Abstract
More than 80 years ago, Pio Del Rio‐Hortega recognized that one of the “main controversial points in regard to the microglia” is “whether it belongs to the reticulo‐endothelial system [i.e. monocytes and macrophages] and possesses the ordinary characteristics of this system or has a more specialized function.” The notion of microglia having functions that are different from those of other macrophages has gained significant support in recent years. The brain represents a unique environment and shows species, developmental and regional specialization. Thus, any consideration of microglial activity has to be thought of in this tissue context. Contexts may be normal (health, physiology) or disease conditions showing either primary or secondary microglial involvement. Subclinical, reversible “soft pathologies” (Kreutzberg) such as pain that involves microglia also exist. Here, we examine a multilayered approach to understanding microglia that illustrates the emergent character of the microglial (population) phenotype. Accordingly, terms such as microglial “activation” and microgliosis, which are of increasing importance for our understanding of neurological disorders, need to be filled with refined meaning. It is suggested that the pathophysiological context guides nomenclatorial considerations; for example, development, trauma or pain‐associated microglia is preferred over the traditional but less distinctive “microglial activation.” This should also help to tease out the different functional subtypes currently hidden under the umbrella term “neuroinflammation,” which is being applied so widely that it has become effectively useless in practice and even inhibits research progress because both true and pseudo‐inflammation are covered by this term.
Keywords: Alzheimer's disease, emergence, inflammation, interactome, microglial activation, neurotoxicity, proteome, systems biology, transcriptome
Introduction
Following Pio Del Rio‐Hortega's work 15, microglia were almost ignored until the mid‐1980s but this has changed radically. Microglia are now recognized as the immune and defense cells of the central nervous system (CNS). Yet, immune and defense functions are only relevant under pathological conditions, and diseases do not represent the most common, normal state. In other words, diseases are the exception, but most contemporary knowledge on microglial cells is derived from disease states. Microglia in neuroinflammation, the topic of this symposium, are particularly popular (Figure 1).
Figure 1.
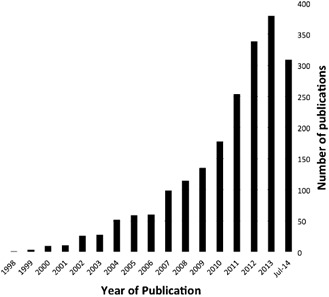
PubMed entries for “microglia” + “neuroinflammation” (2014 until early July).
Microglial cells are highly interactive cells. It is hard to overstate this when it is considered that microglia have been shown to be capable of expressing receptors for neurotransmitters, a large suite of immune receptors 26, and that their processes are constantly in motion, contacting the membranes of synapses, other glia (oligodendrocytes and astrocytes) as well as blood vessels 52 (Figure 2 and Supporting Information). In this article, we try to capture aspects of the microglial phenotype as emergent properties of their interactome. The reverse, that is, effects of the microglia on the system, which they populate, is also considered. Findings on “neuroinflammation” are discussed in this context and we propose a heightened focus on the interactome for microglial research. Thus, a concept of emergent properties as it appears to apply to microglia is introduced.
Figure 2.
