Introduction
If you wrap up different kinds of furniture in enough wrapping paper, you can make them all look the same shape 12. The term “neuroinflammation” has become such a wrapping paper for an increasing number of etiologically distinct central nervous system (CNS) pathologies. As the first article 4 of this symposium explains: “Historically, the term ‘neuroinflammation’ was clearly defined and denoted immune‐driven pathology in the brain. Unfortunately, this clarity has diminished in recent years.” It has in fact diminished to the point that the designation “neuroinflammation” is effectively useless in practice because it denotes both true and pseudo‐inflammation 5. Some recent, attention‐grabbing examples of the latter include air pollution, obesity and sleep loss 2, 3, 16.
As of August 2014, there were 6249 publications and 118 894 citations on the topic of “neuroinflammation” (Figure 1), and the increase is steep. Are most or all of these papers missing the point? Certainly not all, but the problem with a “diagnosis” that has opposite meanings in the literature cannot be solved easily in the face of thousands of publications, many of which are conflicting but cannot realistically be corrected. Therefore, although the term “neuroinflammation” seems doomed for logical reasons alone, a serious effort needs to be made to achieve clarity regarding what the findings reported in these publications mean in the light of state‐of‐the‐art concepts 14, 15.
Figure 1.
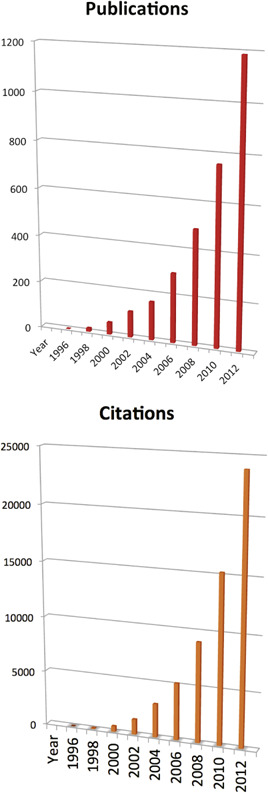
All wrong? Search results for the topic “neuroinflammation”: 6249 new publications and 118 894 citations for the years shown. The increase is extremely rapid. For a discussion, see text. Source: Web of Science™ (Thomson Reuters).
The occurrence of classical inflammation in the CNS is the backdrop against which any consideration of “neuroinflammation” of the non‐autoimmune type 9 has to be viewed and judged. Classical inflammation occurs in the CNS like in any other organ and it shows essentially the same tissue characteristics. Multiple sclerosis is a prime example, as is its animal model, experimental autoimmune encephalomyelitis (EAE). The ensuing idea that inflammation should be recognizable beyond organ borders is strongly supported by recent in silico findings 5. In addition to autoimmune forms, other examples of true neuroinflammation in the CNS include acute and chronic infections, stroke and trauma to name the most important examples. In contrast, the tissue changes observed in Alzheimer's disease, Parkinson's disease and schizophrenia are not even remotely comparable. In other words, they are categorically different as confirmed by unsupervised hierarchical clustering of microarray data 5. Indeed, it has been fairly clear all along that a pathogenetic relationship between these diseases and inflammation was almost exclusively thought of by researchers far away from diagnostic neuropathology—and the relevant tissues to compare observations to. Thus, there can be little doubt that the confusion surrounding “neuroinflammation” is in no small part due to the fact that experienced neuropathologists who are capable of making the distinction between classical inflammation, sterile inflammation and microglial activation, gliosis, etc., are very rare compared with the many neuroscientists who study brain tissue because most countries still do not have the medical specialty. Instead, neuropathology is considered a luxury while funding is spent on more fashionable projects (see below).
The absurdity of the current use of the term “neuroinflammation,” and equally a sign of the hype surrounding it, is perhaps best illustrated by the following quotation: “Neuroinflammation was one of the prominent pathological features described by Alois Alzheimer in his first case report in 1907” 10. Of course, Alois Alzheimer was not only unaware of the term but the findings he reports show the exact opposite of inflammation: “Vascular infiltration is completely lacking. (Eine Infiltration der Gefäße fehlt völlig.)” 1. Anyone who reviews the histological slides of the first case of Alzheimer's disease 7 is able to confirm this.
Neuroimaging studies have played and are playing a major role in the creation of the uncertainty surrounding “neuroinflammation,” which is why articles from the field of neuroimaging 11, 17 feature prominently in this symposium. The papers by Liu et al 11 and Zahr et al 17 question that a specific diagnosis of “neuroinflammation” can be made solely based on magnetic resonance spectroscopy (MRS) 17 and positron emission tomography (PET) 11, respectively. The problem of identifying “neuroinflammation” on the basis of PET results is intricately linked to the assumed functions of the 18‐kDa translocator protein (TSPO) and the definition of microglial activation. We have therefore solicited a large review article on this topic 11.
Microglia are the key resident immune cells of the CNS 8, but their activation does not equal “neuroinflammation.” Numerous publications exist that are based on this unfounded assumption. In healthy CNS and in the vast majority of known CNS diseases, microglia do not present as macrophages and their day‐to‐day function must therefore be different 6. This view is increasingly accepted as demonstrated by a recent article 14: “Microglia can detect, process, and respond to signals in an entirely noninflammatory way. The duality of microglia with noninflammatory as well as inflammatory functions provides a challenge to the concept that all disorders involving microglia are de facto neuroinflammatory disorders. Rather, some CNS disorders, or endophenotypes of disorders, may better be conceptualized as resulting from loss or gain of noninflammatory microglia functions.”
