Abstract
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by loss of memory and cognitive abilities, and the appearance of amyloid plaques composed of the amyloid‐β peptide (Aβ) and neurofibrillary tangles formed of tau protein. It has been suggested that exercise might ameliorate the disease; here, we evaluated the effect of voluntary running on several aspects of AD including amyloid deposition, tau phosphorylation, inflammatory reaction, neurogenesis and spatial memory in the double transgenic APPswe/PS1ΔE9 mouse model of AD. We report that voluntary wheel running for 10 weeks decreased Aβ burden, Thioflavin‐S‐positive plaques and Aβ oligomers in the hippocampus. In addition, runner APPswe/PS1ΔE9 mice showed fewer phosphorylated tau protein and decreased astrogliosis evidenced by lower staining of GFAP. Further, runner APPswe/PS1ΔE9 mice showed increased number of neurons in the hippocampus and exhibited increased cell proliferation and generation of cells positive for the immature neuronal protein doublecortin, indicating that running increased neurogenesis. Finally, runner APPswe/PS1ΔE9 mice showed improved spatial memory performance in the Morris water maze. Altogether, our findings indicate that in APPswe/PS1ΔE9 mice, voluntary running reduced all the neuropathological hallmarks of AD studied, reduced neuronal loss, increased hippocampal neurogenesis and reduced spatial memory loss. These findings support that voluntary exercise might have therapeutic value on AD.
Keywords: Alzheimer disease, amyloid, exercise, neurogenesis, tau
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease with higher incidence in the older population, which is characterized by the formation of senile plaques composed of aggregates of amyloid‐β peptide (Aβ), which is generated after the proteolytic cleavage of the amyloid precursor protein (APP) 33, 72, 84 and neurofibrillary tangles (NFT), formed by intracellular aggregation of hyperphosphorylated tau protein 49. In addition, soluble Aβ oligomers have shown to be responsible for the synaptic dysfunction, an early event observed in AD patients and mouse models of AD 18, 71, 74, 75, 100. Another important characteristic of AD brains is the astrocyte inflammatory response 46, characterized by an increase in astrocyte size in response to brain injury 65, and all these hallmark trigger neuronal loss, responsible of the brain atrophy observed in AD 73. In addition to neuronal loss, an impaired neurogenesis has been observed in the hippocampus of different transgenic models of AD 55, 89.
Most cases of AD are sporadic; however, a set of mutations in APP and presenilin (PS) proteins responsible for the cleavage of APP has been associated with familial AD (FAD) 4, 82. Although there is still no way to prevent the onset of AD, there are several factors that are considered protective against the disease; among them, exercise has emerged as a potential nonpharmacological strategy to prevent neurodegeneration and cognitive decline 50, 78. Physical activity improves memory in humans and animals 9, 47, 62, 98, in mouse models of AD improves cognitive function 7, 31, 59, 60, 93, 99 and decreases amyloid load 2, 48, 68. In the present work, we have extended the analysis of the protective effects of exercise, specifically evaluating in the double transgenic APPswe/PS1ΔE9 mouse model of AD, the effects of voluntary wheel running on different aspects of AD pathology including Aβ deposition, inflammatory reaction, tau phosphorylation and cognitive impairment. APPswe/PS1ΔE9 mice show deficits in spatial learning and memory, and present the histopathological hallmarks of the disease by 7 months old 41, 66, 83. We determined that in transgenic mice aged 5 months at the beginning of the treatment, 10 weeks of voluntary wheel running decreased both cognitive decline and histopathological changes. Moreover, running increased the total number of neurons in the CA3 region and the dentate gyrus; finally, runner APPswe‐PS1ΔE9 mice exhibited increased levels of neurogenesis in the hippocampus.
Material and Methods
Animals and treatments
Wild‐type B6C3F1/J and APPswe/PSEN1ΔE9 mice, which express the Swedish mutation of APP (K595N/M596L) and presinilin‐1 (PS1) with the deletion of exon 9, were obtained from the Jackson Laboratory (Bar Harbor, ME, USA; mouse stocks #100010 and #004462). All animals were housed in temperature‐ and light‐controlled rooms, with food and water ad libitum during the treatment. Mice at 5 months old were randomized into either voluntary running or sedentary (control) conditions for a period of 10 weeks (n = 6 mice/group). All animals were housed in groups of three; voluntary runners were maintained in a cage with three running wheels of 12.5 cm and control mice were housed in a similar cage but without the running wheels. The activity of each experimental group was monitored of visual manner. For the last 3 days of treatment, mice were injected with 50 mg/kg BrdU (Sigma‐Aldrich, St Louis, MO, USA) twice a day with 8 h interval between the first and second injections. Twenty‐four hours after last BrdU injection, mice were anesthetized and transcardially perfused as previously described 1.
Tissue sectioning
After dehydration, brains of all control and runner mice were sectioned on a cryostate in 12 sets of serial coronal sections of 40 μm thickness (Leica Microsystems, Wetzlar, Germany) and collected in ice‐cold‐PBS in multiwell dishes 27.Each set contained 5–7 sections covering the entire length of the hippocampus; thus, each set corresponds to a representative sampling of the whole hippocampus.
Immunohistochemical procedures
Free‐floating immunohistochemical procedures were performed as previously described 16, 67. Washing and dilution of immune‐reagents were performed using 0.01 M PBS with 0.2% Triton X‐100 (PBS‐T). Sections were pretreated with 0.5% H2O2 for 30 minutes to reduce endogenous peroxidase activity followed by treatment with 3% bovine serum albumin (BSA) at room temperature for 1 h to avoid nonspecific binding. Primary antibodies were incubated overnight at 4°C. Detection of primary antibody was performed using the Pierce ABC Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Staining was developed by incubating for 15 minutes with 0.6% diaminobenzidine followed by the addition of H2O2 (0.01% final concentration). After immunostaining, sections were mounted on gelatin‐coated slides, air‐dried, dehydrated cover‐slipped with Canada balsam (Merck, Darmstadt, Germany). Primary antibodies used were mouse anti‐PHF‐tau antibody clone AT8 (MN1020, Thermo Scientific), rabbit anti‐GFAP (DAKO, Carpinteria, CA, USA), mouse anti‐Aβ (4G8) and anti‐oligomer (A11; Chemicon, Temecula, CA, USA). To analyze the Aβ burden (percentage of area in the measurement field occupied by Aβ aggregates), the area positive to the antibody 4G8 was measured and the Aβ burden was calculated by dividing the area positive to Aβ by the total area of hippocampus or cortex. To measure the Aβ oligomer with the antibody A11, the intensity was analyzed using measures of Image‐J software (NIH, Bethesda, MD, USA). AT8‐positive cell for area were determined counting the number of cells in the hippocampus or cortex and, AT8‐positive cells were also quantified in the circular area (r ≈ 100 μm) surrounding amyloid plaques 13, 41. GFAP intensity and the size of the perikaryon were measured. For all assays, n ≥ 5 animals were used and three slices for each mouse were analyzed. Differences between wild‐type and control APPswe/PSEN1ΔE9 stained with A11, 4G8 and GFAP antibodies are showed in the Supporting Information Figure S1.
