Clinical History
A 76‐year‐old man presented to an outside hospital with nausea and vomiting. Further questioning disclosed a two month history of worsening gait instability, dizziness, and frequent falls. His limited past medical history included melanoma of the interscapular region diagnosed six year prior to presentation at an outside hospital.
Magnetic resonance imaging (MRI) of the brain demonstrated a 4.5 centimeter diameter, heterogeneously enhancing, left cerebellar mass with intralesional hemorrhage and possible cystic components (Figure 1). There was significant compression of the fourth ventricle. Imaging of the remainder of the neural axis showed no additional lesions or drop metastases.
Figure 1.
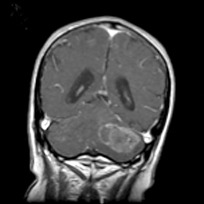
The patient underwent a midline suboccipital craniotomy with gross total resection of the tumor. Intraoperatively, a reddish, fleshy tumor was identified. Intraoperative consultation showed a neoplasm with clear‐cell morphology. The differential diagnoses included an unusual metastatic melanoma but renal cell carcinoma and (less likely) hemangioblastoma were also considerations.
Post‐operative MRI demonstrated no residual enhancing tumor. In addition, comprehensive body imaging of chest, abdomen, and pelvis was negative.
Microscopic Pathology
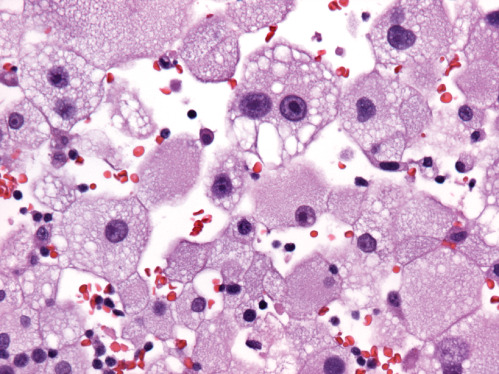
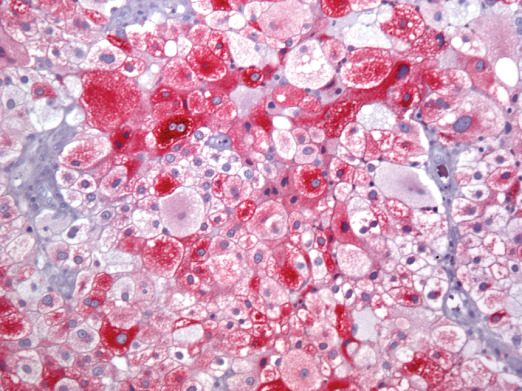
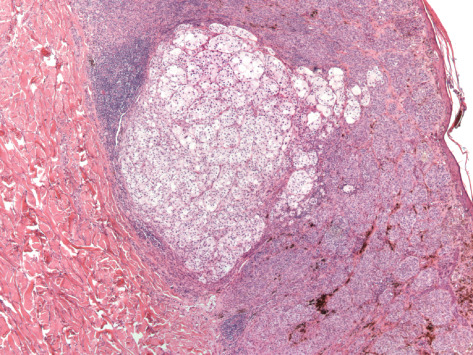
Microscopic examination showed a well‐demarcated metastatic tumor composed of lobular nests and groups of cells with focal central necrosis (Figure 2). High power examination revealed large polygonal to round tumor cells with well‐delimited cell borders (Figure 3) Some interspersed connective tissue septae contained benign lymphocytes and hemosiderin. The voluminous, pale, eosi nophilic cytoplasm was finely vacuolated (Figure 4). The nuclear‐to‐cytoplasmic ratio was low due to the large amount of bubbly cytoplasm. Nuclei were hyperchromatic and contained irregular chromatin and conspicuous nucleoli (Figure 4). Mitotic activity was relatively modest and the MIB‐1 index was 5%. Cytoplasmic melanin pigment was not identified and no typical areas of melanoma were appreciable.
Figure 2.
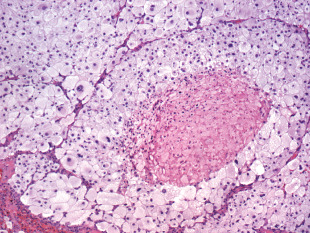
Figure 3.
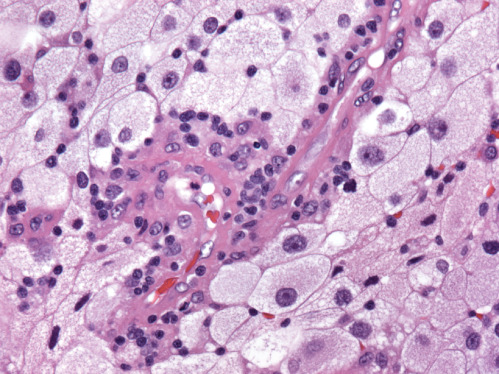
Figure 4.
Immunohistochemical markers for renal cell carcinoma (RCC) antigen, epithelial membrane antigen (EMA), CD10 and cytokeratins 7 and 20 were negative. Tumor cells displayed diffuse positive labeling for S100 and Melan‐A (Figure 5). Immunohistochemistry for HMB‐45 was negative. What is the diagnosis?