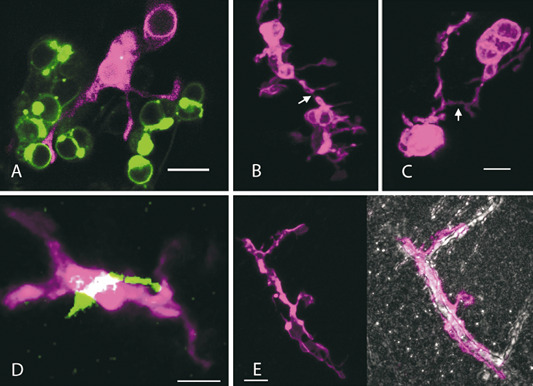
Microglia images showing exemplary zebrafish microglia dynamically in contact with neuronal cell bodies, other microglia, neurites and the vasculature. Microglia in all images are visualized through the mpeg1:mCherry‐CAAX fluorophore transgene (represented as magenta). All images were acquired on a Zeiss LSM 710 confocal microscope with a 20× W‐Plan Apochromat (B, C and E) or a 40× W‐Plan Apochromat objective (A and D). A. Microglia (magenta) with processes contacting neurons (green) expressing the Gal41038, UAS:Synaptophysin‐GFP transgene in the Zebrafish optic tectum at 4 days post fertilization (dpf). Single slice from confocal stack with a z depth of 0.55 μm. Scale = 10 μm. B, C. Processes of adjacent microglia coming into contact. (B) and (C) are maximum projections from different regions of the same stack in the Zebrafish optic tectum at 6 dpf. (B) has a z depth of 23 μm and (C) has a z depth of 25 μm. Scale = 10 μm. D. Microglia membrane co‐localized (white) with an axon (green) in the forebrain from a hypothalamic HCRT neuron expressing HCRT: Synaptophysin‐GFP at 6 dpf. Maximum projection from a z depth of 3.47 μm. Scale = 5 μm. E. Microglial cell profiles in the brain parenchyma covering a blood vessel in the optic tectum at 8 dpf. The vascular tissue is highlighted by a standard deviation projection wherein vasculature appears white and cell masses appear grey/black (photo on the right). Maximum and standard deviation projections from a z depth of 22.6 μm. Scale = 10 μm.
Understanding Microglia Beyond Cellular Markers
There are examples in the neurosciences of how the consideration of single entities such as cell surface molecules, receptor expression and interleukins can lead to flawed conclusions at the phenotype and systemic levels 19, 32. Neuroinflammation represents a particularly illustrative example and conventional thinking in this field currently goes as follows: Interleukin‐X has been found to be upregulated in the context of tissue inflammation and is known to support inflammatory cellular reactions; it is called a pro‐inflammatory cytokine. We see upregulation of pro‐inflammatory interleukin‐X in the brain in Alzheimer's disease. Therefore, as this cytokine is upregulated, there is an inflammatory tissue reaction taking place in the brain affected by Alzheimer's disease. In other words, there is evidence that the brain in Alzheimer's disease is inflamed. We conclude that Alzheimer's disease is an inflammatory disorder. Therefore, patients ought to be treated using anti‐inflammatory drugs. And such trials have indeed taken place. However, not unexpectedly (in view of the above), they have been of very limited success 1, 2, 42.
We know now that at least some of the assumptions that underlie the neuroinflammation hypothesis of psychiatric disorders 35 are incorrect 19. The same likely applies to other diseases where “inflamed microglia” (a nonsensical choice of terms as inflammation is a multicellular process!) have been reported 37, and other “inflammatory” molecules can create similar pitfalls 11. Complement is an example of such a poly‐functional class of molecules. Complement receptors have immunological functions in the CNS under inflammatory conditions, but in healthy and subclinically stressed (“conditioned”) CNS, complement appears to have its main role in synaptic turnover 9, 31, 44, 50. MHC class I is a further example of a classical immune molecule with a neural role in the refinement of synapses during CNS development 25, 29. Let us assume we discover regulation of one of the above molecules in a hippocampal slice preparation or in a transgenic zebrafish screen; if we misidentify its function, the predicted phenotype will be in error.
As unsupervised in silico analyses have demonstrated, progress can be made through the careful analysis of “big data.” Microglia are already the subject of multiple trancriptome profiling efforts 7, 36, 38, 40, and transcriptome and proteome data on neurological diseases are becoming increasingly available. However, putting these data together and extracting useful information from it will not always be simple. There is nothing new in the idea that knowledge of the molecular and genetic components is fundamental to acquiring an understanding of a biological system. What is new is the ability to gather a highly comprehensive and even complete set of these data and analyze it at the vast scale and complexity required to unpack the combinatorial nature of a phenotype. Yet, it would be naïve to think that the field being presented with microglia “big data” will immediately know, without effort and numerous mistakes, how to conceptualize this information. It is clear that new thinking tools are required.
