurate in predicting small vessel disease. J Neuroimaging 23:345–351. [DOI] [PubMed] [Google Scholar]
- 80. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA et al (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Healy BC, Ali EN, Guttmann CRG, Chitnis T, Glanz BI, Buckle G et al (2009) Smoking and disease progression in multiple sclerosis. Arch Neurol 66:858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Heijer T, Den Launer LJ, Prins ND, Van Dijk EJ (2005) Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 64:263–267. [DOI] [PubMed] [Google Scholar]
- 83. Hill J, Rom S, Ramirez SH, Persidsky Y (2014) Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol 9:591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Holland CM, Charil A, Csapo I, Liptak Z, Ichise M, Khoury SJ et al (2012) The relationship between normal cerebral perfusion patterns and white matter lesion distribution in 1,249 patients with multiple sclerosis. J Neuroimaging 22:129–136. [DOI] [PubMed] [Google Scholar]
- 85. Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM et al (2011) Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134:2755–2771. [DOI] [PubMed] [Google Scholar]
- 86. Huang YH, Zhang WW, Lin L, Feng J, Zhao XX, Guo WH, Wei W (2010) Could changes in arterioles impede the perivascular drainage of interstitial fluid from the cerebral white matter in leukoaraiosis? Neuropathol Appl Neurobiol 36:237–247. [DOI] [PubMed] [Google Scholar]
- 87. Huo Y, Ley K (2001) Adhesion molecules and atherogenesis. Acta Physiol Scand 173:35–43. [DOI] [PubMed] [Google Scholar]
- 88. Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80:844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Iadecola C, Anrather J (2012) The immunology of stroke: from mechanism to translation. Nat Med 17:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Janaway BM, Simpson JE, Hoggard N, Highley JR, Forster G, Drew D et al (2014) Brain haemosiderin in older people: pathological evidence for an ischaemic origin of magnetic resonance imaging (MRI) microbleeds. Neuropathol Appl Neurobiol 40:258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jeon S, Kang D, Kim JS, Kwon SU (2014) Homocysteine, small‐vessel disease, and atherosclerosis. Neurology 83:695–701. [DOI] [PubMed] [Google Scholar]
- 92. Jiang T, Cadenas E (2014) Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jokinen H, Gouw aa, Madureira S, Ylikoski R, Van Straaten ECW, Van Der Flier WM et al (2011) Incident lacunes influence cognitive decline: the LADIS study. Neurology 76:1872–1878. [DOI] [PubMed] [Google Scholar]
- 94. Jones EG (1970) On the mode of entry of blood vessels into the cerebral cortex. J Anat 106:507–520. [PMC free article] [PubMed] [Google Scholar]
- 95. Kalaria RN (1996) Cerebral vessels in ageing and Alzheimer's disease. Pharmacol Ther 72:193–214. [DOI] [PubMed] [Google Scholar]
- 96. Kapeller P, Ropele S, Enzinger C, Lahousen T, Strasser‐Fuchs S, Schmidt R, Fazekas F (2005) Discrimination of white matter lesions and multiple sclerosis plaques by short echo quantitative 1H‐magnetic resonance spectroscopy. J Neurol 252:1229–1234. [DOI] [PubMed] [Google Scholar]
- 97. Kappus N, Weinstock‐guttman B, Hagemeier J, Hagemeier J, Kennedy C, Melia R et al (2015) Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 87:181–187. [DOI] [PubMed] [Google Scholar]
- 98. Kiliç T, Akakin A (2008) Anatomy of cerebral veins and sinuses. Front Neurol Neurosci 23:4–15. [DOI] [PubMed] [Google Scholar]
- 99. Kilsdonk I, Steenwijk M, Pouwels P, Zwanenburg J, Visser F, Luijten P et al (2015) Perivascular spaces in MS patients at 7 Tesla MRI: a marker of neurodegeneration?. Mult Scler 21:155–162. [DOI] [PubMed] [Google Scholar]
- 100. Kilsdonk ID, Wattjes MP, Lopez‐Soriano A, Kuijer JP, de Jong MC, de Graaf WL et al (2014) Improved differentiation between MS and vascular brain lesions using FLAIR* at 7 Tesla. Eur Radiol 24:841–849. [DOI] [PubMed] [Google Scholar]
