Abstract
We quantified vascular changes in the frontal lobe and basal ganglia of four inherited small vessel diseases (SVDs) including cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL), hereditary multi‐infarct dementia of Swedish type (Swedish hMID), and hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS). Vascular pathology was most severe in CADASIL, and varied with marginally greater severity in the basal ganglia compared to the frontal lobe. The overall sclerotic index values in frontal lobe were in the order CADASIL ≥ HERNS > PADMAL > Swedish hMID > sporadic SVD, and in basal ganglia CADASIL > HERNS > Swedish hMID > PADMAL> sporadic SVD. The subcortical white matter was almost always more affected than any gray matter. We observed glucose transporter‐1 (GLUT‐1) protein immunoreactivities were most affected in the white matter indicating capillary degeneration whereas collagen IV (COL4) immunostaining was increased in PADMAL cases in all regions and tissue types. Overall, GLUT‐1 : COL4 ratios were higher in the basal ganglia indicating modifications in capillary density compared to the frontal lobe. Our study shows that the extent of microvascular degeneration varies in these genetic disorders exhibiting common end‐stage pathologies but is the most aggressive in CADASIL.
Keywords: CADASIL, hereditary multi‐infarct dementia, HERNS, PADMAL, sclerotic index, small vessel disease
Introduction
In 1977, Sourander and Wålinder described cases of cerebrovascular disease associated with progressive dementia with autosomal dominant inheritance within a Swedish family 18. This was thought to be the first report of the most common form of familial vascular dementia, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) 7, 8, 11. However, we 13 demonstrated that these patients did not exhibit NOTCH3 gene‐linked CADASIL but suffered from hereditary multi‐infarct dementia (hMID) of the Swedish type, a distinct familial small vessel disease (SVD). Another German family with similar progressive dementia showing autosomal dominant inheritance was reported by Hagel et al 5. This family showed similar early onset of SVD‐type dementia, but, like the Swedes, was not linked to the NOTCH3 gene and lacking the hallmark features of CADASIL pathology including granular osmiophilic material (GOM) in vessel walls 5. The family was described to exhibit pontine infarcts and their condition was termed pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL) 3. Any genetic abnormalities involved in both the Swedish and German familial disorders remain unknown. Hereditary endotheliopathy with retinopathy, nephropathy and stroke (HERNS) was first reported in a Chinese family 6, and was later shown to be caused by mutations in the TREX1 gene on chromosome 3p, common to the retinal vasculopathy with cerebral leukodystrophy disorders 16, 17, 20. HERNS is described by progressive neurologic decline, beginning with retinal deterioration in the third or fourth decades, focal neurological deficits beginning around 4–10 years later, and with progression toward SVD‐type dementia; renal dysfunction is also a key indicator for the disease 6.
Until now, the relative degrees of cerebral vascular involvement within the subcortical structures including the white matter and the basal ganglia in CADASIL, HERNS, Swedish hMID and PADMAL have not been explored 20. We therefore quantified vascular changes in these hereditary SVDs in two key brain regions, the frontal lobe and subcortical gray and white matter, with a view to distinguishing different mechanisms of vascular degeneration. Using conventional histopathology and immunohistochemical methods we studied three main components of the arterial structure. The glucose transporter‐1 (GLUT‐1) protein was demonstrated to examine the cerebral endothelium, smooth muscle cell α‐actin (SMA) for vascular smooth muscle cells (VSMCs), and collagen IV (COL4) as a marker of the basement membrane. We also assessed the extent of vascular pathology and the sclerotic index (SI) in the two brain regions 12, 14, 21.
Methods
Subjects
The demographic details of the subjects studied are given in Table 1. The mean ages of the subjects with the familial SVDs were not different except the Swedish hMID group, due to one young subject (IV:1) 22. We also compared the familial disorders with subjects diagnosed with sporadic SVD, and young and older controls without any evident neurological or psychiatric conditions. CADASIL and HERNS diagnoses were confirmed by NOTCH3 and TREX1 sequencing (Table 1). The subject with HERNS, described previously 1, presented with asymptomatic retinopathy, cerebral microvascular disease, nephropathy and raised inflammatory markers. The PADMAL and Swedish hMID cases were known to be wild type for NOTCH3 5, 13 and they were also negative for the known mutations in the HTRA1 and TREX1 genes (Gregor Kuhlenbaeumer et al, unpub. obs.). The available Mini‐Mental State Examination scores for SVD cases ranged from 16 to 27, with a mean score of 21.25. The mean duration of disease in the hereditary SVD cases from onset of first symptom to death ranged from 8.6 to 11.6 years and not different between groups (P > 0.05).
Table 1.
Demographic details of subjects and causes of death. Abbreviations: CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; GI = gastrointestinal; HERNS = hereditary endotheliopathy with retinopathy, nephropathy, and stroke; n = number; PADMAL = pontine autosomal dominant microangiopathy and leukoencephalopathy; SVD = small vessel disease; Swedish hMID = hereditary multi‐infarct dementia of Swedish type; UTI = urinary tract infection; TREX1 = three prime repair exonuclease‐1; N/A = not available
| Young controls | Old controls | SVD|| | CADASIL | PADMAL | Swedish hMID | HERNS | |
|---|---|---|---|---|---|---|---|
| n (total = 50) | 11 | 10 | 10 | 9 | 5 | 4 | 1 |
| Mean age (years) | 57.5 | 83.4 | 81.0 | 57.8 | 50.0 | 42.5 | 39 |
| Range (years) | 49–69 | 78–94 | 67–96 | 44–68 | 41–59 | 30–48 | — |
| Gender (M/F) | 6/5 | 2/8 | 5/5 | 6/3 | 2/3 | 2/2 | 0/1 |
| Mean age at onset (years) | — | — | N/A | 46.9 | 38.4 | 33.9 | 26 |
| Duration of disease (years) | — | — | N/A | 10.9 | 11.6 | 8.6 | 13 |
| Gene mutations | — | — | — | NOTCH3: R133C (2), R141C (3), R153C (1), R169C (3), R558C (1) | — | — | TREX1—3835_3836 insA reference R284 |
| Notable clinical features | — | — | White matter disease, lacunar strokes, dementia | Migraine, TIA, lacunar strokes, dementia | Gait disturbances, emotional disturbances, lacunar strokes, epilepsy in one case, dementia | Gait disturbances, emotional disturbances, lacunar strokes, dementia | Retinopathy, lacunar strokes, cognitive impairment |
| Cause(s) of death | Cardiac arrest, cancer, renal failure, infection | Heart failure, cancer, ischemic bowel infection | Heart failure, cancer, GI bleed, sudden death | Bronchopneumonia, heart failure | Pulmonary embolism, heart failure, suicide | Respiratory failure, cerebral hemorrhage, dementia | UTI |
| Neuropathological features | |||||||
| Braak staging | 0 | 0–4 | 1–4 | 0 | 0 | 0 | 0 |
| CERAD score | 1 | 2 | 0–2 | 0 | 0 | 0 | 0 |
| Median vascular pathology score /10 | 3 | 5.5 | 6 | 8 | 7 | 5.5 | 6 |
| Range | 2–6 | 4–8 | 5–7 | 6–10 | 6–8 | 5–9 | — |
| Sclerotic index | |||||||
| Frontal gray matter† | 0.28 | 0.29 | 0.32 | 0.33* | 0.33* | 0.28 | 0.37 |
| Frontal white matter†† | 0.25 | 0.27 | 0.30 | 0.39** | 0.31* | 0.30 | 0.31 |
| ganglia gray matter‡ | 0.29 | 0.27 | 0.27 | 0.35* | 0.27 | 0.33 | 0.31 |
| Basal ganglia white matter‡‡ | 0.28 | 0.25 | 0.28 | 0.37* | 0.28 | 0.34 | 0.38 |
Mean age of young controls (Y Con) was not different from CADASIL and PADMAL, and the mean age of the old controls (O Con) was not different from SVD group. Mean age of Swedish hMID group was different from Y Con and CADASIL (P < 0.05) but not from PADMAL. The post‐mortem interval between death and immersion fixation of tissue ranged from 9 to 96 h. The length of fixation of tissues prior to paraffin embedding ranged from 1 to 7 months.