Irreducibility and Prediction by Simulation
The chemical and physical interactions going on in a single microglial cell can be likened to the workings of a biophysical computation. As transcriptomes, proteomes, sensomes 24, etc., become available in increasing numbers, it is foreseeable that a near‐complete snapshot and perhaps even real‐time observations of a cell in contextual action will become possible. However, as the phenotype depends on the interactome, availability of this information alone will not inevitably lead to in‐depth understanding of cellular function.
A thought experiment is illustrative: Consider the microglial phenotype influenced by the cytosolic Ca2+ concentration 26. We include the receptor pathways, such as the P2X family 51, the G‐protein‐IP3‐IP3R cascade and ryanodine receptors, that push the Ca2+ concentration up 26. In parallel, we must include pathways, such as the Ca2+ uniporter‐Sigma‐1‐IP3R interaction, that push this concentration down 22, 23, 33. We also have to consider feedback loops built in such as store‐operated Ca2+ entry and voltage‐gated channels 26. In this thought experiment, we take a snapshot and measure the concentration of Ca2+, as well as gathering transcriptome, proteome, localization and all other relevant molecular data. Could we use these data to (i) predict the cytosolic calcium concentration in the next second, or in the next hour? Or (ii) could we predict how the cell will respond to a new extracellular source of ATP or free cellular debris? We could start by powering on our desktop universal computing machine.
Prediction is a shortcut that can be taken when “the computations used in the calculation are more sophisticated than those that the physical system can itself perform” 56. If the computation of our universal computer can be compressed to be smaller and faster than that of the physical system, then time given to the idealized computation can predict a disproportionately greater time of the physical system. For example, the motion of a ball traveling through a vacuum can be compressed to a single differential equation and its future position estimated in a single computation regardless of the travel time for the ball itself. Compression can become quickly overwhelmed by parallel, combinatorial interactions. This is well illustrated by the rapid increase in the complexity and unpredictability of the behavior over time when two, three or n‐bodies are orbiting one another 13. To come back to biology, this type of complexity is perhaps best illustrated by Turing's classic work on morphogens 53 where highly idealized, low‐dimensional chemical diffusion systems produce surprising outcomes, such as oscillatory waves of morphogen concentration and irregular “dappled” patterning.
For the model microglial cell, our universal machine will need to determine the outcome, microsecond to microsecond, of a large set of parallel interactions, each heavily dependent on the outcome of the interactions occurring among its neighbors in the previous microsecond. As such, the problem of prediction becomes a problem of simulation of a computationally irreducible set of interactions (the interactome). To make a prediction at the molecular level (question 1 above) or to make a judgment at the phenotype level (question 2 above) requires moving through a path of interactions. What this thought experiment illustrates is that the interactome constitutes a nontrivial, context‐dependent layer between the molecular and phenotypic. The inherent complexity of this increases when the microglia are considered as a population and further when their functional impact on the CNS environment is taken into account.
We should reconsider the use of “microglial activation” in this context. The term has become very broad, too broad considering the very specific roles microglia (as well as astrocytes) seem to have in distinct disease states. Therefore, it may be more appropriate to include the context when speaking about microglia associated with certain CNS states rather than using the one‐size‐fits‐all term, “microglia activation,” or even worse, “neuroinflammation.” As Ludwig Wittgenstein once said: “If you wrap up different kinds of furniture in enough wrapping paper, you can make them all look the same shape” 46.