- 101. Kirk J, Plumb J, Mirakhur M, McQuaid S (2003) Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J Pathol 201:319–327. [DOI] [PubMed] [Google Scholar]
- 102. Klawiter EC (2013) Current and new directions in MRI in multiple sclerosis. Contin Lifelong Learn Neurol 19:1058–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA et al (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kozinska D, Holland CM, Krissian K, Westin C‐F, Guttmann CR (2004) A method for the analysis of the geometrical relationship between white matter pathology and the vascular architecture of the brain. Neuroimage 22:1671–1678. [DOI] [PubMed] [Google Scholar]
- 105. Kuchling J, Ramien C, Bozin I, Dörr J, Harms L, Rosche B et al (2014) Identical lesion morphology in primary progressive and relapsing‐remitting MS—an ultrahigh field MRI study. Mult Scler J 20:1866–1871. [DOI] [PubMed] [Google Scholar]
- 106. Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR (2008) Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology 55:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kwa VI, Stam J, Blok LM, Verbeeten B (1997) T2‐weighted hyperintense MRI lesions in the pons in patients with atherosclerosis. Amsterdam Vascular Medicine Group. Stroke 28:1357–1360. [DOI] [PubMed] [Google Scholar]
- 108. LF, JAL, PAN, JSW , Freeman L, Lincoln JA, Narayana PA, Wolinsky JS (2015) Evidence of widespread cortical hypoperfusion in MS: a surface‐based MRI study with pseudo‐continuous arterial spin labeling Multiple Sclerosis. 23:794. [Google Scholar]
- 109. Lane J, Bolster B, Campeau N, Welker K, Gilbertson JR (2015) Characterization of multiple sclerosis plaques using susceptibility‐weighted imaging at 1.5 T: can perivenular localization improve specificity of imaging criteria? J Comput Assist Tomogr 39:317–320. [DOI] [PubMed] [Google Scholar]
- 110. Lassmann H (2003) Hypoxia‐like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci 206:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lassmann H (2010) Review: The architecture of inflammatory demyelinating lesions: implications for studies on pathogenesis. Neuropathol Appl Neurobiol 37:698–710. [DOI] [PubMed] [Google Scholar]
- 112. Lassmann H, Brück W, Lucchinetti CF (2007) The immunopathology of multiple sclerosis: an overview. Brain Pathol 17:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lau LW, Cua R, Keough MB, Haylock‐Jacobs S, Yong VW (2013) Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci 14:722–729. [DOI] [PubMed] [Google Scholar]
- 114. Laule C, Pavlova V, Leung E, Zhao G, MacKay AL, Kozlowski P et al (2013) Diffusely abnormal white matter in multiple sclerosis: further histologic studies provide evidence for a primary lipid abnormality with neurodegeneration. J Neuropathol Exp Neurol 72:42–52. [DOI] [PubMed] [Google Scholar]
- 115. Launer LJ, Lewis CE, Schreiner PJ, Sidney S, Battapady H, Jacobs DR et al (2015) Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS ONE 10:e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee M. a, Smith S, Palace J, Narayanan S, Silver N, Minicucci L et al (1999) Spatial mapping of T2 and gadolinium‐enhancing T1 lesion volumes in multiple sclerosis: evidence for distinct mechanisms of lesion genesis? Brain 122:1261–1270. [DOI] [PubMed] [Google Scholar]