||In most of the subsequent quantitative analysis as indicated we used n = 8 SVD cases.
Sclerotic index: *P < 0.05, different from Y Con; **P < 0.05, different from all groups. ANOVA and post‐hoc analysis, †P = 0.41, CADASIL and PADMAL different from Y Con, old controls (O Con) and Swedish hMID; ††P = 0.000, all groups; ‡P < 0.002, CADASIL was different from all groups, except Swedish hMID, but Swedish hMID was different from O Con and SVD; ‡‡P = 0.000, CADASIL was different from all groups, except Swedish hMID, but Swedish was different from O Con.
Post‐mortem brain tissues
Coronal blocks of formalin‐fixed brain tissues containing the superior frontal lobe and basal ganglia at level of the anterior commissure from the hereditary SVD subjects were obtained from several sources. Sources of the CADASIL and Swedish hMID cases were described previously 13, 21. The PADMAL cases were provided by Christian Hagel 5. The HERNS case was characterized and provided by Catriona McLean 1. Unless otherwise stated, all the tissues were processed for fixation in 10% buffered formalin and embedding in paraffin following standard protocols established in clinical histopathology services. As previous studies have suggested, these two regions have the most profound pathology in CADASIL. We focused on these two brain regions also given that samples from other brain regions were not consistently available (Table 1). Similarly, brain tissue was obtained from the Newcastle Brain Tissue Resource from cognitively normal controls with similar ages to hereditary SVD cases, subjects with pathologically confirmed SVD 9, and cognitively normal subjects age matched to the SVD group.
Histopathology and immunohistochemistry
Paraffin‐embedded tissue blocks containing the basal ganglia and frontal cortex with underlying white matter were serially cut into 10‐μm‐thick sections. For vascular pathology and SI analysis, sections were stained with Luxol Fast Blue (LFB), Cresyl Fast Violet (CFV), and Hematoxylin and Eosin (H&E), respectively. For immunohistochemistry of vascular markers, tissue sections were stained and analyzed as previously described [6]. Antibodies used included smooth muscle anti‐actin for VSMCs (SMA, 1 in 1000 dilution, clone 1A4 Dako), GLUT‐1 for vascular endothelial cells (1:200 dilution, antibody 21041, Thermo Scientific, Rockford, IL, USA), and anti‐COL4 for basement membrane (1 in 1000 dilution, antibody C1926, Sigma, Dorset, UK). Immunohistochemical stains were assessed for the percent area of an image covered by the stain to indicate vascular density using Image Pro Plus (V.4.0, Media Cybernetics, Silver Spring, MD, USA). None of the cases and controls met pathological criteria for any other neurodegenerative disease. There was no evidence for high burden of amyloid or tau accumulation in any of the familial SVDs 5, 13, 20. To decipher if there were differences in vascular smooth muscle degeneration in arterioles in the familial disorders, we also stained sections with antibodies to ubiquitin (1:200 dilution, Dako Cytomation, Carpinteria, CA, USA), fractin (1:100, Millipore, Billerica, MA, USA) and HSP27 (1:100, NovoCastra, Bucks, UK) 21. Microglia and macrophages were immunostained with antibodies to CD68 (1:200, Dako Cytomation) and CD45 (1:100, Dako Cytomation).
Vascular pathology scores
Cerebrovascular lesions including SVD pathology were assessed as described previously 2. A score out of 10 was applied to each case, calculated from a subtotal score out of 6 in the frontal region and 4 in the basal ganglia. Vascular scores were not significantly different between the two operators (VD and RNK) with over 90% agreement. The mean of the two sets of scores was used in the final analysis. Cross‐tabulations were performed to identify frequency of cases per score using seven parameters assessed in the frontal region and four parameters in the basal ganglia (data not shown).
Sclerotic index
SI was determined as described previously 21. SI value of greater than 0.3 is considered to be a state of mild SVD, and an SI greater than 0.5 is considered to be severe 12. Arterioles with external diameter between 35 and 350 μm within the region of interest were imaged using a Zeiss Axioplan 2 microscope (Carl Zeiss Microscopy, Thornwood, NY, USA) and Image capture software (Infinity Capture V4.6.0, Lumenera Corporation, Ontario, Canada) using 10‐μm sections stained with H&E. To provide consistency in sampling between sections, regions of interest were limited to gray (caudate and putamen) and white matter (internal and external capsules) of the basal ganglia, and one gyrus of frontal cortex with the underlying white matter (approximate Brodmann areas 8 or 9 of dorsolateral prefrontal cortex). It was expected that the vessels sampled in these regions would provide a representative sample of vessels for comparative purposes. Changes in SI of blood vessels as they penetrate through the cortex into white matter and the underlying deeper white matter tracts have been previously reported 15.
Statistical analysis
Results were analyzed using SPSS (IBM, V 19.0, IBM Corporation, Armonk, NY, USA). Graphical data are presented as means ± 2SEM and values of P < 0.05 were considered significant. Where appropriate following analysis of variance (ANOVA), post‐hoc analysis was performed using least significant difference (LSD). Vascular scores used ordinal data and were analyzed for between‐group effects using Kruskal–Wallis H test. The HERNS case was omitted from the ANOVA analysis for between‐group effects on SI and immunohistochemical staining, but included in the Kruskal–Wallis H test for vascular scores and in the regression analysis, which used SI values for all vessels analyzed per case. A linear regression model was used to assess the relationship between SI and potential predictive values age, gender, brain region (using dummy variables of frontal, 1; basal ganglia, 0), tissue type (dummy variable gray matter, 1; white matter, 0) and 1/external diameter of vessel.