The term “activated microglial cells” was first used when an early molecular marker enabled detection of this comparatively subtle (see below) phenotypic change 20. In contrast, the neuropathological designation “microgliosis” refers to population changes of the microglia that are often characterized by increased cell numbers and altered nuclear shape (e.g. comma or rod; the latter is not to be confused with classical cortical rod cells that show linear elongated cell processes as well), which occur in various disease settings but usually not in isolation. Microgliosis is characteristically accompanied by astrogliosis but may be dominant (hence the name). Thus, the term microgliosis is descriptive and not tied to any specific pathology or molecular mechanism. It is thus as unspecific as “microglial activation” but predates it as it stems from classical histology where tinctorial properties of nervous tissue based on the use of chemical dyes originally allowed the delineation of neuropathological tissue alterations. “Microgliosis” generally indicates more severe pathology than “microglial activation,” which may not be detectable in routine histological stains. Both terms should be abandoned in favor of more precise contextual definitions.
Adopting New Thinking Tools
The professionals of thinking on thinking are the philosophers and it is from their discourse that we may obtain a start upon the conceptual tools we are seeking. The concept of emergence provides a “toolbox” for dealing with multilevel interactions and complexity, often with input to and from computational science. In particular, emergence helps with the understanding of systems biology. We therefore propose to use emergence as a thinking toolbox for dealing with “big data” and the complex and highly plastic as well as dynamic microglial phenotype in particular.
What is an emergent property? Let us consider an example provided by John Searle 48: “Suppose we have a system, S, made up of elements a, b, c… For example, S might be a stone and the elements might be molecules. In general, there will be features of S that are not, or not necessarily, features of a, b, c… For example, S might weigh ten pounds, but the molecules individually do not weigh ten pounds. Let us call such features ‘system features’. The shape and the weight of the stone are system features. Some system features can be deduced or figured out or calculated from the features of a, b, c … just from the way these are composed and arranged (and sometimes from their relations to the rest of the environment). Examples of these would be shape, weight, and velocity. But some other system features cannot be figured out just from the composition of the elements and environmental relations; they have to be explained in terms of the causal interactions among the elements. Let's call these ‘causally emergent system features’. Solidity, liquidity, and transparency are examples of causally emergent system features.” A visual representation of these ideas adapted to pathways is shown in Figure 3.
Figure 3.
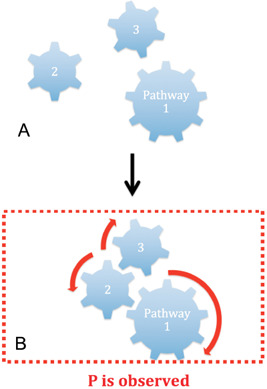
A property, P, is an emergent property of a mereologically complex object shown in (A) if and only if P supervenes on properties and relations of the pathways of the object. P is not observed in any of the parts of the object and the object has a downward functional causal influence over its parts, constraining their relations in space‐time so that the pattern of constraints realizes (arrow) as shown in (B) and, thus, explains P [modified after 18].
The behavioral effects of microglial sculpting of synapses during development 39, 41, 44, 57, microglial sex differences and their apparent impact on behavior 10, 30, 45, microglial systemic defense functions, and their population changes in response to peripheral cytokine storms during peripheral infections that may reach the brain and may cause delirium 12, 14, 21 can all be viewed as features of individual microglia cells just as liquidity and the capacity to form waves or snowflakes are features of water molecules.
Therefore, we are not merely interested in creating a catalog of microglial properties and functions but to try and understand that what we observe is a complex combination of individual properties, with interactions at a high enough level that makes it appropriate to use the language of emergent states. Dealing with microglia, and their functions as a population in particular, now requires this in our opinion. Such a point of view emphasizes the need for more transcriptome and proteome level experiments on both individual cell types and whole tissues coupled with pathway and interactome analyses to fill in the layer(s) between molecular and phenotypic (Box 1).
Box 1. Suggested new thinking tools: definition of entities.