- 117. Lengfeld J, Cutforth T, Agalliu D (2014) The role of angiogenesis in the pathology of multiple sclerosis. Vasc Cell 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Liu X (2014) A meta‐analysis of association between cerebral microbleeds and cognitive impairment. Med Sci Monit 20:2189–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Loos CMJ, Klarenbeek P, van Oostenbrugge RJ, Staals J (2015) Association between perivascular spaces and progression of white matter hyperintensities in lacunar stroke patients. PLoS ONE 10:e0137323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lourenço CF, Ledo A, Dias C, Barbosa RM, Laranjinha J (2016) Neurovascular and neurometabolic derailment in aging and Alzheimer's disease. Front Aging Neurosci 7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Love S, Chalmers K, Ince P, Esiri M, Attems J, Jellinger K et al, (2014) Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post‐mortem brain tissue. Am J Neurodegener Dis 3:19–32. [PMC free article] [PubMed] [Google Scholar]
- 123. Lublin FD (2014) New multiple sclerosis phenotypic classification. Eur Neurol 72(Suppl. 1):1–5. [DOI] [PubMed] [Google Scholar]
- 124. Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14:183–193. [DOI] [PubMed] [Google Scholar]
- 125. Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS et al (2009) Mitochondrial changes within axons in multiple sclerosis. Brain 132:1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Maki T, Liang AC, Miyamoto N, Lo EH, Arai K (2013) Mechanisms of oligodendrocyte regeneration from ventricular‐subventricular zone‐derived progenitor cells in white matter diseases. 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Marrie RA, Cohen J, Stuve O, Trojano M, Sørensen PS, Reingold S et al (2015) A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler 21:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Marrie RA, Elliott L, Marriott J, Cossoy M, Blanchard J, Leung S, Yu N (2015) Effect of comorbidity on mortality in multiple sclerosis. Neurology 85:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Marrie RA, Horwitz RI (2010) Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol 9:820–828. [DOI] [PubMed] [Google Scholar]
- 130. Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Cutter G et al (2015) A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler 21:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T (2010) Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 74:1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marshall O, Lu H, Brisset J‐C, Xu F, Liu P, Herbert J et al (2014) Impaired cerebrovascular reactivity in multiple sclerosis. JAMA Neurol 71:1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Messner B, Bernhard D (2014) Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 34:509–515. [DOI] [PubMed] [Google Scholar]
- 134. Miki Y, Grossman RI, Udupa JK, Wei L, Kolson DL, Mannon LJ, Grossman M (1998) Isolated U‐fiber involvement in MS: preliminary observations. Neurology 50:1301–1306. [DOI] [PubMed] [Google Scholar]
- 135. Miller H, Newell DJ, Ridley A (1961) Multiple sclerosis. Trials of maintenance treatment with prednisolone and soluble aspirin. Lancet 1:127–129. [DOI] [PubMed] [Google Scholar]
- 136. Mistry N, Abdel‐Fahim R, Samaraweera A, Mougin O, Tallantyre E, Tench C et al (2015) Imaging central veins in brain lesions with 3‐T T2*‐weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler J 22:1289–1296. [DOI] [PubMed] [Google Scholar]
- 137. Miwa K, Okazaki S, Sakaguchi M, Mochizuki H, Kitagawa K (2016) Interleukin‐6, interleukin‐6 receptor gene variant, small‐vessel disease and incident dementia. Eur J Neurol 23:656–663. [DOI] [PubMed] [Google Scholar]
- 138. Mohamed FB, Vinitski S, Gonzalez CF, Faro SH, Lublin FA, Knobler R, Gutierrez JE (2001) Increased differentiation of intracranial white matter lesions by multispectral 3D‐tissue segmentation: preliminary results. Magn Reson Imaging 19:207–218. [DOI] [PubMed] [Google Scholar]
- 139. Mohan H, Krumbholz M, Sharma R, Eisele S, Junker A, Sixt M et al (2010) Extracellular matrix in multiple sclerosis lesions: fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol 20:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Molyneux PD, Miller DH, Filippi M, Yousry T, Kappos L, Gasperini C et al (2000) The use of magnetic resonance imaging in multiple sclerosis treatment trials: power calculations for annual lesion load measurement. J Neurol 247:34–40. [DOI] [PubMed] [Google Scholar]