Svahn et al 15 suggest that the pathophysiological context guide nomenclatorial considerations. Thus, the designations development, trauma or pain‐associated microglia are preferred over the traditional but less distinctive “microglial activation.” This proposal may offer a simple solution for the nomenclatorial problem addressed in this editorial.
There is one additional sociological aspect that must not be forgotten in the context of this debate: the universal commercialization of biomedical research. This is a relatively new development and only a few decades old. More and more subspecialty journals have been founded and they need to fill their pages. Journalistic rather than scientific criteria often determine how these public attention‐grabbing publications are run. The structure of research articles has also been modified by some journals in order to combine results and discussion sections. However, this fatally mixes careful description with subjective interpretation. While the former should be objective and timeless, the latter is necessarily biased and time‐dependent. In addition, contemporary funding systems worldwide favor popular and/or intellectual property generating rather than fundamental (blue‐sky) research. Only the latter, however, is designed to get to the bottom of a scientific problem rather than to primarily maximize chances of funding support. These new priorities in research are also reflected by the attempt of science administrators to re‐brand research income as research productivity, a development of Orwellian quality 13. Furthermore, drug companies readily capitalize on the semantic linkage between inflammation and common diseases such as dementia as well as aging.
Taken together, cleaning up the “neuroinflammation” literature will require parsing publications for various conflicts of interest. It is significant that some of the strongest supporters of the attempt to generalize the “neuroinflammation” concept are also paid by drug companies. This particular point deserves detailed attention because the declaration of a conflict of interest does not remove it. It is also not possible to serve two masters at the same time, and no public relations campaign will be able to change that.
The good news is that the large numbers of publications that are being produced (Figure 1) contain many intriguing observations. They form the substance of these publications and the observations are what will matter in the long run rather than their current interpretation. So what is next? The journals specializing in the field of “neuroinflammation” have a key role in organizing and aiding the intelligent reassessment of the finding made so far. For instance, although it may not be called inflammation, the affinity of activated microglial cells for synapses is undeniable and deserves further scrutiny. Perhaps, it is the microglial cells’ interaction and that of astrocytes with synapses that explains most of what is currently being called “neuroinflammation.”
In conclusion, the idea for this mini‐symposium is based on the question whether the presently popular, very broad concept of “neuroinflammation” could be largely due to a lack of access to neuropathological information, relevant tissues and thus represent a misunderstanding. We argue yes, and that it is highly counterproductive. However, as major commitments and investments have been made by a number of parties, ranging from the re‐branding of entire research divisions and laboratories to supranational funding decisions, unanimity cannot be expected quickly.
Acknowledgments
Funding from the Cure Brain Cancer Foundation, Australia, to MBG is gratefully acknowledged.
References
- 1. Alzheimer A (1907) Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr 64:146–148. [Google Scholar]
- 2. Block ML, Calderon‐Garciduenas L (2009) Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C (2014) Obesity induces neuroinflammation mediated by altered expression of the renin‐angiotensin system in mouse forebrain nuclei. Physiol Behav. doi: 10.1016/jphysbeh201401016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estes M, McAllister AK (2014) Alterations in immune cells and mediators in the brain: it's not always neuroinflammation! Brain Pathol 24:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Filiou MD, Arefin AS, Moscato P, Graeber MB (2014) “Neuroinflammation” differs categorically from inflammation: transcriptomes of Alzheimer's disease, Parkinson's disease, schizophrenia and inflammatory diseases compared. Neurogenetics 15:201–212. [DOI] [PubMed] [Google Scholar]
- 6. Graeber MB (2010) Changing face of microglia. Science 330:783–788. [DOI] [PubMed] [Google Scholar]
- 7. Graeber MB, Kosel S, Grasbon‐Frodl E, Moller HJ, Mehraein P (1998) Histopathology and APOE genotype of the first Alzheimer disease patient, Auguste D. Neurogenetics 1:223–228. [DOI] [PubMed] [Google Scholar]
- 8. Graeber MB, Streit WJ (1990) Microglia: immune network in the CNS. Brain Pathol 1:2–5. [DOI] [PubMed] [Google Scholar]
- 9. Kleinewietfeld M, Hafler DA (2014) Regulatory T cells in autoimmune neuroinflammation. Immunol Rev 259:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C et al (2012) Systemic immune challenges trigger and drive Alzheimer‐like neuropathology in mice. J Neuroinflammation 9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu G‐J, Middleton RJ, Hatty CR, Kam WW‐Y, Chan R, Pham T et al (2014) The 18 kD translocator protein, microglia and neuroinflammation. Brain Pathol 24:631–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludwig Wittgenstein cited in Searle JR (1992) The Rediscovery of the Mind. MIT Press: Cambridge, MA, p. 126. ISBN 9780262691543. [Google Scholar]
- 13. Orwell G (1946) Politics and the English language. Available at: https://www.mtholyoke.edu/acad/intrel/orwell46.htm (accessed 18 Aug 2014).
- 14. Salter MW, Beggs S (2014) Sublime microglia: expanding roles for the guardians of the CNS. Cell 158:15–24. [DOI] [PubMed] [Google Scholar]
- 15. Svahn AJ, Becker TS, Graeber MB (2014) Emergent properties of microglia. Brain Pathol 24:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wisor JP, Schmidt MA, Clegern WC (2011) Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. Sleep 34:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zahr NM, Mayer D, Rohlfing T, Sullivan EV, Pfefferbaum A (2014) Imaging neuroinflammation? A perspective from MR spectroscopy. Brain Pathol 24:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]