Results
General vascular pathology
Limiting the analysis to the frontal and basal ganglia regions, the overall burden of vascular pathology was variable depending on the type of disorder (Figure 1). Quantification revealed vascular changes were most severe in CADASIL, followed by PADMAL and sporadic SVD with less pathology, and the Swedish hMID cases showing an even lower degree of pathology (Table 2). As expected, young and older controls exhibited low scores (scores ≤5 compared to >5 in groups exhibiting dementia). In the basal ganglia region, high vascular pathology scores were more common in CADASIL and PADMAL compared to the SVD, Swedish hMID and HERNS case (Table 2)
Figure 1.
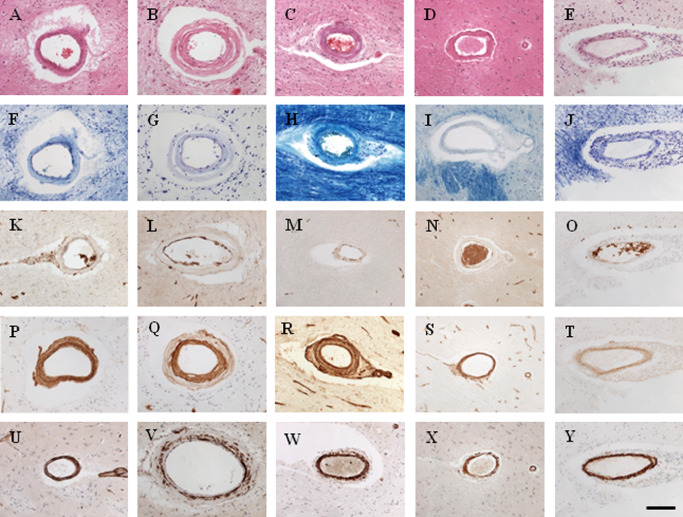
Representative images of cerebral vessels (arterioles) in familial SVDs, and young and old controls. Key to panel: images in column A, sporadic SVD; column B, CADASIL; column C, PADMAL; column D, Swedish hMID; column E, HERNS. Row: conventional stains and immunostains as follows: A–E, H&E; F–J, Luxol Fast Blue‐Cresyl Violet; K–O, GLUT‐1; P–T, COL4; U–Y, smooth muscle cell α‐actin (SMA). Magnification bar: 50 μm
Table 2.
Relative degrees of severity of vascular pathology in different disorders. Abbreviations: Asymp. sig = level of significance; d.f. = degrees of freedom; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; SVD = small vessel disease; PADMAL = pontine autosomal dominant microangiopathy and leukoencephalopathy; Swedish hMID = hereditary multi‐infarct dementia of Swedish type; HERNS = hereditary endotheliopathy with retinopathy, nephropathy, and stroke
| Mean rank/Kruskal–Wallis | Frontal summary score/6 | Overall group rank | Basal ganglia summary score/4 | Overall group rank | Total score/10 | Overall group rank |
|---|---|---|---|---|---|---|
| Young control | 9.50 | 7 | 13.32 | 7 | 7.31 | 7 |
| Old control | 18.50 | 6 | 22.10 | 6 | 17.44 | 6 |
| SVD | 23.00 | 2 | 22.63 | 5 | 21.07 | 4 |
| CADASIL | 30.88 | 1 | 38.00 | 1 | 33.88 | 1 |
| PADMAL | 23.00 | 2 | 32.90 | 2 | 28.00 | 2 |
| Swedish hMID | 22.75 | 5 | 24.50 | 3 | 20.75 | 5 |
| HERNS | 23.00 | 2 | 23.00 | 4 | 21.50 | 3 |
| Total (n cases) | 41 | 50 | 41 | |||
| Chi‐square | 19.181 | 19.131 | 23.052 | |||
| d.f. | 6 | 6 | 6 | |||
| Asymp. sig. | 0.004 | 0.004 | 0.001 |
Numbers computed for the Kruskal–Wallis H test for mean rank scores for vascular pathology. The cases were different, overall ranks for each group were listed in order of severity from highest rank score = 1 to lowest score = 7. Scoring based on modified methods was described by Deramecourt et al [18].
In the frontal region, the extent of hyalinosis was highest in the CADASIL group followed by SVD then the PADMAL subjects. Dilated perivascular space (PVS) in white matter and juxtacortical regions were most severe and frequent in CADASIL cases, followed by PADMAL, SVD and HERNS. CADASIL, SVD, and PADMAL groups and the HERNS case were found to have the most severe white matter loss. Microinfarcts were most common in the CADASIL group, while two CADASIL cases [CAD7 and CAD9 in 21] and one Swedish hMID case had larger infarcts of >0.5 cm. Focal hemosiderin deposits possibly representing microbleeds were found to be present in all disease groups and cognitively normal older controls, although CADASIL cases clearly exhibited the highest frequency.
In the basal ganglia, hyalinosis was also severe in the CADASIL cases followed by SVD cases, while PADMAL, Swedish hMID and HERNS scored similar to cognitively normal controls. Dilated PVS was most severe in the CADASIL and PADMAL groups but SVD, Swedish hMID and HERNS groups were comparable to cognitively normal controls, and the lowest scores were observed in the young Swedish hMID case. The HERNS case was also found to exhibit severe thickening of the vessel wall, although the vessel walls were found to be infiltrated with CD45 and CD68 positive macrophages as opposed to hyalinosis as observed in the other hereditary SVDs (Supporting Information Figure S1). This influx of inflammatory cells may be due to systemic sepsis as a result of the primary urinary tract infection. As expected, the presence of multiple microinfarcts was most frequent in the CADASIL cases, whereas only three of the five PADMAL cases showed multiple infarcts, and the Swedish hMID and HERNS case had even fewer.
We used the Kruskal–Wallis H test to compare vascular scores for the frontal and basal ganglia regions and then the total score out of 10. We found differences between groups in all three parameters (P = 0.004, 0.004 and 0.001, respectively, Table 2). CADASIL cases were ranked with the highest levels of pathology, followed by PADMAL, then Swedish hMID and HERNS; SVD ranked second with PADMAL in the frontal region but was ranked lower in the basal ganglia and overall scores, while old controls and young controls exhibited least pathology on this comparative basis (Table 2).