- 141. Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z et al (2015) Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Moore GRW, Laule C, MacKay A, Leung E, Li DK, Zhao G et al (2008) Dirty‐appearing white matter in multiple sclerosis. J Neurol 255:1802–1811. [DOI] [PubMed] [Google Scholar]
- 143. Moulton CD, Costafreda SG, Horton P, Ismail K, Fu CH (2015) Meta‐analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav 9:651–662. [DOI] [PubMed] [Google Scholar]
- 144. Munger KL, Fitzgerald KC, Freedman MS, Miller DH, Montalbán X, Edan G et al (2015) No association of multiple sclerosis activity and progression with EBV or tobacco use in BENEFIT. Neurology 85:1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Naik P, Fofaria N, Prasad S, Sajja RK, Weksler B, Couraud P‐O et al (2014) Oxidative and pro‐inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine‐free products really safe? BMC Neurosci 15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Narayana PA, Zhou Y, Hasan KM, Datta S, Sun X, Wolinsky JS (2014) Hypoperfusion and T1‐hypointense lesions in white matter in multiple sclerosis. Mult Scler 20:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Nitkunan A, Lanfranconi S, Charlton RA, Barrick TR, Markus HS (2011) Brain atrophy and cerebral small vessel disease a prospective follow‐up study. Stroke 42:133–138. [DOI] [PubMed] [Google Scholar]
- 148. Nofer J‐R, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V et al (2007) FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low‐density lipoprotein receptor‐deficient mice. Circulation 115:501–508. [DOI] [PubMed] [Google Scholar]
- 149. Oakley R, Tharakan B (2014) Vascular hyperpermeability and aging. Aging Dis 5:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Öztoprak B, Öztoprak İ, Topalkara K, Erkoç MF, Şalk İ (2014) Role of thalamic diffusion for disease differentiation between multiple sclerosis and ischemic cerebral small vessel disease. Neuroradiology 57:339–347. [DOI] [PubMed] [Google Scholar]
- 151. Paling D, Thade Petersen E, Tozer DJ, Altmann DR, Wheeler‐Kingshott CA, Kapoor R et al (2014) Cerebral arterial bolus arrival time is prolonged in multiple sclerosis and associated with disability. J Cereb Blood Flow Metab 34:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9:689–701. [DOI] [PubMed] [Google Scholar]
- 153. Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO (2012) A quantitative magnetic resonance imaging from infancy to late adulthood. Arch Neurol 51:874–887. [DOI] [PubMed] [Google Scholar]
- 154. Plumb J, McQuaid S, Mirakhur M, Kirk J (2002) Abnormal endothelial tight junctions in active lesions and normal‐appearing white matter in multiple sclerosis. Brain Pathol 12:154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A et al (2008) Urinary complaints in nondisabled elderly people with age‐related white matter changes: the Leukoaraiosis And DISability (LADIS) study. J Am Geriatr Soc 56:1638–1643. [DOI] [PubMed] [Google Scholar]
- 156. Prakash R, Carmichael ST (2015) Blood−brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol 28:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Prins ND, Scheltens P (2015) White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 11:157–165. [DOI] [PubMed] [Google Scholar]
- 158. Putman T, Adler A (1937) Vascular architecture of the lesions of multiple sclerosis. Arch Neurol Psychiatry 38:1–15. [Google Scholar]
- 159. Putnam T, Chiavacci L, Hoff H, Weizen H (1947) Results of treatment of multiple sclerosis with dicoumarin. Arch Neurol Psychiatry 57:1–13. [DOI] [PubMed] [Google Scholar]