At its core, emergence 49 holds a fundamental idea that is of great relevance to all of the life sciences: interaction is an entity of the system. In order to define some terms: any object that is capable of receiving input, processing this input and producing an output is an entity. The input, internal processing and output are all processes. Emergence, then, is a phenomenon wherein the output processes of two or more entities interact to form a new output, distinct from the output of any one entity involved. The new output is not reducible to any one entity; it comes into existence only out of the interaction between output processes. Emergence elevates this interaction to the status of entity, imbued with its own inputs, processes and outputs. The action of raising the interaction from process to entity is simple, but key. Through this we can begin to consider the interaction as an object that has unique features. It can be turned around and examined. A basic illustration of what constitutes an emergent property is shown in Figure 3. Some suggestions for further reading are included 3, 4, 5, 6, 8, 16, 17, 27, 28, 43, 47, 48, 54, 55.
Concluding Remarks
Transcriptomes 7, 24 and proteomes 34 will increasingly become the standard readout of microglia experimentation. Emergence appears very useful as a concept when the phenotype being observed is composed of irreducible context‐dependent interactions. Emergence can be wielded as a thinking tool to deal with intracellular and intercellular microglial interactions at an appropriate conceptual level, that is, comparable to their molecular components. As a result, the role of the microglia in both health and disease should become clearer and the impact of the microglia on behavior and how their life in an electrical organ differs from that of other macrophages better understood.
Supporting information
Movie S1. An example of an mpeg1:EGFP expressing microglial cell quickly morphing between ramified and amoeboid morphologies in the optic tectum at 4 dpf. Note how the morphology can change dramatically in a single time frame. Depth = 31.5 μm. Scale bar = 10 μm. Time = 2 h 56.8 mins. Each frame = 5 mins 12 s. Reproduced from (52).
Movie S2. Example of a ramified mpeg1:mCherry expressing microglial cell in the optic tectum at 11 dpf. Dynamic activity occurs at the endings of the thin processes while the cell body remains sessile. Depth = 25 μm. Scale bar = 10 μm. Time = 45 mins 26 s. Each frame = 2 mins 16 s. Reproduced from (52).
Acknowledgments
Funding from the Cure Brain Cancer Foundation, Australia, to MBG is gratefully acknowledged.
References
- 1. Aisen PS (2002) The potential of anti‐inflammatory drugs for the treatment of Alzheimer's disease. Lancet Neurol 1:279–284. [DOI] [PubMed] [Google Scholar]
- 2. Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL et al (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289:2819–2826. [DOI] [PubMed] [Google Scholar]
- 3. Balch WE, Roth DM, Hutt DM (2011) Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harb Perspect Biol 3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar Yam Y (2004) A mathematical theory of strong emergence using multiscale variety. Complexity 9:15–24. [Google Scholar]
- 5. Bedau MA (1997) Weak emergence. Noûs 31:375–399. [Google Scholar]
- 6. Bedau MA, Humphreys PE (2008) Emergence: Contemporary Readings in Philosophy and Science. The MIT Press: Cambridge, MA. ISBN 9780262524759. [Google Scholar]
- 7. Beutner C, Linnartz‐Gerlach B, Schmidt SV, Beyer M, Mallmann MR, Staratschek‐Jox A et al (2013) Unique transcriptome signature of mouse microglia. Glia 61:1429–1442. [DOI] [PubMed] [Google Scholar]
- 8. Bhalla US, Iyengar R (1999) Emergent properties of networks of biological signaling pathways. Science 283:381–387. [DOI] [PubMed] [Google Scholar]
- 9. Bialas AR, Stevens B (2013) TGF‐beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Bilbo SD, Schwarz JM (2012) The immune system and developmental programming of brain and behavior. Front Neuroendocrinol 33:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boulanger LM (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64:93–109. [DOI] [PubMed] [Google Scholar]
- 12. Calsavara AC, Rodrigues DH, Miranda AS, Costa PA, Lima CX, Vilela MC et al (2013) Late anxiety‐like behavior and neuroinflammation in mice subjected to sublethal polymicrobial sepsis. Neurotox Res 24:103–108. [DOI] [PubMed] [Google Scholar]
- 13. Cartwright J (2013) Physicists discover a whopping 13 new solutions to three‐body problem. Available at: http://news.sciencemag.org/physics/2013/03/physicists‐discover‐whopping‐13‐new‐solutions‐three‐body‐problem (accessed August 6, 2014).