Amyloid plaques staining
Thioflavine‐S (Th‐S) is a fluorescent dye used to stain amyloid plaques 26, 95. Th‐S staining was carried out in sections mounted on gelatin‐coated slices as previously described 16, 19, 40. After dehydration and rehydration in ethanol and xilol batteries, slices were incubated in distilled water for 10 minutes and then immersed in the Th‐S solution (0.1% ThS in 70% ethanol) for 5 minutes. Then, slices were washed twice in 70% ethanol for 30 s and cover‐slipped with Fluoromont‐G (Electron Microscopy Sciences, Hatfield, PA, USA). Th‐S burden was obtained using the Image‐J software, specifically with the macros for Th‐S, which is a particle counter. For all assays, n ≥ 5 animals were used and three slices for each mice were analyzed. The absence of amyloid plaques in wild‐type mice in comparison with APPswe/PSEN1ΔE9 is observed in the Supporting Information Figure S1.
Nissl staining
Mounted sections were defatted in xylene and hydrated in ethyl alcohol and water series. Nissl staining (0.3% cresyl violet) was performed as previously described 67. The number of cells was evaluated in the different zones from the hippocampus.
Immunofluorescence staining
Immunodetection of BrdU and neuronal markers in tissue sections was carried out as described in 96 with some modifications. Sections were denatured in 2N HCl for 15 minutes and incubated in blocking solution (3% donkey serum, 3% BSA, 0.5% Triton X‐100 in PBS) for 6 h and then incubated overnight with primary antibodies diluted in blocking solution. Primary antibodies used were: rat anti‐BrdU (AbCam Inc, Cambridge, USA), rabbit anti‐doublecortin (Cell Signaling Technology Inc., Beverly, MA, USA), monoclonal anti‐NeuN (Millipore, Billerica, MA, USA). After washing with PBS, the sections were incubated with secondary antibodies in PBS 3% BSA, 0.5% Triton X‐100. Alexa (Molecular Probes) and Dy Light (Abcam) conjugated secondary antibodies were used. Slices were mounted on gelatin‐coated slides with Fluoromont‐G (Electron Microscopy Sciences, Hatfield, England).
Image analysis
Stained brain sections were photographed using an Olympus BX51 microscope (Tokyo, Japan) coupled to a Micro‐publisher 3.3 RTV camera (QImaging, Surrey, BC, Canada). The luminance of the incident light and the time of exposure were calibrated to assign pixel values ranging from 0 to 255 in RGB image (no‐light to full‐light transmission), which were used along all preparations. The images were analyzed with Image‐J software (NIH). Selection of areas for measurement was performed by manual threshold adjustment or by direct manual selection of ROIs in heterogeneous stains. For BrdU staining, positive cells were counted using the OLIMPUS AMERICA INC. Melville, NY, USA fluorescence microscope. Cells were counted in all sections of one set (see tissue sectioning), and total cells counted in one set were multiplied by the total number of sets to obtain an estimation of the total number of BrdU+ cells in the whole hippocampus. Double‐labeled sections were analyzed by confocal laser microscopy (Olympus FV 1000). Z‐Projections were made with ImageJ software.
Behavioral test
The Morris water maze (MWM) test was performed as previously described 83. Briefly, all animals (six from each experimental group) were trained in a circular water maze (1.2 m diameter, 50 cm deep, 19–21°C, 9 cm platform 1 cm below water colored opaque with nontoxic white paint); the maximum trial duration was 60 s and mice were kept 5 s on the platform at the end of trials. Each animal was trained for one pseudo‐random location of the platform per day, for 4 days, with a new platform location each day. Training was conducted up to 15 trials per day, until the criterion of three successive trials with escape latency below 20 s was met. On completion of testing, the mouse was removed from the maze, dried and returned to its cage. The following day, animals were tested for the next location. Data were collected using a video tracking system for water maze (HVS Imagen, Hampton, UK).
Statistical analyses
For the comparison of means, analysis of variance (ANOVA) was used followed by Tukey's HSD post‐hoc test or two‐tailed Student's t‐test. For non‐parametric analysis, Kruskal–Wallis test followed by the pair‐wise Mann–Whitney U test was used. In all cases, P < 0.05 was considered statistically significant.
Results
Voluntary running decreased Aβ burden and tau phosphorylation
We aimed to evaluate the effect of voluntary running on the AD‐like histopathological changes in the APPswe/PSEN1ΔE9 mouse model of AD. For this purpose, 5‐month‐old APPswe/PSEN1ΔE9 mice were housed for 10 weeks in cages with (Tg runner) or without (Tg control) running wheels. First, we evaluated the relative amounts of Aβ oligomers by using the specific antibody A11 (Figure 1A). The relative intensity of A11 in wild‐type mice in comparison with APPswePSEN1ΔE9 is showed in Supporting Information Figure S1. Brain sections from runner mice showed a reduction in the relative intensity of A11 staining in the hippocampus compared with control transgenic mice (Figure 1C), suggesting that running decreased the levels of Aβ oligomers. To evaluate the effect of running on Aβ peptide deposition, brain sections from wild‐type (Supporting Information Figure S1), control and runner APPswe/PSEN1ΔE9 mice were immunostained against total Aβ peptide using the 4G8 antibody (Figure 1B); Aβ burden was measured in the cortex and hippocampus. A significant reduction in Aβ burden was observed in the hippocampus of runner mice (Figure 1D), whereas no changes were observed in the cortex area (Figure 1E). Amyloid deposits were also analyzed in the hippocampus and cortex by Th‐S staining (Figure 2A); wild‐type mice do not show Th‐S‐positive staining indicating the specificity of Th‐S signal (Supporting Information Figure S1). A detailed analysis of Aβ plaque size was also carried out (Figure 2B) and presented as the percentage of senile plaques for size (μm2; Figure 2C), which revealed that runner APPswe/PSEN1ΔE9 mice present smaller plaques in the hippocampus compared with control transgenic mice. Digital quantification also revealed that in the cortex running did not affect the area occupied by amyloid deposits (Tg 0.34 ± 0.03; Tg runner 0.29 ± 0.01; P‐value = 0.0537) (Figure 2D) but decreased the number of senile plaques (Tg 52 ± 0.3; Tg runner 39 ± 0.4; P‐value = 0.0419; Figure 2E), whereas in the hippocampus, voluntary running decrease both Th‐S burden (Tg 0.40 ± 0.02; Tg runner 0.29 ± 0.02; P‐value = 0.309; Figure 2F) and plaque number (Tg 33 ± 0.4; Tg runner 22 ± 0.2; P‐value = 0.0211; Figure 2G). These results indicate that voluntary running decreases hippocampal Aβ load suggesting that it reduces amyloid pathology, at least in the hippocampus.
Figure 1.

Voluntary running reduces the intensity of Aβ oligomers, Aβ burden and staining in brain sections from APPswe/PS1ΔE9 mice. A. Immunohistochemical analysis of Aβ oligomers stained using the specific antibody A11 in control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 brains. B. Representative immunohistochemical analysis in brain slices from control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 mice using the antibody 4G8 to analyze total Aβ deposits. C. Mean relative intensity of A11 staining. D, E. Mean area fraction positive for Aβ in the hippocampus (D) and cortex (E). Bars represent mean ± SE (n ≥ 5). *P < 0.05; **P < 0.01.