- 160. Rae‐grant AD, Wong C, Bernatowicz R, Fox RJ (2014) Observations on the brain vasculature in multiple sclerosis: a historical perspective. Mult Scler Relat Disord 3:156–162. [DOI] [PubMed] [Google Scholar]
- 161. Ranadive SM, Yan H, Weikert M, Lane AD, Linden MA, Baynard T et al (2012) Vascular dysfunction and physical activity in multiple sclerosis. Med Sci Sports Exerc 44:238–243. [DOI] [PubMed] [Google Scholar]
- 162. Rashid W, Parkes LM, Ingle GT, Chard DT, Toosy AT, Altmann DR et al (2004) Abnormalities of cerebral perfusion in multiple sclerosis. J Neurol Neurosurg Psychiatry 75:1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Raz L, Knoefel J, Bhaskar K (2015) The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab 36:172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Reidel MA, Stippich C, Heiland S, Storch‐Hagenlocher B, Jansen O, Hahnel S (2003) Differentiation of multiple sclerosis plaques, subacute cerebral ischaemic infarcts, focal vasogenic oedema and lesions of subcortical arteriosclerotic encephalopathy using magnetisation transfer measurements. Neuroradiology 45:289–294. [DOI] [PubMed] [Google Scholar]
- 165. Renard D, Castelnovo G, Bousquet PJ, de Champfleur N, de Seze J, Vermersch P, Labauge P (2010) Brain MRI findings in long‐standing and disabling multiple sclerosis in 84 patients. Clin Neurol Neurosurg 112:286–290. [DOI] [PubMed] [Google Scholar]
- 166. Revesz T, Hawkins CP, du Boulay EP, Barnard RO, McDonald WI (1989) Pathological findings correlated with magnetic resonance imaging in subcortical arteriosclerotic encephalopathy (Binswanger's disease). J Neurol Neurosurg Psychiatry 52:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Reynolds E (2006) Vitamin B12, folic acid, and the nervous system. Lancet Neurol 5:949–960. [DOI] [PubMed] [Google Scholar]
- 168. Reynolds EH (1992) Multiple sclerosis and vitamin B12 metabolism. J Neuroimmunol 40:225–230. [DOI] [PubMed] [Google Scholar]
- 169. Rice GPA, Hartung H, Calabresi PA (2005) Anti‐ alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 64:1336–1342. [DOI] [PubMed] [Google Scholar]
- 170. Rindfleisch E (1863) Histologisches Detail zu der grauen Degeneration von Gehirn und Rückenmark (Zugleich ein Beitrag zu der Lehre von der Entstehung und Verwandlung der Zelle). Arch Pathol Anat Physiol Klin Med 26:474–483. [Google Scholar]
- 171. Ritz M‐F, Grond‐Ginsbach C, Kloss M, Tolnay M, Fluri F, Bonati LH et al (2016) Identification of inflammatory, metabolic, and cell survival pathways contributing to cerebral small vessel disease by postmortem gene expression microarray. Curr Neurovasc Res 13:58–67. [DOI] [PubMed] [Google Scholar]
- 172. Rosenberg GA, Wallin A, Wardlaw JM, Markus HS, Montaner J, Wolfson L et al (2016) Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab 36:6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Roshanisefat H, Bahmanyar S, Hillert J, Olsson T, Montgomery S (2014) Multiple sclerosis clinical course and cardiovascular disease risk—Swedish cohort study. Eur J Neurol 21:1353–1e88. [DOI] [PubMed] [Google Scholar]
- 174. Rostrup E, Gouw AA, Vrenken H, van Straaten EC, Ropele S, Pantoni L et al (2012) The spatial distribution of age‐related white matter changes as a function of vascular risk factors—results from the LADIS study. Neuroimage 60:1597–1607. [DOI] [PubMed] [Google Scholar]
- 175. Rouhl RPW, Damoiseaux JGMC, Lodder J, Theunissen R, Knottnerus IL, Staals J et al (2012) Vascular inflammation in cerebral small vessel disease. Neurobiol Aging 33:1800–1806. [DOI] [PubMed] [Google Scholar]
- 176. Saindane a. M, Law M, Ge Y, Johnson G, Babb JS, Grossman RI (2007) Correlation of diffusion tensor and dynamic perfusion MR imaging metrics in normal‐appearing corpus callosum: support for primary hypoperfusion in multiple sclerosis. Am J Neuroradiol 28:767–772. [PMC free article] [PubMed] [Google Scholar]