Sclerotic index
The extent of arterial vessel wall changes varied across the SVD‐related dementia groups and between brain regions. We examined cross‐sectional profiles of >5200 randomly selected blood vessels to determine the SI of arterioles in gray and white matter of both frontal and basal ganglia regions (Table 3). It was readily evident that some vessels in all groups had an SI equivalent to disease state (SI > 0.3, [13]). However, both the hereditary and sporadic SVD groups exhibited vessels with higher SI values than those observed in cognitively normal controls (Table 1). Our results showed that SI was not strongly associated with increasing age even when incorporating all samples in regression models (see below). CADASIL cases showed the most severe sclerosis, with a greater proportion of blood vessels showing not only disease state, but large numbers of severely affected vessels with an SI value of ≥0.5 (Table 3). The majority of vessels in cognitively normal controls had normal SI values of less than 0.3, representing 64.2% in the frontal region and 60.1% basal ganglia of the young controls, and 61.2% and 61.6% of the same regions in aged controls. This fraction was markedly lower in disease states, where less than 45% of blood vessels in both regions of CADASIL and HERNS had vessel walls with SI ≤ 0.3.
Table 3.
Stratified sclerotic index values within brain regions relative to total vascular profiles. Abbreviations: GM = gray matter; WM = white matter; SVD = small vessel disease; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; PADMAL = pontine autosomal dominant microangiopathy and leukoencephalopathy; Swedish hMID = hereditary multi‐infarct dementia of Swedish type; HERNS = hereditary endotheliopathy with retinopathy, nephropathy, and stroke
| Frontal lobe | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of vessels measured | All vessels | Vessels ≤0.3 | Vessels >0.3 and <0.5 | Vessels ≥0.5 | ||||||||
| Group | Total | GM | WM | Total | GM | WM | Total | GM | WM | Total | GM | WM |
| Young control | 495 | 220 | 275 | 318 | 113 | 205 | 167 | 69 | 98 | 10 | 9 | 1 |
| Old control | 531 | 242 | 289 | 325 | 133 | 192 | 195 | 92 | 103 | 11 | 6 | 5 |
| SVD | 567 | 283 | 284 | 276 | 124 | 152 | 251 | 136 | 115 | 36 | 23 | 13 |
| CADASIL | 244 | 137 | 107 | 107 | 61 | 46 | 119 | 49 | 70 | 18 | 6 | 12 |
| PADMAL | 287 | 123 | 164 | 137 | 55 | 82 | 142 | 78 | 64 | 8 | 4 | 4 |
| Swedish hMID | 182 | 77 | 105 | 105 | 49 | 56 | 69 | 42 | 27 | 8 | 1 | 7 |
| HERNS | 62 | 32 | 30 | 22 | 7 | 15 | 36 | 14 | 22 | 4 | 3 | 1 |
| % of vessels measured | All vessels | Vessels ≤0.3 | Vessels >0.3 and <0.5 | Vessels ≥0.5 | ||||||||
| Group | GM | WM | Total | GM | WM | Total | GM | WM | Total | GM | WM | |
| Young control | 44.4 | 55.6 | 64.2 | 22.8 | 41.4 | 33.7 | 13.9 | 19.8 | 2.0 | 1.8 | 0.2 | |
| Old control | 45.6 | 54.4 | 61.2 | 25.0 | 36.2 | 36.7 | 17.3 | 19.4 | 2.1 | 1.1 | 0.9 | |
| SVD | 49.9 | 50.1 | 48.7 | 21.9 | 26.8 | 44.3 | 24.0 | 20.3 | 6.3 | 4.1 | 2.3 | |
| CADASIL | 56.1 | 43.9 | 43.9 | 25.0 | 18.9 | 48.8 | 20.1 | 28.7 | 7.4 | 2.5 | 4.9 | |
| PADMAL | 42.9 | 57.1 | 47.7 | 19.2 | 28.6 | 49.5 | 27.2 | 22.3 | 2.8 | 1.4 | 1.4 | |
| Swedish hMID | 42.3 | 57.7 | 57.7 | 26.9 | 30.8 | 37.9 | 23.1 | 14.8 | 4.4 | 0.5 | 3.8 | |
| HERNS | 51.6 | 48.4 | 35.5 | 11.3 | 24.2 | 58.1 | 22.6 | 35.5 | 6.5 | 4.8 | 1.6 | |
| Basal ganglia | ||||||||||||
| Number of vessels measured | All vessels | Vessels ≤0.3 | Vessels >0.3 and <0.5 | Vessels ≥0.5 | ||||||||
| Group | Total | GM | WM | Total | GM | WM | Total | GM | WM | Total | GM | WM |
| Young control | 642 | 354 | 288 | 386 | 204 | 182 | 241 | 141 | 100 | 15 | 9 | 6 |
| Old control | 513 | 250 | 263 | 316 | 143 | 173 | 186 | 100 | 86 | 11 | 7 | 4 |
| SVD | 427 | 201 | 226 | 253 | 122 | 131 | 143 | 69 | 74 | 31 | 10 | 21 |
| CADASIL | 548 | 296 | 252 | 177 | 99 | 78 | 262 | 149 | 113 | 109 | 48 | 61 |
| PADMAL | 360 | 170 | 90 | 208 | 103 | 105 | 150 | 66 | 84 | 2 | 1 | 1 |
| Swedish hMID | 351 | 194 | 157 | 182 | 95 | 87 | 140 | 84 | 56 | 29 | 15 | 14 |
| HERNS | 84 | 46 | 38 | 29 | 20 | 9 | 50 | 26 | 24 | 5 | 0 | 5 |
| % of vessels measured | All vessels | Vessels ≤0.3 | Vessels >0.3 and <0.5 | Vessels ≥0.5 | ||||||||
| Group | GM | WM | Total | GM | WM | Total | GM | WM | Total | GM | WM | |
| Young control | 55.1 | 44.9 | 60.1 | 31.8 | 28.3 | 37.5 | 22.0 | 15.6 | 2.3 | 1.4 | 0.9 | |
| Old control | 48.7 | 51.3 | 61.6 | 27.9 | 33.7 | 36.3 | 19.5 | 16.8 | 2.1 | 1.4 | 0.8 | |
| SVD | 47.1 | 52.9 | 59.3 | 28.6 | 30.7 | 33.5 | 16.2 | 17.3 | 7.3 | 2.3 | 4.9 | |
| CADASIL | 54.0 | 46.0 | 32.3 | 18.1 | 14.2 | 47.8 | 27.2 | 20.6 | 19.9 | 8.8 | 11.1 | |
| PADMAL | 47.2 | 25.0 | 57.8 | 28.6 | 29.2 | 41.7 | 18.3 | 23.3 | 0.6 | 0.3 | 0.3 | |
| Swedish hMID | 55.3 | 44.7 | 51.9 | 27.1 | 24.8 | 39.9 | 23.9 | 16.0 | 8.3 | 4.3 | 4.0 | |
| HERNS | 54.8 | 45.2 | 34.5 | 23.8 | 10.7 | 59.5 | 31.0 | 28.6 | 6.0 | 0.0 | 6.0 | |
Number of vessels measured to determine sclerosis in the regions of brain stratified for vessels with SI values of either ≤0.3, between 0.3 and 0.5, and ≥0.5. Numbers below expressed as a percent of total vessels measured. The bold % numbers indicate in range the high to low values for different groups.