Figure 2.
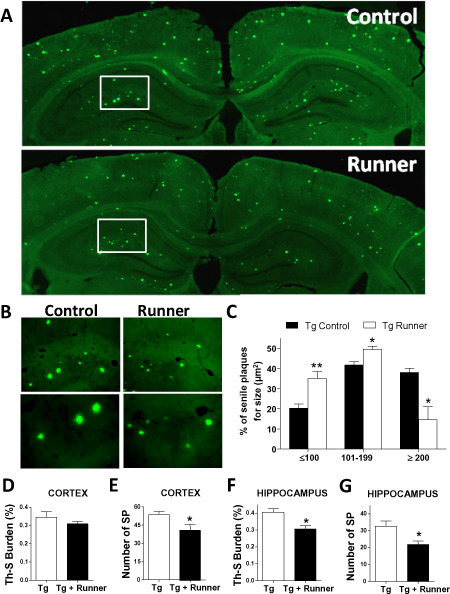
Running reduces the levels of Th‐S burden in APPswe/PS1ΔE9 mice brain. A. Amyloid plaques detected with Th‐S staining in control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 mice brain. Images represent a reconstruction of the hippocampus and cortex of control and runner transgenic mice. B. Magnification of senile plaques. C. The graphs classified the senile plaques of control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 mice for size (mm2). Amyloid burden was quantified with the Th‐S staining and the percentage of the area covered by amyloid plaques (D) and the number of senile plaques (E) in cortex and hippocampus, respectively (F,G), was plotted. Bars represent mean ± SE (n ≥ 5). *P < 0.05; **P < 0.01.
Tau pathology, characterized by the intracellular aggregation of hyperphosphorylated tau protein, is also a classic hallmark of AD 25, 33, 49 that is present in APPswe/PSEN1ΔE9 mice 14, 40. Therefore, we analyzed the effect of running on the phosphorylation of the tau. The appearance of the AD‐associated tau AT8 epitope was studied using an antibody that recognizes phosphorylated Ser‐202 and Thr‐205 34. Cells positive for AT8 staining were observed in brain sections of control APPswe/PSEN1ΔE9 mice (Figure 3A), and the density of these cells was significantly lower in the cortex (Figure 3B) but not in the hippocampus (Figure 3C) of runner transgenic mice. It has been described that cytoskeletal changes, tau phosphorylation, glial fibrillar acidic protein (GFAP) protein activation and synapse loss mainly occur nearby Aβ plaques 35, 49, 72. Therefore, and as previously described 13, 41, AT8‐positive cells were quantified in the circular area (r ≈ 100 μm) surrounding amyloid plaques stained with Th‐S (Figure 3D, arrows). Voluntary runners had fewer AT8‐positive neurons next to amyloid deposits compared with control APPswe/PSEN1ΔE9 mice (Figure 3E). These data show that voluntary running decreases tau phosphorylation, which is a later event in the progression of AD neuropathology.
Figure 3.
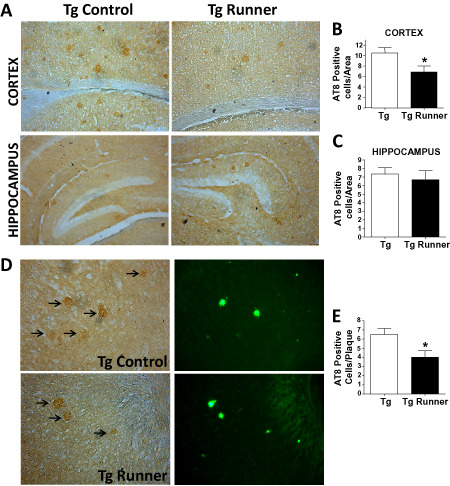
Running reduces the levels of phosphorylated tau protein in the brain of APPswe/PS1ΔE9 mice. A. AT8‐positive cells were detected in control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 mice in the cortex (top panel) and hippocampus (bottom panel). Graph shows the quantification of AT8‐positive cell per area in the cortex (B) and hippocampus (C). D. Images show representative images of the immunodetection of AT8‐positive cells (left panels, arrows) near amyloid plaques identified by Th‐S stain (right panels). E. Quantification of the number of AT8‐positive neurons per plaque. Bars represent mean ± SE (n ≥ 5). *P < 0.05.
Voluntary exercise decreases inflammation in the brain of APPswe/PSEN1ΔE9 mice
To analyze the inflammatory reaction, brain sections from wild‐type (Supporting Information Figure S1), control and runner APPswe/PSEN1ΔE9 mice were immunostained against GFAP (Figure 4A). It was previously reported that APPswe/PSEN1ΔE9 mice show increased GFAP staining than age‐matched wild‐type animals 83. The GFAP staining was significantly reduced in the cortex and hippocampus of runner APPswe/PSEN1ΔE9 mice compared with control transgenic mice (Figure 4A). Runners showed lower average GFAP intensity in the cortical and hippocampal regions (Figure 4B,C) and in the perikaryon area of the astrocytes (Figure 4D,E), indicating that voluntary running prevents the neuroinflammatory reaction characteristic of AD.
Figure 4.
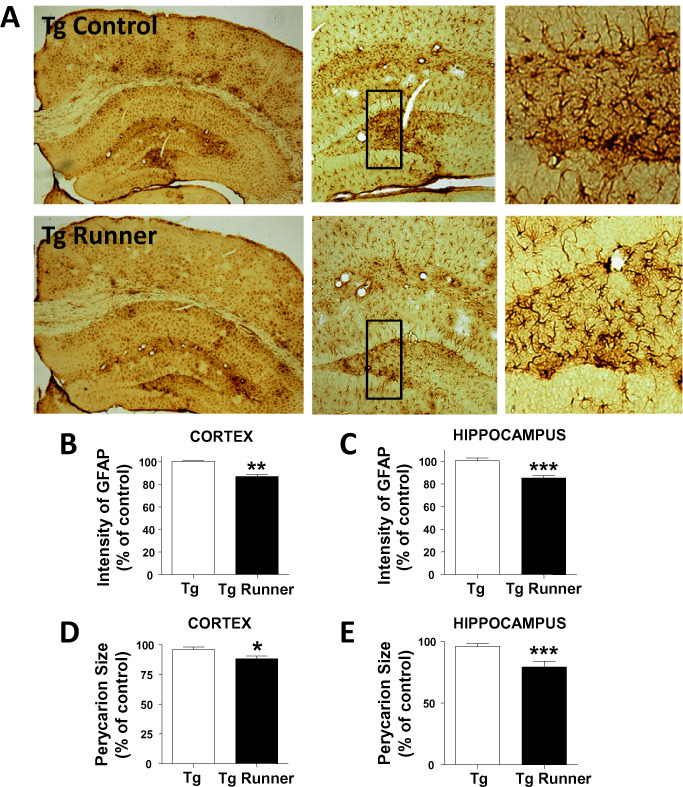
Voluntary exercise reduces astrogliosis in the brain of APPswe/PS1ΔE9 mice. A. Immunohistochemistry against GFAP in brain slices from control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 mice. Right panels show full resolution images of astrocytes in both experimental groups. B, C. Analysis of the intensity GFAP staining (relative to control) in cortex (B) and hippocampus (C). D, E. Perikaryon size of glial cells in the hippocampus (D) and cortex (E). Quantification was normalized to control APPswe/PS1ΔE9 mice. Bars represent mean ± SE (n ≥ 5). *P < 0.05; **P < 0.01; ***P < 0.001.