- 177. Salvetti M, Landsman D, Schwarz‐Lam P, Comi G, Thompson AJ, Fox RJ (2015) Progressive MS: from pathophysiology to drug discovery. Mult Scler 21:1376–1384. [DOI] [PubMed] [Google Scholar]
- 178. Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC (2014) Onset of secondary progressive phase and long‐term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry 85:67–75. [DOI] [PubMed] [Google Scholar]
- 179. Schmidt R, Berghold A, Jokinen H, Gouw AA, van der Flier WM, Barkhof F et al, (2012) White matter lesion progression in LADIS: frequency, clinical effects, and sample size calculations. Stroke 43:2643–2647. [DOI] [PubMed] [Google Scholar]
- 180. Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F (2006) Subcortical vascular cognitive impairment: Similarities and differences with multiple sclerosis. J Neurol Sci 245:3–7. [DOI] [PubMed] [Google Scholar]
- 181. Schutzer WE, Xue H, Reed J, Oyama T, Beard DR, Anderson S, Mader SL (2015) Age‐related β‐adrenergic receptor‐mediated vasorelaxation is changed by altering G protein receptor kinase 2 expression. Vascul Pharmacol 55:178–188. [DOI] [PubMed] [Google Scholar]
- 182. Segarra M, Kirchmaier BC, Acker‐Palmer A (2015) A vascular perspective on neuronal migration. Mech Dev 138(1):17–25. [DOI] [PubMed] [Google Scholar]
- 183. Sepehrdad R, Chander PN, Oruene A, Rosenfeld L, Levine S, Stier CT (2003) Amiloride reduces stroke and renalinjury in stroke‐prone hypertensive rats. Am J Hypertens 16:312–318. [DOI] [PubMed] [Google Scholar]
- 184. Seze J, De Delalande S, Michelin E, Gauvrit JY, Mackowiak MA, Ferriby D (2005) Brain MRI in late‐onset multiple sclerosis. Eur J Neurol 12:241–244. [DOI] [PubMed] [Google Scholar]
- 185. Shin S, Walz KA, Archambault AS, Sim J, Bollman BP, Koenigsknecht‐Talboo J et al (2014) Apolipoprotein E mediation of neuro‐inflammation in a murine model of multiple sclerosis. J Neuroimmunol 271:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Smallwood A, Oulhaj A, Joachim C, Christie S, Sloan C, Smith AD, Esiri M (2012) Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol 38:337–343. [DOI] [PubMed] [Google Scholar]
- 187. Solaro C, Ponzio M, Moran E, Tanganelli P, Pizio R, Ribizzi G et al (2015) The changing face of multiple sclerosis: prevalence and incidence in an aging population. Mult Scler 21:1244–1250. [DOI] [PubMed] [Google Scholar]
- 188. Sowa P, Bjørnerud A, Nygaard GO, Damangir S, Spulber G, Celius EG et al, (2015) Reduced perfusion in white matter lesions in multiple sclerosis. Eur J Radiol 84:2605–2612. [DOI] [PubMed] [Google Scholar]
- 189. Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J et al, (2015) Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 36:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Staessen JA, Wang J, Bianchi G, Birkenhäger WH (2003) Essential hypertension. Lancet 361:1629–1641. [DOI] [PubMed] [Google Scholar]
- 191. Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW (2014) Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol 10:459–468. [DOI] [PubMed] [Google Scholar]
- 192. Sternberg Z, Leung C, Sternberg D, Li F, Karmon Y, Chadha K, Levy E (2013) The prevalence of the classical and non‐classical cardiovascular risk factors in multiple sclerosis patients. CNS Neurol Disord Drug Targets 12:104–111. [DOI] [PubMed] [Google Scholar]
- 193. Szolnoki Z, Szekeres M, Szaniszlo I, Balda G, Bodor A, Kondacs A et al (2015) Decreased number of mitochondria in leukoaraiosis. Arch Med 46:604–608. [DOI] [PubMed] [Google Scholar]
- 194. Szolnoki Z, Szolnoki Z (2010) Common genetic variants of the mitochondrial trafficking system and mitochondrial uncoupling proteins affect the development of two slowly developing demyelinating disorders, leukoaraiosis and multiple sclerosis. Curr Med Chem 17:3583–3590. [DOI] [PubMed] [Google Scholar]