In the frontal region, the overall burden of arteriolosclerosis was similar between groups, with CADASIL and HERNS exhibiting the greatest number of vessels with SI ≥ 0.5 (7.4 and 6.5%, respectively). In the PADMAL cases, the proportion of vessels with severe sclerosis was estimated to be 2.8% but still not as extensive as those in the SVD and Swedish hMID cases (6.3 and 4.4%, respectively). The highest burden of sclerosis was evident in the basal ganglia of CADASIL patients, where 19.9% of vessel walls had SI ≥ 0.5 with progressively lower frequencies in sporadic SVD (7.3%), Swedish hMID (8.3%) and HERNS (6.0%) cases, and, surprisingly, even fewer in PADMAL (0.6%). Despite this, 41.7% of vessels in PADMAL were found to have an SI value of >0.3, indicating a significant burden of arteriosclerosis. Overall, these results indicated that the presence of severely sclerosed vessels with SI ≥ 0.5 varied between different hereditary and sporadic SVDs but the greatest distinction between regions was in PADMAL. This analysis also indicated that SI value of >0.3 in a significant density of blood vessels was associated with SVD‐related dementias.
We also performed ANOVA of the mean SI values for gray matter and white matter in each region of the brain (Table 1). The SI value for the HERNS case is shown for comparison but was not included in the ANOVA. The mean SI values in the frontal gray matter showed a trend to be different between groups (ANOVA P = 0.041), where post‐hoc analysis between groups indicated that CADASIL and PADMAL vessels were significantly higher than young controls, old controls and the Swedish hMID (P < 0.05). However, the mean SI of arterioles in SVD patients was not significantly different from any other group. SI values in the subcortical white matter were significantly different between groups (P = 0.000), whereas CADASIL vessels showed significantly more sclerosis compared to all other groups (P < 0.05). Additionally, vessels in the frontal white matter of PADMAL and SVD groups showed a higher degree of sclerosis in vessels compared to young controls (P < 0.05). In the basal ganglia region, arterioles of CADASIL and Swedish hMID patients showed the highest degree of sclerosis, where the mean SI of vessels in SVD and PADMAL cases was not greater than 0.3 (Table 1). SI values in both gray and white matter of the basal ganglia were significantly different between groups (ANOVA, P = 0.002 and 0.000, respectively). Post‐hoc testing revealed CADASIL vessels showed significantly more sclerosis compared to all groups except Swedish hMID in both gray matter and white matter. The arterioles of Swedish hMID patients were also more sclerosed than vessels of both old controls and SVD groups in gray matter, and compared to old controls only in the white matter (P < 0.05).
We used linear regression analysis to test for variables considered as predictors of SI. This required transformation of the external diameter data to create a linear relationship. We found an inverse relationship between the extent of sclerosis and the external diameter of the vessel, for example, smaller arteriole exhibited greater SI values indicating the effect of vessel wall thickening has a greater effect on smaller vessels. There was a positive linear correlation in all groups (P < 0.05) between SI and the inverse of the external diameter (1/external diameter, Spearman's rho, P < 0.000, data not shown). In order to investigate which parameters could contribute to variation in sclerosis of arterioles, we used a linear regression model to test the effect of 1/external vessel diameter, brain region, tissue type (gray vs. white matter), age and gender within each group. A linear regression model using all five factors showed the variability in SI could be explained by the combination of these factors for 12.3% in old controls and up to 22.8% in PADMAL group (Table 4, P < 0.001).
Table 4.
Sclerotic index tested against various factors in regression models. Abbreviations: ANOVA P = analysis of variance probability values; SVD = small vessel disease; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; PADMAL = pontine autosomal dominant microangiopathy and leukoencephalopathy; Swedish hMID = hereditary multi‐infarct dementia of Swedish type; HERNS = hereditary endotheliopathy with retinopathy, nephropathy, and stroke
| Model A | 1/external diameter | Age | Dummy tissue type | Dummy brain region | Gender | Adjusted R square | ANOVA P |
|---|---|---|---|---|---|---|---|
| Young control | 0.475*** | 0.056* | 0.059* | −0.231*** | 0.003 | 0.202 | 0.000 |
| Old control | 0.353*** | 0.066 | 0.052 | −0.081* | −0.035 | 0.123 | 0.000 |
| SVD | 0.426*** | 0.024 | −0.026 | −0.074* | −0.226*** | 0.175 | 0.000 |
| CADASIL | 0.345*** | 0.020 | −0.099** | −0.250*** | 0.009 | 0.124 | 0.000 |
| PADMAL | 0.438*** | 0.216*** | −0.077* | 0.099** | 0.229*** | 0.228 | 0.000 |
| Swedish hMID | 0.311*** | −0.309*** | −0.084* | −0.116** | 0.023 | 0.184 | 0.000 |
| Model B | 1/external diameter | Dummy tissue type | Dummy brain region | Adjusted R square | ANOVA P | ||
|---|---|---|---|---|---|---|---|
| Young control | 0.474*** | 0.059* | −0.239*** | 0.201 | 0.000 | ||
| Old control | 0.357*** | 0.050 | −0.098** | 0.119 | 0.000 | ||
| SVD | 0.393*** | −0.072* | −0.021 | 0.127 | 0.000 | ||
| CADASIL | 0.345*** | −0.099** | −0.256*** | 0.126 | 0.000 | ||
| PADMAL | 0.440*** | −0.052 | 0.101** | 0.213 | 0.000 | ||
| Swedish hMID | 0.285*** | −0.044 | −0.134** | 0.089 | 0.000 | ||
| HERNS | 0.493*** | −0.241** | 0.016 | 0.201 | 0.000 |
Model A: Predictors: (constant), gender, dummy tissue type, age, inverse external diameter, dummy brain region; dependent variable: SI. Model B: Predictors: (constant), dummy brain region, inverse external diameter, dummy tissue type; dependent variable: SI. Significance: *P < 0.05, **P < 0.01, ***P < 0.001.