Voluntary exercise prevents neuronal cell loss in the hippocampus and increases neurogenesis in the dentate gyrus of APPswe/PSEN1ΔE9 mice
We analyzed whether voluntary running could prevent neuronal cell loss; the number of neurons was assessed by Nissl staining and the number of cells present in different hippocampal zones was counted 67. As observed in Table 1, control transgenic mice have less neurons in the CA1‐CA2 (*P = 0.0213); CA3 (*P = 0.0189) regions and dentate gyrus (*P = 0.0309) in comparison with wild‐type mice, and voluntary running significantly prevented neuronal cell loss in the dentate gyrus (# P = 0.0290) and in the CA3 region (# P = 0.0394) of APPswe/PSEN1ΔE9 mice, showing nonsignificant differences with wild‐type mice; however, running did not affect the number of neurons in the CA1 region. These results suggest a neuroprotective role of voluntary running against hippocampal neuronal loss.
Table 1.
Number of neurons in hippocampus (cells/mm2)
| CA–CA2 | CA3 | Dentate gyrus | |
|---|---|---|---|
| WT | 85.13 ± 6.017 | 186.1 ± 4.112 | 81.07 ± 8.927 |
| Tg control | 57.00 ± 8.103* | 118.3 ± 9.280* | 52.53 ± 4.208* |
| Tg runner | 58.86 ± 4.783 | 157.0 ± 8.554† | 70.40 ± 7.119† |
* significant differences of Tg control in comparison with WT mice.
† significant differences of Tg control in comparison with Tg runner mice.
Then, we evaluated whether voluntary running could stimulates neurogenesis in the hippocampus of APPswe/PSEN1ΔE9 mice, as has been described previously running induces hippocampal neurogenesis in young and aged mice 86, 87. In the subgranular zone (SGZ) of the adult dentate gyrus, there are neural progenitor cells that proliferate and differentiate into granule cells that integrate into the hippocampal network 52, 101. To evaluate cell proliferation control and runner APPswe/PSEN1ΔE9 mice received serial i.p. injections of the nucleotide analog BrdU (50 mg/kg twice a day for the last 3 days of the experiment) and were sacrificed 24 h after the final BrdU injection. Total number of proliferating cells (BrdU‐positive, BrdU+) was estimated in the whole dentate gyrus by BrdU immunoreactivity 1. It is known from previous reports that APPswe/PSEN1ΔE9 mice show a decreased proliferation in the SGZ as compared with age‐matched wild‐type animals 1, 38, 90; in agreement, fewer BrdU+ cells are observed in the SGZ of transgenic mice compared with wild‐type animals (Supporting Information Figure S2). Running increased almost threefold the total number of BrdU+ cells in the SGZ of transgenic mice (Figure 5A,B), being the total number of BrdU+ cells in transgenic runners very similar to that of aged‐matched wild‐type mice that received a similar BrdU administration protocol 1. This result indicates that running stimulated cell proliferation in the hippocampus of diseased mice. To assess neural differentiation, double staining of BrdU and the immature neuron marker doublecortin (DCX) was carried out (Figure 5A). We previously reported that the percentage of BrdU+ cells positive for DCX staining is decreased in APPswe/PSEN1ΔE9 mice compared with aged‐matched wild‐type mice 1; here, we observed that this percentage was significantly increased in voluntary runners reaching around 60% of the BrdU‐positive cells expressing DCX (Figure 5C), which is comparable with wild‐type mice of similar age and BrdU administration protocol 1. In sum, these results indicate that running prevented neuronal cell loss and increased neurogenesis in the hippocampus of APPswe/PSEN1ΔE9 mice.
Figure 5.
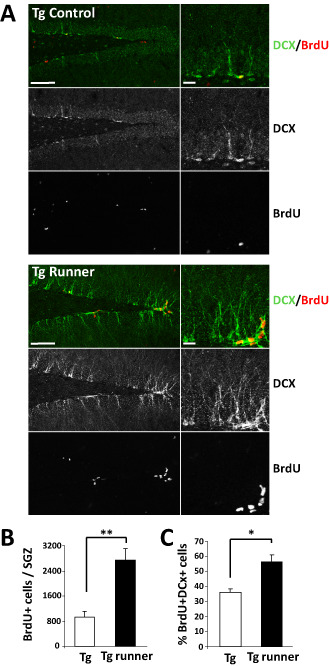
Voluntary running increases cell proliferation and neurogenesis in the SGZ of APPswe/PS1ΔE9 mice. A. Immunodetection of BrdU and the immature neuronal protein DCX in control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 mice. B. Quantification of the total number of BrdU + cells in the SGZ. C. Quantification of the percentage of BrdU + cells that is also positive for DCX. Bars represent mean ± SE (n ≥ 5). *P < 0.05; **P < 0.01.
Voluntary running improves cognitive performance of APPswe/PSEN1ΔE9 mice
We aimed to evaluate effect of voluntary running on the cognitive deficit associated with AD. For this purpose, we used a water maze protocol in which the animal learns every day a new location of the hidden platform 20. Thus, once the animal has learned the location of the platform, it is moved to a novel location, and so on. This task in which mice should learn and recall the successive platform locations requires memory flexibility, which is an element of episodic‐like memory, and is sensitive to AD 20, 83. Previous studies have shown spatial learning impairment in APPswe/PSEN1ΔE9 mice by 7 months of age 30. In order to evaluate acquisition memory, animals were subjected to the test with no previous training. As shown in Figure 6A, at the fourth day of the memory flexibility test, runner mice reached the criteria in fewer trials than control mice. In addition, runner animals swam shorter distances to reach de platform (Figure 6B,C). Importantly, no differences were observed in the swimming speed during the test indicating similar locomotor activity in the different experimental groups (Figure 6D).These results indicate that voluntary running prevented impairment in spatial acuity and memory acquisition in APPswe/PSEN1ΔE9 mice.
Figure 6.