Figure 5.
Diagnosis
Balloon cell melanoma, metastatic to cerebellum.
Discussion
Metastatic melanoma is one of the most common nervous system metastatic tumor types encountered by pathologists, but this balloon cell variant is exceedingly unusual as a CNS metastasis and can be challenging to diagnose correctly, especially at the time of intraoperative consultation or when the slides from the original cutaneous tumor are not available for direct comparison, both of which were true in this case.
Even in primary cutaneous sites, balloon cell melanoma is one of the rarest variants, with more than 95% of primary cutaneous melanomas fitting into one of the more common, classic subtypes 8. Balloon cell melanoma has been the subject of only sporadic case reports, even as a primary lesion 5, 9. This case illustrates the additional challenges when this rare subtype is encountered as a metastasis, especially to the CNS and especially to the cerebellum, where either metastatic renal cell carcinoma or hemangioblastoma are more likely.
There have been few reports of metastatic balloon cell melanoma and even fewer cases which involve the central nervous system (CNS). One prior report documented two cases balloon cell melanoma arising from the choroid of the eye with associated metastatic disease 6. Balloon cell melanoma has also been reported as a primary lesion of the CNS arising from the leptomeninges 1. We were able to identify only a single prior case report of metastatic balloon cell melanoma to the cerebellum 3.
This case underscores the value in requesting pathology reports, and optimally, the actual glass slides from any known primary lesion on a patient suspected of harboring a metastatic brain tumor. Only after obtaining, and reviewing this patient's actual slides from his original skin lesion was it apparent that the primary cutaneous melanoma had contained a subpopulation of tumor cells with a balloon cell phenotype (Figure 6). By the time of the cerebellar metastasis, the tumor had evolved into a pure balloon cell appearance.
Figure 6.
Pathologically, balloon cell melanoma is characterized by nests and sheets of large, abundantly vacuolated cells that result in a “clear‐cell” appearance on microscopic analysis 5, 7. Balloon cell melanomas typically label with several or all of the immunohistochemical markers for melanoma, S100, Melan‐A and HMB‐45. There was strong immunoreactivity for S100 and Melan‐A in our case.
The exact nature of the ballooning change remains incompletely understood but was originally thought to represent a degenerative process. Recent studies propose that the balloon cells are, in fact, metabolically active, as they demonstrate abundant ribosomes and RNA on electron microscopy as well as positive immunohistochemical labeling for HMB‐45 (an antigen with an established predilection for metabolically active and neoplastic melanocytes) 5. Of note, our case was HMB‐45 negative. Balloon cell melanomas possess the same propensity for metastasis as other melanomas and the extent of ballooning does not influence the metastatic potential. Recent theories, therefore, attribute the ballooning change to some sort of aberrancy in the maturation of melanosomes rather than a degenerative change 5.
The incidence of melanoma in the United States is rising 4 and associated morbidity and mortality for CNS metastatic disease are high 10. Chemotherapy and radiotherapy only provide modest clinical benefit, with approaches utilizing a combination of surgery and chemotherapy offering the longest survival advantage 2. As always, correct treatment is predicated on accurate pathological diagnosis.
Abstract
We report a case of balloon cell melanoma metastatic to the cerebellum; the clear cell morphology prompted initial differential diagnostic considerations of metastatic renal cell carcinoma and hemangioblastoma in this site. To our knowledge this is only the second case of metastatic balloon cell melanoma to the CNS.
References
- 1. Adamek D, Kaluza J, Stachura K (1995) Primary balloon cell malignant melanoma of the right temporo‐parietal region arising from meningeal naevus. Clin Neuropathol 14(1):29–32. [PubMed] [Google Scholar]
- 2. Bafaloukos D, Gogas H (2004) The treatment of brain metastases in melanoma patients. Cancer Treat Rev 30(6):515–520. [DOI] [PubMed] [Google Scholar]
- 3. Ferracini R, Manetto V, Minghetti G, Lanzanova G (1982) Cerebellar balloon‐cell metastasis of a melanoma. Tumori 68(2):177–180. [DOI] [PubMed] [Google Scholar]
- 4. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 5. Kao GF, Helwig EB, Graham JH (1992) Balloon cell malignant melanoma of the skin. A clinicopathologic study of 34 cases with histochemical, immunohistochemical, and ultrastructural observations. Cancer 69(12):2942–2952. [DOI] [PubMed] [Google Scholar]
- 6. Khalil MK (1983) Balloon cell malignant melanoma of the choroid: ultrastructural studies. Br J Ophthalmol 67(9):579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magro CM, Crowson AN, Mihm MC (2006) Unusual variants of malignant melanoma. Mod Pathol 19 Suppl 2:S41–70. [DOI] [PubMed] [Google Scholar]
- 8. Perniciaro C (1997) Dermatopathologic variants of malignant melanoma. Mayo Clin Proc 72(3):273–279. [DOI] [PubMed] [Google Scholar]
- 9. Peters MS, Su WP (1985) Balloon cell malignant melanoma. J Am Acad Dermatol 13(2 Pt 2):351–354. [DOI] [PubMed] [Google Scholar]
- 10. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF (1998) Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 88(1):11–20. [DOI] [PubMed] [Google Scholar]