- 195. Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Wu K et al (2013) A longitudinal study of age‐ and gender‐related annual rate of volume changes in regional gray matter in healthy adults. Hum Brain Mapp 34:2292–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Tallantyre EC, Dixon JE, Donaldson I, Owens T, Morgan PS, Morris PG, Evangelou N (2011) Ultra‐high‐field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 76:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Tettey P, Simpson S, Taylor BV, van der Mei IAF (2014) Vascular comorbidities in the onset and progression of multiple sclerosis. J Neurol Sci 347:23–33. [DOI] [PubMed] [Google Scholar]
- 198. Teunissen CE, Killestein J, Kragt JJ, Polman CH, Dijkstra CD, Blom HJ (2008) Serum homocysteine levels in relation to clinical progression in multiple sclerosis. J Neurol Neurosurg Psychiatry 79:1349–1353. [DOI] [PubMed] [Google Scholar]
- 199. Theocharakis P, Glotsos D, Kalatzis I, Kostopoulos S, Georgiadis P, Sifaki K et al (2009) Pattern recognition system for the discrimination of multiple sclerosis from cerebral microangiopathy lesions based on texture analysis of magnetic resonance images. Magn Reson Imaging 27:417–422. [DOI] [PubMed] [Google Scholar]
- 200. Thomas T, Miners S, Love S (2015) Post‐mortem assessment of hypoperfusion of cerebral cortex in Alzheimer's disease and vascular dementia. Brain 138:1059–1069. [DOI] [PubMed] [Google Scholar]
- 201. Tourbah A, Frenay‐Lebrun C, Edan G, Clanet M, Papeix C, Vukusic S,D et al (2016) MD1003 (high‐dose biotin) for the treatment of progressive multiple sclerosis: a randomized, double‐blind, placebo‐controlled study. Mult Scler 22:1719–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285. [DOI] [PubMed] [Google Scholar]
- 203. Tsau S, Emerson M, Lynch S, LeVine S (2015) Aspirin and multiple sclerosis. BMC Med 13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Tsivgoulis G, Sergentanis TN, Chan A, Voumvourakis K, Triantafyllou N, Psaltopoulou T et al (2014) Chronic cerebrospinal venous insufficiency and multiple sclerosis: a comprehensive meta‐analysis of case‐control studies. Ther Adv Neurol Disord 7:114–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tubbs S, Blouir MC, Singh R, Lachman N, D'Antoni AV, Loukas M et al (2015) Relationship between regional atherosclerosis and adjacent spinal cord histology. Cureus 7:e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Tuladhar AM, Reid AT, Shumskaya E (2015) Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke 46:425–432. [DOI] [PubMed] [Google Scholar]
- 207. Tutuncu M, Tang J, Zeid NA, Kale N, Crusan DJ, Atkinson E et al (2013) Onset of progressive phase is an age‐dependent clinical milestone in multiple sclerosis. Mult Scler 19:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. van Horssen J, Bö L, Vos CMP, Virtanen I, de Vries HE (2005) Basement membrane proteins in multiple sclerosis‐associated inflammatory cuffs: potential role in influx and transport of leukocytes. J Neuropathol Exp Neurol 64:722–729. [DOI] [PubMed] [Google Scholar]
- 209. Verhaaren BF, Debette S, Bis JC, Smith JA, Ikram MK, Adams HH et al (2015) Multi‐ethnic genome‐wide association study of cerebral white matter hyperintensities on MRI. Circ Cardiovasc Genet 8:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Von Sarnowski B, Putaala J, Grittner U, Gaertner B, Schminke U, Curtze S et al (2013) Lifestyle risk factors for ischemic stroke and transient ischemic attack in young adults in the stroke in young fabry patients study. Stroke 44:119–125. [DOI] [PubMed] [Google Scholar]
- 211. Vukusic S, Confavreux C (2007) Natural history of multiple sclerosis: risk factors and prognostic indicators. Curr Opin Neurol 20:269–274. [DOI] [PubMed] [Google Scholar]