In a second model, controlling for 1/external diameter, brain region, and gray or white matter, 1/external diameter showed consistently significant correlations with a high adjusted R square value in all disease groups (Table 4). This model was able to explain 8.9% of variation in the Swedish hMID group, but accounted for over 20% of variation in SI in the young control, PADMAL and HERNS groups. Overall, gray or white matter and brain region showed some dependence in both models; however, the strongest and most consistent contributor to SI was 1/external diameter, showing that the vessels that are most vulnerable or affected by sclerosis are those with smaller external diameters. Surprisingly, age did not play a significant contribution to SI values, although there was some contribution in the Swedish hMID and PADMAL groups, but this may be confounded due to the sudden early deaths of cases included in our cohort.
Immunohistochemical analysis of markers of vessel structure
Although endothelial abnormalities and blebbing were evident in individual CADASIL and HERNS cases, GLUT‐1 immunostaining densities were not different in the frontal gray matter or white matter (ANOVA P = 0.532 and P = 0.109, respectively, Figure 2A). There was no apparent change in GLUT‐1 immunostaining in the gray matter of the basal ganglia (P = 0.671), but a trend was observed in the white matter (P = 0.063, Figure 2B). COL4 immunostaining was significantly different in the frontal gray matter (ANOVA P < 0.001) where the density of staining was increased in PADMAL cases compared to other groups (P < 0.05, Figure 2C). In the frontal subcortical white matter, there was also a clear change (P = 0.046), where post‐hoc tests revealed SVD had higher staining compared to the old control group, and PADMAL had higher staining compared to the young control groups. In the basal ganglia, the PADMAL group showed increased COL4 staining compared to all groups in both gray and white matter (ANOVA P = 0.000 for both gray and white matter, post‐hoc comparison P < 0.05, Figure 2D). COL4 was significantly higher in the lenticular nucleus of Swedish hMID compared to all groups except CADASIL (LSD, P < 0.05), but there was no significant effect observed in the white matter of the basal ganglia.
Figure 2.
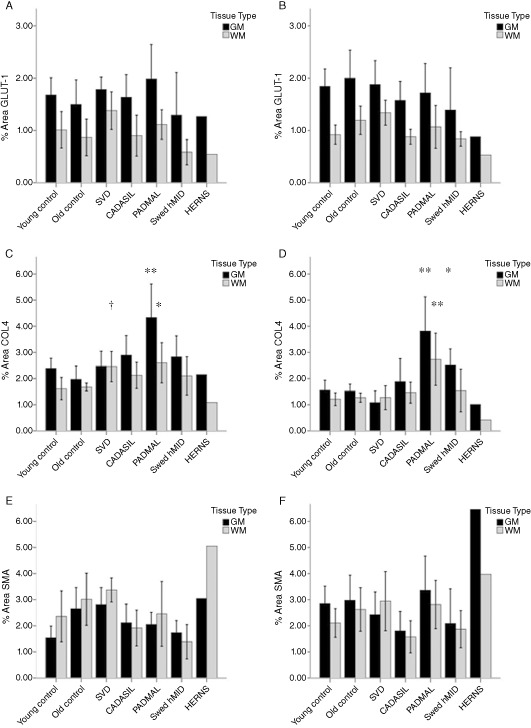
Mean (±2SEM) percent area stained with vascular markers in all groups and brain regions. Key: black bars are gray matter and gray bars are white matter. Post‐hoc tests performed following ANOVA: *P < 0.05, different from young control group; **P < 0.01, significantly different from all groups. †Different from old control group. A. GLUT‐1 % area in frontal region; B. GLUT‐1 % area in basal ganglia; C. COL4 percent area in frontal region; D. COL4 in basal ganglia; E. SMA % area in frontal region; F. SMA % area in basal ganglia.
The percent area of SMA stain was different in the gray matter but did not reach significance in the white matter in the frontal regions (P = 0.036 and P = 0.096, respectively, Figure 2E), where post‐hoc tests showed SMA was increased in the frontal gray matter of older cognitively normal controls and sporadic SVD group compared to the young controls (P < 0.05). This effect was not apparent in the basal ganglia where the ANOVA did not show significance (Figure 2F) (P = 0.319 and 0.219 for gray and white matter, respectively). There appeared to be an increase in the PADMAL group, and some reduction in SMA staining in the CADASIL and Swedish hMID groups, although this did not reach significance. Cerebral vessels immunostained with antibodies to fractin, ubiquitin and HSP27 showed variable globular to punctuate reactivities within the walls of arterioles with degenerating VSMCs that could not be readily distinguished between the four familial disorders (data not shown).
GLUT‐1 : COL4 ratios were altered in the frontal region, where Swedish hMID had lower GLUT‐1 : COL4 ratios than young and old controls in the white matter (ANOVA P = 0.036, post‐hoc LSD, P < 0.05), and although the GLUT‐1 : COL4 ratios were lower in hereditary SVDs in the frontal white matter, this did not reach significance (ANOVA P = 0.138, Figure 3A). The overall GLUT‐1 : COL4 ratios were higher in the basal ganglia, indicating a larger area of capillary density in this brain region; however, there was no significant effect between groups, even though again the PADMAL group did have numerically lower ratios compared to other groups (P = 0.141 and 0.358 for gray matter and white matter, respectively, Figure 3B).
Figure 3.
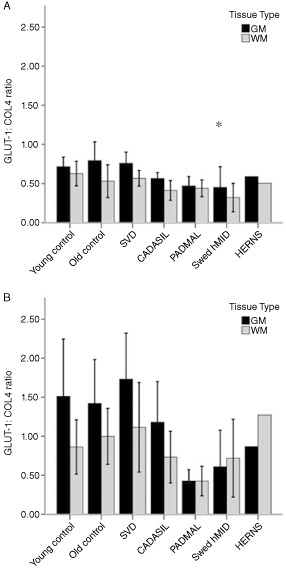
Mean (±2SEM) GLUT‐1 : COL4 ratios in all groups and brain regions. Key: black bars are gray matter and gray bars are white matter. A. Frontal region; B. Basal ganglia. *P < 0.05 indicates different from young controls by post‐hoc testing by LSD.
Discussion
Our results provide unique insights into the burden of vascular pathology in sporadic SVD and four genetic forms of SVDs with dementia. First, we have shown that CADASIL exhibits the most aggressive vascular pathology, and affects vessels in the frontal cortical regions as much as it affects those located in the deeper subcortical structures involving the basal ganglia. Second, we have shown that PADMAL and Swedish hMID differ from CADASIL in that the extent of vascular pathology and the sclerosis of arterioles are not as exaggerated. Third, the degree of vascular burden varies between brain regions, where frontal lobe areas are affected more in PADMAL but less so in the hMID of the Swedish type, and yet it is the reverse in the basal ganglia. PADMAL and Swedish hMID familial dementias undergo a less severe disease process but they may be of similar level to that observed in sporadic late onset dementia of SVD type, depending on brain regions examined. However, the SVD group was affected in the frontal region more so than in the basal ganglia, showing similar disease distribution to PADMAL cases. Because of differences in sampling, only some brain regions were available for investigation in all cases, and thus we could not include other pathological areas such as the pons, which is most affected in PADMAL 5, but not commonly involved in other vascular disorders.