- 14. Cerejeira J, Firmino H, Vaz‐Serra A, Mukaetova‐Ladinska EB (2010) The neuroinflammatory hypothesis of delirium. Acta Neuropathol 119:737–754. [DOI] [PubMed] [Google Scholar]
- 15. Del Rio‐Hortega P (1932) Cytology and Cellular Pathology of the Nervous System. Paul B Hoeber: New York, pp. 481–534. [Google Scholar]
- 16. Del Negro CA, Morgado‐Valle C, Feldman JL (2002) Respiratory rhythm: an emergent network property? Neuron 34:821–830. [DOI] [PubMed] [Google Scholar]
- 17. Efroni S, Harel D, Cohen IR (2007) Emergent dynamics of thymocyte development and lineage determination. PLoS Comput Biol 3:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El‐Hani CN, Emmeche C (2000) On some theoretical grounds for an organism‐centered biology: property emergence, supervenience, and downward causation. Theory Biosci 119:234–275. [Google Scholar]
- 19. Filiou MD, Arefin AS, Moscato P, Graeber MB (2014) “Neuroinflammation” differs categorically from inflammation: transcriptomes of Alzheimer's disease, Parkinson's disease, schizophrenia and inflammatory diseases compared. Neurogenetics 15:201–212. [DOI] [PubMed] [Google Scholar]
- 20. Graeber MB, Streit WJ, Kreutzberg GW (1988) Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res 21:18–24. [DOI] [PubMed] [Google Scholar]
- 21. Gyoneva S, Davalos D, Biswas D, Swanger SA, Garnier‐Amblard E, Loth F et al (2014) Systemic inflammation regulates microglial responses to tissue damage in vivo . Glia 62:1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hall AA, Herrera Y, Ajmo CT, Cuevas J, Pennypacker KR (2009) Sigma receptors suppress multiple aspects of microglial activation. Glia 57:744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayashi T, Su T‐P (2007) Sigma‐1 receptor chaperones at the ER‐mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610. [DOI] [PubMed] [Google Scholar]
- 24. Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang L, Means TK, El Khoury J (2013) The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16:1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ (2000) Functional requirement for class I MHC in CNS development and plasticity. Science 290:2155–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91:461–553. [DOI] [PubMed] [Google Scholar]
- 27. Kim J (2006) Emergence: core ideas and issues. Synthese 151:547–559. [Google Scholar]
- 28. Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J (2008) Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci 9:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee H, Brott BK, Kirkby LA, Adelson JD, Cheng S, Feller MB et al (2014) Synapse elimination and learning rules co‐regulated by MHC class I H2‐Db. Nature 509:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lenz KM, McCarthy MM (2014) A starring role for microglia in brain sex differences. Neuroscientist. 2014 May 28. pii: 1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linnartz B, Kopatz J, Tenner AJ, Neumann H (2012) Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor‐3‐mediated removal by microglia. J Neurosci 32:946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loo D (2011) In situ detection of apoptosis by the TUNEL assay: an overview of techniques. Chapter 1. In: DNA Damage Detection In Situ, Ex Vivo, and In Vivo. Didenko VV (ed.), pp. 3–13. Humana Press: Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 33. Maurice T, Su T‐P (2009) The pharmacology of sigma‐1 receptors. Pharmacol Ther 124:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLaughlin P, Zhou Y, Ma T, Liu J, Zhang W, Hong J‐S et al (2006) Proteomic analysis of microglial contribution to mouse strain‐dependent dopaminergic neurotoxicity. Glia 53:567–582. [DOI] [PubMed] [Google Scholar]
- 35. Monji A (2012) [The neuroinflammation hypothesis of psychiatric disorders]. Seishin Shinkeigaku Zasshi 114:124–133. [PubMed] [Google Scholar]
- 36. Moran LB, Duke DC, Turkheimer FE, Banati RB, Graeber MB (2004) Towards a transcriptome definition of microglial cells. Neurogenetics 5:95–108. [DOI] [PubMed] [Google Scholar]