- 212. Wakefield AJ, More LJ, Difford J, McLaughlin JE (1994) Immunohistochemical study of vascular injury in acute multiple sclerosis. J Clin Pathol 47:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Wang T, Li Y, Guo X, Huang D, Ma L, Wang DJ, Lou X (2016) Reduced perfusion in normal‐appearing white matter in mild to moderate hypertension as revealed by 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging 43:635–643. [DOI] [PubMed] [Google Scholar]
- 214. Wang Y, Bos SD, Harbo HF, Thompson WK, Schork AJ, Bettella F et al (2016) Genetic overlap between multiple sclerosis and several cardiovascular disease risk factors. Mult Scler (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ward RJ, Zucca Fa, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Wardlaw JM (2010) Blood–brain barrier and cerebral small vessel disease. J Neurol Sci 299:66–71. [DOI] [PubMed] [Google Scholar]
- 217. Wardlaw JM, Smith C, Dichgans M (2013) Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Weber C, Noels H (2011) Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17:1410–1422. [DOI] [PubMed] [Google Scholar]
- 219. Weinstock‐Guttman B, Zivadinov R, Mahfooz N, Carl E, Drake A, Schneider J et al (2011) Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Neuroinflammation 8(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Wiesner D, Merdian I, Lewerenz J, Ludolph AC, Dupuis L, Witting A (2013) Fumaric acid esters stimulate astrocytic VEGF expression through HIF‐1α and Nrf2. PLoS ONE 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Wiseman SJ, Doubal FN, Chappell FM, Valdés‐Hernández MC, Wang X, Rumley A et al (2015) Plasma biomarkers of inflammation, endothelial function and hemostasis in cerebral small vessel disease. Cerebrovasc Dis 40:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Wong CHY, Heit B, Kubes P (2010) Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc Res 86:183–191. [DOI] [PubMed] [Google Scholar]
- 223. Wu H, Roks AJM (2014) Genomic instability and vascular aging: a focus on nucleotide excision repair. Trends Cardiovasc Med 24:61–68. [DOI] [PubMed] [Google Scholar]
- 224. Zamboni P, Galeotti R, Menegatti E, Malagoni aM, Tacconi G, Dall'Ara S et al (2009) Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 80:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Zha AM, Di Napoli M, Behrouz R (2015) Prevention of stroke in rheumatoid arthritis. Curr Neurol Neurosci Rep 15:77. [DOI] [PubMed] [Google Scholar]
- 226. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood–brain barrier. Cell 163:1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Zivadinov R, Ramasamy DP, Benedict RR, Polak P, Hagemeier J, Magnano C et al (2016) Cerebral microbleeds in multiple sclerosis evaluated on susceptibility‐weighted images and quantitative susceptibility maps: a case–control study. Radiology 281:884–895. [DOI] [PubMed] [Google Scholar]
- 228. Zivadinov R, Weinstock‐Guttman B, Hashmi K, Abdelrahman N, Stosic M, Dwyer M et al (2009) Smoking is associated with increased lesion volumes and brain atrophy in multiple sclerosis. Neurology 73:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12:723–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site:
Figure S1. SVD and MS interaction review. Search strategy: arterioscleros*, basal, basal ganglia hemorrhage, bleed*, brain*, cereb*, cerebral small vessel diseases, crani*disease*,disseminated, ganglia, haemorrhag*, hemorrhag*, intra‐crani*, intracrani*, intracranial, intracranial arteriosclerosis, intracranial hemorrhage, hypertensive, lacun*, leukoaraios*, leukoaraiosis, micro‐bleed*, microangiopath*, microbleed, ms, multiple, multiple sclerosis, multiple sclerosis, chronic progressive, multiple sclerosis, relapsing‐remitting, scleros*, small, stroke*, stroke, lacunar, vessel.
Table S1. Brain MRI studies on the impact of vascular risk factors on MS brain lesions.