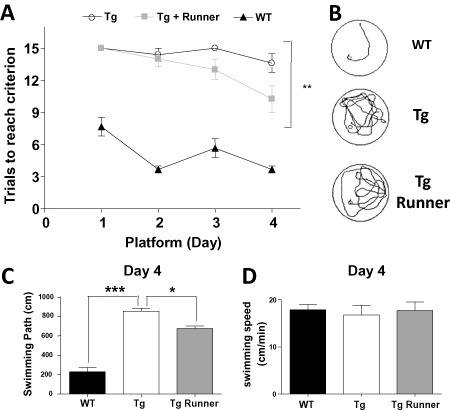
Voluntary wheel running decreased the spatial memory impairment in APPswe/PS1ΔE9 transgenic mice. A. Memory flexibility test in the MWM shows the number of trials necessary to reach the criterion in wild‐type mice (WT control), control (Tg) and runner (Tg runner) APPswe/PS1ΔE9 mice. Exercise reduces the number of trials necessary to reach the criterion. B, C. Representative swimming tracks (B) and analysis of the swimming path of the last day of testing (C). D. Analysis of the swimming speeds shows no differences between the experimental groups. Bars represent mean ± SE (n ≥ 5). *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In the present study, we determined that 10 weeks of voluntary running improved short‐term memory and decreased the development of AD‐like neuropathological markers in APPswe/PSEN1ΔE9 mice. Specific neuroprotective effects of running observed in this study includes decreased Aβ burden and increased cell proliferation and neurogenesis in the dentate gyrus. These findings are consistent with the previous studies on the effects of running and exercise 2, 59, 64, 87, 93. Additionally, we observed that running decreased: (i) the intensity of Aβ oligomers, (ii) the levels of astrogliosis, (iii) tau phosphorylation in the proximity of amyloid plaques, (iv) and reduced neuronal cell loss in the hippocampus. Altogether, these results indicate that voluntary running could be used as potential therapeutic treatment on AD.
AD is characterized by the loss of cognitive abilities including learning and memory 4, 17, which is observed in different mouse models of the disease, including double transgenic APPswe/PSEN1ΔE9 mice 66, which shows impaired spatial learning at 7 months old 40, 41. Here we showed that 10 weeks of voluntary running (since 5 months of age) decreased cognitive impairment in APPswe/PSEN1ΔE9 mice as evaluated by spatial memory flexibility in the MWM. Previous reports have shown that exercise improves cognitive function in rodents and more importantly in humans, particularly in the aged population 8, 10, 23, 37, 69, 70, 87, 91, 94. Our results provide evidence that voluntary running also reduces the cognitive impairment in AD mice.
Our results show a correlation between cognitive improvement and histopathological data. According to the amyloid cascade hypothesis, Aβ formation is a critical step in AD pathogenesis 56, and Aβ peptide accumulation is thought to have a key function in the cognitive deficit associated to the disease 72. We determined a decreased Aβ load in the brains of runner mice, which was evidenced by a decreased Aβ burden in the hippocampus. APPswe/PSEN1ΔE9 mice develop Aβ plaques in the cortex and hippocampus in an age‐dependent manner 30. We demonstrated that running decreased the amyloid deposits in the hippocampus, and decreased the size of the plaques in the cortex and hippocampus. In addition, we observed a lower intensity of A11 staining in the hippocampus of runner mice compared with control AD mice, suggesting that voluntary running decreased the levels of oligomeric Aβ species. Importantly, Aβ oligomers are relevant for the synaptotoxicity associated to the cognitive decline 45, 97. Therefore, our results indicate that running decreased synaptotoxic aggregates which may underlie the cognitive improvement observed. Overall, these results suggest that voluntary running for 10 weeks is sufficient to ameliorate the development of the amyloid pathology and these results are consistent with a previous study that has shown that voluntary wheel running for 16 weeks, and not forced treadmill running, decreased Th‐S‐stained plaques in brain of Tg2576 mouse model of AD 99. However, we did not observed significant differences in cortical Aβ burden. Previously, it was shown in the TgCRND8 mouse model of AD that voluntary wheel running for 5 months significantly decreased amyloid plaques in the cortex 2. This suggests that although we observed a decrease in the total number of Th‐S aggregates in runner mice, a longer exposure to voluntary running might be necessary to significantly decrease the total area occupied by senile plaques in the cortex.
We cannot eliminate the possibility that exercise decreased APP processing required for the generation of Aβ, as a previous study in TgCRND8 mice showed that 5 months of voluntary exercise resulted in decreased extracellular Aβ plaques in the frontal cortex (at the level of the hippocampus), and the hippocampus by mechanisms that appear to be mediated by changes in the processing of APP 2. Additional studies are needed to elucidate whether the effect of running involves APP processing and/or Aβ clearance.
Tau pathology is also a hallmark of AD. Fibrils composed of hyper‐phosphorylated tau protein accumulate within neuron cell bodies and dendrites in AD brain, and form the paired‐helical filaments (PHFs) that coalesce into NFTs 5, 12, 49, 92. Runner APPswe/PSEN1ΔE9 mice showed reduced number of AT8‐positive cells in the cortex and decreased number of AT8‐positive cell in close proximity to amyloid plaques, suggesting that running decreased a late event in the progression of AD. This result is very important because it shows that short‐term voluntary running decreases this neuropathological lesion of AD. Neurofibrillary degeneration starts in the allocortex of the medial temporal lobe (entorhinal cortex) and spreads to the associative isocortex and to a lesser extent to primary sensory, motor, and visual areas 73. A severe involvement of striatum and substantia nigra can occur during the late iso‐cortical stage 3, 11, 39. Our results suggest that voluntary exercise decreased AD‐associated phosphorylation of tau protein in the brain region where NFTs first appear. Also, the reduction of AT8‐positive cells could be related to the decreased accumulation of Aβ and reduced inflammatory response, or may be a consequence of modifications in the signaling molecules involved in the phosphorylation of tau such as glycogen synthase kinase‐3β (GSK‐3β), cyclin‐dependent kinase 5 (Cdk5), extracellular signal‐related kinase 2 (ERK2) and microtubule affinity‐regulating kinase (MARK). It is known for example that protein kinase C (PKC), mitogen‐activated protein kinase (MAPK) and phospholipase C (PLC) pathways are activated by exercise 53, 76. Activation of the Wnt signaling pathway has also been associated with running 6, 43, 61, 77, which may reduces the activity of GSK‐3β as part of the signaling cascade 21. Interestingly, Aβ treatment in cultured neurons increased GSK‐3β activity 80, 81, which enhanced tau phosphorylation 81. Moreover, active GSK‐3β has been found in AD brains with NFT 80, 81, and its distribution seems to co‐occur with the development of neurofibrillary changes 63.Therefore, running may induce the activation of the Wnt signaling pathway in the brain and thus inhibit GSK‐3β and the consequent phosphorylation of tau. The activation of the Wnt signaling pathway may also underlie other protective effects of running in transgenic animals as previously observed by in vivo activation of the Wnt pathway in AD mice 29, 83.
Another histopathological feature of AD brain is the presence of reactive astrocytes associated with senile plaques 36. The glial cell reaction has been suggested to play a role in the neurodegenerative events that take place in the disease 51, 65. Interestingly, we demonstrated that voluntary running decreased the activation of glial cells, decreasing the intensity and size of activated astrocytes in the cortex and hippocampus. This is a novel finding and suggests that running for 10 weeks prevented the chronic inflammatory response observed in AD brain.
Recent reports have suggested or hypothesized that the interaction between Aβ oligomers and tau protein could cause the damage in AD brains, and might cause neuronal cell death 24, 42, 54. A decrease in neuronal loss in the hippocampus was observed in runner APPswe/PSEN1ΔE9 mice, specifically in the dentate gyrus and CA3 region. These results suggest that running prevented or attenuated the events that trigger neuronal cell death. This is in agreement with a previous report showing that in the Tg‐NSE7PS2m mouse model of AD, exercise suppressed neuronal death in the hippocampus of aged mice 85.