- 37. Neher JJ, Neniskyte U, Brown GC (2012) Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front Pharmacol 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I et al (2014) Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging 35:1–14. [DOI] [PubMed] [Google Scholar]
- 39. Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P et al (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. [DOI] [PubMed] [Google Scholar]
- 40. Parakalan R, Jiang B, Nimmi B, Janani M, Jayapal M, Lu J et al (2012) Transcriptome analysis of amoeboid and ramified microglia isolated from the corpus callosum of rat brain. BMC Neurosci 13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parkhurst CN, Yang G, Ninan I, Savas JN, Yates I, John R et al (2013) Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell 155:1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR et al (2004) Rofecoxib: no effect on Alzheimer's disease in a 1‐year, randomized, blinded, controlled study. Neurology 62:66–71. [DOI] [PubMed] [Google Scholar]
- 43. Schadt EE (2009) Molecular networks as sensors and drivers of common human diseases. Nature 461:218–223. [DOI] [PubMed] [Google Scholar]
- 44. Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R et al (2012) Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schwarz JM, Sholar PW, Bilbo SD (2012) Sex differences in microglial colonization of the developing rat brain. J Neurochem 120:948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Searle JR (1992) The Rediscovery of the Mind. MIT Press: Cambridge, MA, p. 126. ISBN 9780262691543. [Google Scholar]
- 47. Searle JR (1995) Consciousness, the brain and the connection principle: a reply. Philos Phenomenol Res 55:217–232. [Google Scholar]
- 48. Searle JR (2008) Reductionism and the irreducibility of consciousness. Chapter 3. In: Emergence. Bedau MA, Humphreys P (eds), pp. 69–80. The MIT Press: Cambridge, MA. ISBN 9780262524759. [Google Scholar]
- 49. Stanford encyclopedia of philosophy. Available at: http://plato.stanford.edu/entries/properties‐emergent/ (accessed August 6, 2014).
- 50. Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N et al (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178. [DOI] [PubMed] [Google Scholar]
- 51. Surprenant A, North RA (2009) Signaling at purinergic P2X receptors. Annu Rev Physiol 71:333–359. [DOI] [PubMed] [Google Scholar]
- 52. Svahn AJ, Graeber MB, Ellett F, Lieschke GJ, Rinkwitz S, Bennett MR, Becker TS (2012) Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev Neurobiol 73:60–71. [DOI] [PubMed] [Google Scholar]
- 53. Turing AM (1953) The chemical basis of morphogenesis. Bull Math Biol 52:153–197, discussion 19–52. [DOI] [PubMed] [Google Scholar]
- 54. Varela FJ, Coutinho A (1991) Second generation immune networks. Immunol Today 12:159–166. [DOI] [PubMed] [Google Scholar]
- 55. Witherington DC (2011) Taking emergence seriously: the centrality of circular causality for dynamic systems approaches to development. Hum Dev 54:66–92. [Google Scholar]
- 56. Wolfram S (1985) Undecidability and intractability in theoretical physics. Phys Rev Lett 54:735–738. [DOI] [PubMed] [Google Scholar]
- 57. Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F et al (2014) Deficient neuron‐microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17:400–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. An example of an mpeg1:EGFP expressing microglial cell quickly morphing between ramified and amoeboid morphologies in the optic tectum at 4 dpf. Note how the morphology can change dramatically in a single time frame. Depth = 31.5 μm. Scale bar = 10 μm. Time = 2 h 56.8 mins. Each frame = 5 mins 12 s. Reproduced from (52).
Movie S2. Example of a ramified mpeg1:mCherry expressing microglial cell in the optic tectum at 11 dpf. Dynamic activity occurs at the endings of the thin processes while the cell body remains sessile. Depth = 25 μm. Scale bar = 10 μm. Time = 45 mins 26 s. Each frame = 2 mins 16 s. Reproduced from (52).