The importance of brain region on vascular pathology was indicated in our regression analyses and this is consistent with differential degree of regional vascular degeneration in other studies 14. These suggested that the causes of cortical infarcts were due to stenosed vessels, whereas the infarcts in deeper structures such as those in the basal ganglia are more likely to be of hemodynamic origin 14. Uehara et al 19 reported that clinical risk factors for silent infarcts in basal ganglia and cerebral white matter were different, where age, female gender and hypertension increased risk of cortical white matter infarcts. Alternatively, the same authors suggested ischemic heart disease and carotid artery stenosis, two factors that may reduce global cerebral blood flow, were implicated in basal ganglia infarcts 19.
Miao et al 14 investigated vessels in the lenticular nucleus (putamen and globus pallidus) and compared to the frontal cortex and underlying white matter in CADASIL cases of a similar age to those included in the present study. Our results are in agreement with Miao and colleagues who also found that vessels in the lenticular nucleus were more sclerosed than in the cortical gray matter, but not as severely affected as the white matter underlying the cortex.
Surprisingly, the level of pathology observed in our SVD group was not always significantly higher than that observed in our age‐matched cognitively normal controls, indicating that the level of pathology differentiating between dementia and cognitively normal cases is subtle, and in some cases may be lower than expected in those showing signs of dementia. However, we acknowledge the limitations in our study with only two brain regions, as it is highly likely that other brain regions that are involved in sporadic SVD and preserved in cognitively normal controls may contribute to differentiating dementia and retained cognition 2. Conversely, our results emphasize the vascular burden involved in the disease progression of CADASIL, where cases have a much younger onset and yet have the highest vascular pathology and undergo the most severe vessel sclerosis. The HERNS case, which showed similar levels of vascular pathology in terms of arteriolosclerosis to CADASIL, confirmed this but the proportion of vessels involved was not as pronounced, particularly in the basal ganglia. This case provided an interesting comparison, in that the causal gene TREX1 is not necessarily associated with vascular development or disease as in CADASIL, but the resulting cerebral vascular disease burden is high.
Our SI values were slightly lower than other studies have previously reported 13, 14, which may be due to the nature of our cohort, as a previous study by our laboratory using the temporal lobe of the same cases reported similar values 21. Lammie et al 12 evaluated the SI of vessels in varying patients diagnosed with SVD and reported SI values of between 0.2 and 0.3 in cases of mild SVD, which they described as within “normal” vessel range. The majority of vessels measured from our cases of young and old cognitively normal controls were also within this range, although our young controls had higher SI values in the basal ganglia than those found in the old controls (Table 1), but this may be a result of a “survival factor” in those individuals that survived until old age, or indeed an artifact of the ageing process on cerebral vessels. A further confounding issue in the measurement of SI is that the measurement is only viable for blood vessels with an intact vessel wall and the method cannot account for vessels that were diseased to a degree which could not be measured, for example, vessels that have undergone “segmental arteriolar disorganisation” 4, an indicator of severity of SVD 12. Our results do demonstrate a more complete picture of the burden of diseased blood vessels in varying disease states and a small cohort of cognitively normal controls ranging from 49 to 94 years of age. It is surprising that that there was no clear effect of ageing, but this was supported by the study performed by Lammie et al 12, who explained this by the effective treatment of hypertension in the population and therefore reduced incidence of hypertensive vessel damage.
We found higher levels of GLUT‐1 staining in the white matter of SVD cases in both the frontal and basal ganglia regions when compared to the hereditary SVD groups, indicating a differential disease progression between these disorders, where hereditary SVD cases had lower capillary density than that observed in sporadic SVD. This was also observed with SMA staining in the white matter of the frontal region, although similar age older controls showed a similar trend, and therefore this may be an ageing effect as opposed to the disease state. This suggests differential mechanisms on the disease process in SVD and hereditary SVDs with younger onset, although further work needs to be done on a larger cohort to fully evaluate this hypothesis. Immunostaining of COL4 showed a significant increase in the PADMAL group, where the level of staining was largely increased in the gray matter of both frontal and basal ganglia vessels. The immunoreactivity in vessels in the gray matter of basal ganglia in the Swedish hMID group also exhibited increased COL4 reactivity, and this coincides with the higher SI values for this region in this disease group. In contrast, CADASIL cases demonstrated the most severe sclerosis, and yet this group had lower reactivity for COL4 than the PADMAL and Swedish hMID groups. An increase in COL4 immunoreactivity was previously reported in these disorders 5, 20; however, this has not been quantified relative to other disorders. There were less obvious changes in the staining of SMA, although there was an effect toward an increase in the white matter of the sporadic SVD group, which seemed to be closely followed in the older cognitively normal control group. Unlike in CADASIL and Swedish hMID, this implies a tendency for an increase in VSMCs with age, possibly to enable larger arterioles. There was also evidence for an increase in SMA staining in the PADMAL group, although this did not reach significance, and this may further differentiate this hereditary SVD from the other three familial disorders included in this study. Further work using a larger cohort or using other brain areas is needed to elucidate these effects, as it may provide insight into the component of cerebral blood vessels that are involved in the ageing and sporadic SVD process, which may be distinct to that observed in hereditary SVDs. A loss of VSMCs in CADASIL is already well established 10, and this may also be the case in Swedish hMID although it is less aggressive 13. Our results suggest that there are measurable collective effects in the capillary bed in SVDs, and this will likely have a significant impact on local blood flow in the regions explored here.
In summary, we have shown that hereditary SVDs of different genetic origin have significantly different vascular pathologies. These are brain region and tissue specific. While CADASIL is the most aggressive hereditary SVD in terms of overall vascular pathology and severe arteriolosclerosis in both frontal and basal ganglia regions, other hereditary SVDs differ in regional involvement and extent of vessel changes. PADMAL seems to affect the vessels of the frontal region more than the basal ganglia, while the Swedish hMID shows the opposite effect. When these vascular changes were further investigated using immunohistochemical stains, the mechanisms of vessel degeneration were further differentiated, with vessels in PADMAL showing increased COL4 deposition, in agreement with previous reports 5, and yet this was not associated with a severe state of vessel hyalinosis, as PADMAL cases had the lowest incidence of vessels with an SI in the “severe” disease state of SI ≥ 0.5. Our results further underline the differential genetic involvement in mechanisms of vascular degeneration in SVDs of the brain. We acknowledge the limitations of this study in terms of a small sample group for the hereditary SVDs; however, the large number of blood vessels that were assessed in the SI analysis strengthens our conclusions. Tissues from other hereditary SVDs were not available for this study, as there are several other uncharacterized hereditary SVDs, which would have been interesting to compare using our methods 21. Further work to elucidate the disease processes within cerebral blood vessels may unveil novel treatment strategies for both sporadic and hereditary SVDs.