Finally, we demonstrated that running increased neurogenesis in the dentate gyrus of APPswe/PSEN1ΔE9 mice. This transgenic mouse shows decreased neurogenesis in the hippocampus compared with age‐matched wild‐type mice 1. We determined that runner mice show increased cell proliferation in the SGZ and increased generation of immature neurons or neuroblasts. It has been well established that running is a strong inducer of neurogenesis in young and aged mice 86, 87. Our results indicate that voluntary running is also able to stimulate neurogenesis in the diseased AD brains. The mechanism underlying this effect may involve signaling pathways such as the Wnt signaling cascade, which is a positive regulator of hippocampal neurogenesis (reviewed in 88) activated by running, or brain‐derived neurotrophic factor (BDNF) signaling 22, 32, 58, 60, which plays important roles in control and the diseased brain 57. Also, exercise induces vascular endothelial growth factor (VEGF) that may mediate the effects of running on neurogenesis 15, 28, 44, 79. Interestingly, by using magnetic resonance imaging measurements, it was determined that among all hippocampal subregions, exercise has a primary effect on dentate gyrus cerebral blood volume, which correlated with postmortem measurements of neurogenesis 64. By using the same MRI technologies in humans, it was shown that exercise also has a primary effect on dentate gyrus cerebral blood volume. These findings suggested that exercise differentially targets the dentate gyrus, which may underlie part of the effects observed in our study including decreased neuronal cell death and increased neurogenesis 64.
Taken together, our data provide both cellular and molecular insights into the cognitive and neuropathological benefits of voluntary running for AD pathology. The results show that voluntary running in an early pathological state decreased the development of several neuropathological markers, indicating that there is a global effect on AD pathogenesis. Moreover, voluntary running was able to decrease neuronal cell death and to increase the generation of new neurons in the hippocampus. These effects could be the cause of the improvements in plasticity and memory performance induced by exercise in in vivo models of AD. Overall, this study provides compelling evidence that running may be relevant for preventing the development and progression of AD pathology.
Conflict of Interest
The authors declare that they have no conflict of interest.
Supporting information
Figure S1. Compares the histopathological procedures of wild‐type mice with control APPswe/PS1ΔE9 mice, using the A11, 4G8 and GFAP antibodies and the Th‐S staining.
Figure S2. Shows the BrdU+ cells in the SGZ of transgenic mice compared with wild‐type animals.
Acknowledgments
This work was supported by the National Commission of Science and Technology of Chile (CONICYT) through FONDECYT (N°1120156) and the Basal Center of Excellence in Aging and Regeneration (CONICYT‐PFB12/2007) to NCI; FONDECYT (N°11110012) to LV‐N; and Pre‐doctoral Fellowship from FONDECYT to CTR.
References
- 1. Abbott AC, Calderon Toledo C, Aranguiz FC, Inestrosa NC, Varela‐Nallar L (2013) Tetrahydrohyperforin increases adult hippocampal neurogenesis in wild‐type and APPswe/PS1DeltaE9 mice. J Alzheimers Dis 34:873–885. [DOI] [PubMed] [Google Scholar]
- 2. Adlard PA, Perreau VM, Pop V, Cotman CW (2005) Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci 25:4217–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold S, Hyman B, Flory J, Damasio A, Van Hoesen G (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex 1:103–116. [DOI] [PubMed] [Google Scholar]
- 4. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer's disease. Lancet 377:1019–1031. [DOI] [PubMed] [Google Scholar]
- 5. Ballatore C, Lee VM, Trojanowski JQ (2007) Tau‐mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8:663–672. [DOI] [PubMed] [Google Scholar]
- 6. Bayod S, Menella I, Sanchez‐Roige S, Lalanza JF, Escorihuela RM, Camins A et al (2014) Wnt pathway regulation by long‐term moderate exercise in rat hippocampus. Brain Res 1543:38–48. [DOI] [PubMed] [Google Scholar]
- 7. Belarbi K, Burnouf S, Fernandez‐Gomez FJ, Laurent C, Lestavel S, Figeac M et al (2011) Beneficial effects of exercise in a transgenic mouse model of Alzheimer's disease‐like Tau pathology. Neurobiol Dis 43:486–494. [DOI] [PubMed] [Google Scholar]
- 8. Berkman LF, Seeman TE, Albert M, Blazer D, Kahn R, Mohs R et al (1993) High, usual and impaired functioning in community‐dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol 46:1129–1140. [DOI] [PubMed] [Google Scholar]
- 9. Bherer L, Erickson KI, Liu‐Ambrose T (2013) A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res 2013:657508. 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blomquist KB, Danner F (1987) Effects of physical conditioning on information‐processing efficiency. Percept Mot Skills 65:175–186. [DOI] [PubMed] [Google Scholar]
- 11. Braak H, Braak E (1991) Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 82:239–259. [DOI] [PubMed] [Google Scholar]
- 12. Brunden KR, Ballatore C, Crowe A, Smith AB 3rd, Lee VM, Trojanowski JQ (2010) Tau‐directed drug discovery for Alzheimer's disease and related tauopathies: a focus on tau assembly inhibitors. Exp Neurol 223:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cancino GI, Toledo EM, Leal NR, Hernandez DE, Yevenes LF, Inestrosa NC, Alvarez AR (2008) STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer's beta‐amyloid deposits. Brain 131 (Pt 9):2425–2442. [DOI] [PubMed] [Google Scholar]
- 14. Cancino GI, Perez de Arce K, Castro PU, Toledo EM, von Bernhardi R, Alvarez AR (2011) c‐Abl tyrosine kinase modulates tau pathology and Cdk5 phosphorylation in AD transgenic mice. Neurobiol Aging 32:1249–1261. [DOI] [PubMed] [Google Scholar]
- 15. Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835. [DOI] [PubMed] [Google Scholar]
- 16. Carvajal F, Zolezzi J, Tapia‐Rojas C, Godoy J, Inestrosa N (2013) Tetrahydrohyperforin decreases cholinergic markers associated with amyloid‐β plaques, 4‐hydroxynonenal formation, and caspase‐3 activation in AβPP/PS1 mice. J Alzheimers Dis 36:99–118. [DOI] [PubMed] [Google Scholar]
- 17. Castellani RJ, Rolston RK, Smith MA (2010) Alzheimer disease. Dis Mon 56:484–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cerpa W, Dinamarca MC, Inestrosa NC (2008) Structure‐function implications in Alzheimer's disease: effect of Abeta oligomers at central synapses. Curr Alzheimer Res 5:233–243. [DOI] [PubMed] [Google Scholar]
- 19. Chacon MA, Barria MI, Soto C, Inestrosa NC (2004) Beta‐sheet breaker peptide prevents Abeta‐induced spatial memory impairments with partial reduction of amyloid deposits. Mol Psychiatry 9:953–961. [DOI] [PubMed] [Google Scholar]
- 20. Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ et al (2000) A learning deficit related to age and beta‐amyloid plaques in a mouse model of Alzheimer's disease. Nature 408:975–979. [DOI] [PubMed] [Google Scholar]
- 21. Clevers H, Nusse R (2012) Wnt/beta‐catenin signaling and disease. Cell 149:1192–1205. [DOI] [PubMed] [Google Scholar]
- 22. Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R et al (2014) Acute aerobic exercise increases brain‐derived neurotrophic factor levels in elderly with Alzheimer's disease. J Alzheimers Dis 39:401–408. [DOI] [PubMed] [Google Scholar]