Author contribution
LC was responsible for sample assessment, data collection and analysis, and manuscript preparation. RH and JYS were responsible for sample preparation and technical assistance. CG, GK, AB‐H, CH, HK, CMcL and RK were responsible for clinical and neuropathological analyses, provision of post‐mortem brain tissue, and general advice on analysis. VD provided the vascular pathological scores. AEO and YY assisted with data analysis and experimental design. RNK was also responsible for vascular pathology scores, experimental design and manuscript preparation.
Conflict of interest
None declared.
Supporting information
Figure S1. Representative images of blood vessels from HERNS case stained for CD45 and CD68, showing extensive blood‐borne macrophage infiltration possibly due to systemic sepsis from the urinary tract infection which was cited as cause of death.
Acknowledgments
We are very grateful to the patients, families and clinical house staff for their cooperation in the investigation of this study. We thank Michelle Widdrington, Carein Todd, Jean Scott, Deborah Lett and Anne Nicholson for assistance in managing the cases. Our work is supported by grants to Newcastle Centre for Brain Ageing and Vitality (BBSRC, EPSRC, ESRC and MRC, LLHW) and Alzheimer's Research (UK). Tissue for this study was collected by the Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK MRC (G0400074), by the Newcastle NIHR Biomedical Research Centre in Ageing and Age Related Diseases award to the Newcastle upon Tyne Hospitals NHS Foundation Trust, and by a grant from the Alzheimer's Society and ARUK as part of the Brains for Dementia Research Project.
References
- 1. Cohn AC, Kotschet K, Veitch A, Delatycki MB, McCombe MF (2005) Novel ophthalmological features in hereditary endotheliopathy with retinopathy, nephropathy and stroke syndrome. Clin Experiment Ophthalmol 33:181–183. [DOI] [PubMed] [Google Scholar]
- 2. Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, Kalaria RN (2012) Staging and natural history of cerebrovascular pathology in dementia. Neurology 78:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding X‐Q, Hagel C, Ringelstein EB, Buchheit S, Zeumer H, Kuhlenbäumer G et al (2010) MRI features of pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL). J Neuroimaging 20:134–140. [DOI] [PubMed] [Google Scholar]
- 4. Fisher CM (1968) The arterial lesions underlying lacunes. Acta Neuropathol 12:1–15. [DOI] [PubMed] [Google Scholar]
- 5. Hagel C, Stavrou D, Colmant HJ, Groden C, Niemeyer R (2004) Subcortical angiopathic encephalopathy in a German kindred suggests an autosomal dominant disorder distinct from CADASIL. Acta Neuropathol 108:231–240. [DOI] [PubMed] [Google Scholar]
- 6. Jen J, Cohen AH, Yue Q, Stout JT, Vinters HV, Nelson S, Baloh RW (1997) Hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS). Neurology 49:1322–1330. [DOI] [PubMed] [Google Scholar]
- 7. Joutel A (2010) Pathogenesis of CADASIL: transgenic and knock‐out mice to probe function and dysfunction of the mutated gene, Notch3, in the cerebrovasculature. Bioessays 33:73–80. [DOI] [PubMed] [Google Scholar]
- 8. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P et al (1996) Notch3 mutations in CADASIL, a hereditary adult‐onset condition causing stroke and dementia. Nature 383:707–710. [DOI] [PubMed] [Google Scholar]
- 9. Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T (2004) Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 226:75–80. [DOI] [PubMed] [Google Scholar]
- 10. Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T (2004) The pathogenesis of CADASIL: an update. J Neurol Sci 226:35–39. [DOI] [PubMed] [Google Scholar]
- 11. Kalimo H, Ruchoux M‐M, Viitanen M, Kalaria RN (2002) CADASIL: a common form of hereditary arteriopathy causing brain infarcts. Brain Pathol 12:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lammie GA, Brannan F, Slattery J, Warlow C (1997) Nonhypertensive cerebral small‐vessel disease: an autopsy study. Stroke 28:2222–2229. [DOI] [PubMed] [Google Scholar]
- 13. Low WC, Junna M, Börjesson‐Hanson A, Morris CM, Moss TH, Stevens DL et al (2007) Hereditary multi‐infarct dementia of the Swedish type is a novel disorder different from NOTCH3 causing CADASIL. Brain 130:357–367. [DOI] [PubMed] [Google Scholar]
- 14. Miao Q, Paloneva T, Tuisku S, Roine S, Poyhonen M, Viitanen M, Kalimo H (2006) Arterioles of the lenticular nucleus in CADASIL. Stroke 37:2242–2247. [DOI] [PubMed] [Google Scholar]
- 15. Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, Kalimo H (2004) Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol 14:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ophoff RA, DeYoung J, Service SK, Joosse M, Caffo NA, Sandkuijl LA et al (2001) Hereditary vascular retinopathy, cerebroretinal vasculopathy, and hereditary endotheliopathy with retinopathy, nephropathy, and stroke map to a single locus on chromosome 3p21.1‐p21.3. Am J Hum Genet 69:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richards A, Van Den Maagdenberg AMJM, Jen JC, Kavanagh D, Bertram P, Spitzer D et al (2007) C‐terminal truncations in human 3′‐5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat Genet 39:1068–1070. [DOI] [PubMed] [Google Scholar]
- 18. Sourander P, Walinder J (1977) Hereditary multi‐infarct dementia. Morphological and clinical studies of a new disease. Acta Neuropathol 39:247–254. [DOI] [PubMed] [Google Scholar]
- 19. Uehara T, Tabuchi M, Mori E (1999) Risk factors for silent cerebral infarcts in subcortical white matter and basal ganglia. Stroke 30:378–382. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Craggs L, Baumann M, Kalimo H, Kalaria RN (2011) Review: molecular genetics and pathology of hereditary small vessel diseases of the brain. Neuropathol Appl Neurobiol 37:94–113. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto Y, Ihara M, Tham C, Low WC, Slade JY, Moss T et al (2009) Neuropathological correlates of temporal pole white matter hyperintensities in CADASIL. Stroke 40:2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang WW, Ma KC, Andersen O, Sourander P, Tollesson PO, Olsson Y (1994) The microvascular changes in cases of hereditary multi‐infarct disease of the brain. Acta Neuropathol 87:317–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative images of blood vessels from HERNS case stained for CD45 and CD68, showing extensive blood‐borne macrophage infiltration possibly due to systemic sepsis from the urinary tract infection which was cited as cause of death.