- 23. Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: a meta‐analytic study. Psychol Sci 14:125–130. [DOI] [PubMed] [Google Scholar]
- 24. Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet 19 (R1):R12–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dickson DW (2004) Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: cause or effect? J Clin Invest 114:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH (1988) Alzheimer's disease. A double‐labeling immunohistochemical study of senile plaques. Am J Pathol 132:86–101. [PMC free article] [PubMed] [Google Scholar]
- 27. Encinas JM, Enikolopov G (2008) Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol 85:243–272. [DOI] [PubMed] [Google Scholar]
- 28. Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N et al (2003) VEGF is necessary for exercise‐induced adult hippocampal neurogenesis. Eur J Neurosci 18:2803–2812. [DOI] [PubMed] [Google Scholar]
- 29. Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F (2010) Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS ONE 5:e14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia‐Alloza M, Robbins EM, Zhang‐Nunes SX, Purcell SM, Betensky RA, Raju S et al (2006) Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 24:516–524. [DOI] [PubMed] [Google Scholar]
- 31. Garcia‐Mesa Y, Lopez‐Ramos JC, Gimenez‐Llort L, Revilla S, Guerra R, Gruart A et al (2011) Physical exercise protects against Alzheimer's disease in 3xTg‐AD mice. J Alzheimers Dis 24:421–454. [DOI] [PubMed] [Google Scholar]
- 32. Garcia‐Mesa Y, Pareja‐Galeano H, Bonet‐Costa V, Revilla S, Gomez‐Cabrera MC, Gambini J et al (2014) Physical exercise neuroprotects ovariectomized 3xTg‐AD mice through BDNF mechanisms. Psychoneuroendocrinology 45:154–166. [DOI] [PubMed] [Google Scholar]
- 33. Goedert M, Spillantini MG (2006) A century of Alzheimer's disease. Science 314:777–781. [DOI] [PubMed] [Google Scholar]
- 34. Goedert M, Jakes R, Vanmechelen E (1995) Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 189:167–169. [DOI] [PubMed] [Google Scholar]
- 35. Grundke‐Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule‐associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089. [PubMed] [Google Scholar]
- 36. Heneka MT, O'Banion MK (2007) Inflammatory processes in Alzheimer's disease. J Neuroimmunol 184:69–91. [DOI] [PubMed] [Google Scholar]
- 37. Heyn P, Abreu BC, Ottenbacher KJ (2004) The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta‐analysis. Arch Phys Med Rehabil 85:1694–1704. [DOI] [PubMed] [Google Scholar]
- 38. Hu YS, Xu P, Pigino G, Brady ST, Larson J, Lazarov O (2010) Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer's disease‐linked APPswe/PS1DeltaE9 mice. FASEB J 24:1667–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hyman B, Van Hoesen G, Damasio A, Barnes C (1984) Alzheimer's disease: cell‐specific pathology isolates the hippocampal formation. Science 225:1168–1170. [DOI] [PubMed] [Google Scholar]
- 40. Inestrosa NC, Tapia‐Rojas C, Griffith TN, Carvajal FJ, Benito MJ, Rivera‐Dictter A et al (2011a) Tetrahydrohyperforin prevents cognitive deficit, Abeta deposition, tau phosphorylation and synaptotoxicity in the APPswe/PSEN1DeltaE9 model of Alzheimer's disease: a possible effect on APP processing. Transl Psychiatry 1:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inestrosa N, Godoy J, Vargas J, Arrazola M, Rios J, Carvajal F et al (2013) Nicotine prevents synaptic impairment induced by amyloid‐β oligomers through α7‐nicotinic acetylcholine receptor activation. Neuromolecular Med 15:549–569. [DOI] [PubMed] [Google Scholar]
- 42. Ittner LM, Gotz J (2011) Amyloid‐beta and tau—a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci 12:65–72. [DOI] [PubMed] [Google Scholar]
- 43. Jang M, Bonaguidi M, Kitabatake Y, Sun J, Song J, Kang E et al (2013) Secreted frizzled‐related protein 3 regulates activity‐dependent adult hippocampal neurogenesis. Cell Stem Cell 12:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 99:11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL et al (2004) Synaptic targeting by Alzheimer's‐related amyloid beta oligomers. J Neurosci 24:10191–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT et al (2011) Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer's disease. Curr Alzheimer Res 8:67–80. [DOI] [PubMed] [Google Scholar]
- 47. Loprinzi PD, Herod SM, Cardinal BJ, Noakes TD (2013) Physical activity and the brain: a review of this dynamic, bi‐directional relationship. Brain Res 1539:95–104. [DOI] [PubMed] [Google Scholar]
- 48. Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Hayashida N et al (2012) Exercise is more effective than diet control in preventing high fat diet‐induced β‐amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J Biol Chem 287:23024–23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mandelkow EM, Mandelkow E (2012) Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med 2:a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marques‐Aleixo I, Oliveira P, Moreira P, Magalhães J, Ascensão A (2012) Physical exercise as a possible strategy for brain protection: evidence from mitochondrial‐mediated mechanisms. Prog Neurobiol 99(2):149–162. [DOI] [PubMed] [Google Scholar]
- 51. Meda L, Baron P, Scarlato G (2001) Glial activation in Alzheimer's disease: the role of Abeta and its associated proteins. Neurobiol Aging 22:885–893. [DOI] [PubMed] [Google Scholar]
- 52. Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Molteni R, Ying Z, Gomez‐Pinilla F (2002) Differential effects of acute and chronic exercise on plasticity‐related genes in the rat hippocampus revealed by microarray. Eur J Neurosci 16:1107–1116. [DOI] [PubMed] [Google Scholar]
- 54. Morris M, Maeda S, Vossel K, Mucke L (2011) The many faces of tau. Neuron 70:410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener 6:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid beta‐protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2:a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Murer MG, Yan Q, Raisman‐Vozari R (2001) Brain‐derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol 63:71–124. [DOI] [PubMed] [Google Scholar]
- 58. Neeper SA, Gomez‐Pinilla F, Choi J, Cotman CW (1996) Physical activity increases mRNA for brain‐derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726:49–56. [PubMed] [Google Scholar]
- 59. Nichol KE, Parachikova AI, Cotman CW (2007) Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res 184:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW (2009) Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement 5:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T (2011) Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J 25:3570–3582. [DOI] [PubMed] [Google Scholar]
- 62. Pawlowski J, Dixon‐Ibarra A, Driver S (2013) Review of the status of physical activity research for individuals with traumatic brain injury. Arch Phys Med Rehabil 94:1184–1189. [DOI] [PubMed] [Google Scholar]
- 63. Pei JJ, Braak E, Braak H, Grundke‐Iqbal I, Iqbal K, Winblad B, Cowburn RF (1999) Distribution of active glycogen synthase kinase 3beta (GSK‐3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 58:1010–1019. [DOI] [PubMed] [Google Scholar]
- 64. Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM et al (2007) An in vivo correlate of exercise‐induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A 104:5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pike CJ, Cummings BJ, Cotman CW (1995) Early association of reactive astrocytes with senile plaques in Alzheimer's disease. Exp Neurol 132:172–179. [DOI] [PubMed] [Google Scholar]
- 66. Reiserer RS, Harrison FE, Syverud DC, McDonald MP (2007) Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav 6:54–65. [DOI] [PubMed] [Google Scholar]
- 67. Reyes AE, Chacon MA, Dinamarca MC, Cerpa W, Morgan C, Inestrosa NC (2004) Acetylcholinesterase‐Abeta complexes are more toxic than Abeta fibrils in rat hippocampus: effect on rat beta‐amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am J Pathol 164:2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Richter H, Ambree O, Lewejohann L, Herring A, Keyvani K, Paulus W et al (2008) Wheel‐running in a transgenic mouse model of Alzheimer's disease: protection or symptom? Behav Brain Res 190:74–84. [DOI] [PubMed] [Google Scholar]
- 69. Rogers RL, Meyer JS, Mortel KF (1990) After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc 38:123–128. [DOI] [PubMed] [Google Scholar]
- 70. Schweitzer NB, Alessio HM, Berry SD, Roeske K, Hagerman AE (2006) Exercise‐induced changes in cardiac gene expression and its relation to spatial maze performance. Neurochem Int 48:9–16. [DOI] [PubMed] [Google Scholar]
- 71. Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298:789–791. [DOI] [PubMed] [Google Scholar]
- 72. Selkoe D, Mandelkow E, Holtzman D (2012) Deciphering Alzheimer disease. Cold Spring Harb Perspect Med 2:a011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Serrano‐Pozo A, Frosch M, Masliah E, Hyman B (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shankar GM, Walsh DM (2009) Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener, 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shankar GM, Li S, Mehta TH, Garcia‐Munoz A, Shepardson NE, Smith I et al (2008) Amyloid‐beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 14:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shen H, Tong L, Balazs R, Cotman CW (2001) Physical activity elicits sustained activation of the cyclic AMP response element‐binding protein and mitogen‐activated protein kinase in the rat hippocampus. Neuroscience 107:219–229. [DOI] [PubMed] [Google Scholar]
- 77. Stranahan A, Lee K, Becker K, Zhang Y, Maudsley S, Martin B et al (2010) Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging 31:937–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stranahan AM, Martin B, Maudsley S (2012) Anti‐inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer's disease. Curr Alzheimer Res 9:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA (2006) Vascular endothelial growth factor‐B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol 289:329–335. [DOI] [PubMed] [Google Scholar]
- 80. Takashima A, Noguchi K, Michel G, Mercken M, Hoshi M, Ishiguro K, Imahori K (1996) Exposure of rat hippocampal neurons to amyloid beta peptide (25–35) induces the inactivation of phosphatidyl inositol‐3 kinase and the activation of tau protein kinase I/glycogen synthase kinase‐3 beta. Neurosci Lett 203:33–36. [DOI] [PubMed] [Google Scholar]
- 81. Takashima A, Honda T, Yasutake K, Michel G, Murayama O, Murayama M et al (1998) Activation of tau protein kinase I/glycogen synthase kinase‐3beta by amyloid beta peptide (25–35) enhances phosphorylation of tau in hippocampal neurons. Neurosci Res 31:317–323. [DOI] [PubMed] [Google Scholar]
- 82. Tanzi R (2013) A brief history of Alzheimer's disease gene discovery. J Alzheimers Dis 33 (Suppl. 1):S5–S13. [DOI] [PubMed] [Google Scholar]
- 83. Toledo EM, Inestrosa NC (2010) Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry 15:272–285, 28. [DOI] [PubMed] [Google Scholar]
- 84. Ubhi K, Masliah E (2013) Alzheimer's disease: recent advances and future perspectives. J Alzheimers Dis 33 (Suppl. 1):S185–S194. [DOI] [PubMed] [Google Scholar]
- 85. Um HS, Kang EB, Koo JH, Kim HT, Jin L, Kim EJ et al (2011) Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci Res 69:161–173. [DOI] [PubMed] [Google Scholar]
- 86. van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. [DOI] [PubMed] [Google Scholar]
- 87. van Praag H, Shubert T, Zhao C, Gage FH (2005) Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25:8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Varela‐Nallar L, Inestrosa NC (2013) Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci 7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Varela‐Nallar L, Aranguiz FC, Abbott AC, Slater PG, Inestrosa NC (2010) Adult hippocampal neurogenesis in aging and Alzheimer's disease. Birth Defects Res C Embryo Today 90:284–296. [DOI] [PubMed] [Google Scholar]
- 90. Varela‐Nallar L, Rojas‐Abalos M, Abbott AC, Moya EA, Iturriaga R, Inestrosa NC (2014) Chronic hypoxia induces the activation of the Wnt/beta‐catenin signaling pathway and stimulates hippocampal neurogenesis in wild‐type and APPswe‐PS1DeltaE9 transgenic mice in vivo. Front Cell Neurosci 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vaynman S, Ying Z, Gomez‐Pinilla F (2004) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20:2580–2590. [DOI] [PubMed] [Google Scholar]
- 92. Vincent I, Rosado M, Kim E, Davies P (1994) Increased production of paired helical filament epitopes in a cell culture system reduces the turnover of tau. J Neurochem 62:715–723. [DOI] [PubMed] [Google Scholar]
- 93. Walker JM, Klakotskaia D, Ajit D, Weisman GA, Wood WG, Sun GY et al (2014) Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer's disease mouse model. J Alzheimers Dis 44(2):561–572. [DOI] [PubMed] [Google Scholar]
- 94. Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F (2004) Physical activity, including walking, and cognitive function in older women. JAMA 292:1454–1461. [DOI] [PubMed] [Google Scholar]
- 95. Wisniewski HM, Wen GY, Kim KS (1989) Comparison of four staining methods on the detection of neuritic plaques. Acta Neuropathol 78:22–27. [DOI] [PubMed] [Google Scholar]
- 96. Wojtowicz JM, Kee N (2006) BrdU assay for neurogenesis in rodents. Nat Protoc 1:1399–1405. [DOI] [PubMed] [Google Scholar]
- 97. Xia W (2010) Brain amyloid beta protein and memory disruption in Alzheimer's disease. Neuropsychiatr Dis Treat 6:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yin M, Wang W, Sun J, Liu S, Liu X, Niu Y et al (2013) Paternal treadmill exercise enhances spatial learning and memory related to hippocampus among male offspring. Behav Brain Res 253 (C):297–304. [DOI] [PubMed] [Google Scholar]
- 99. Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM et al (2009) Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis 35:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang W, Hao J, Liu R, Zhang Z, Lei G, Su C et al (2011) Soluble Abeta levels correlate with cognitive deficits in the 12‐month‐old APPswe/PS1dE9 mouse model of Alzheimer's disease. Behav Brain Res 222:342–350. [DOI] [PubMed] [Google Scholar]
- 101. Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Compares the histopathological procedures of wild‐type mice with control APPswe/PS1ΔE9 mice, using the A11, 4G8 and GFAP antibodies and the Th‐S staining.
Figure S2. Shows the BrdU+ cells in the SGZ of transgenic mice compared with wild‐type animals.


