Abstract
Intracellular inclusions consisting of TAR DNA binding protein‐43 (TDP‐43 pathology) are present in up to 57% of Alzheimer's disease (AD) cases and follow a distinct topographical pattern of progression described in the TDP‐43 in AD staging scheme. This scheme has not been applied to the assessment of TDP‐43 pathology in dementia with Lewy bodies (DLB) and aged controls. We investigated TDP‐43 pathology prevalence and severity in AD, DLB, mixed AD/DLB (Mx AD/DLB) and aged controls. One hundred and nineteen human post‐mortem brains were included, neuropathologically diagnosed as AD: 46, DLB: 15, Mx AD/DLB: 19 and aged controls: 39. Paraffin sections inclusive of the amygdala, hippocampus, striatum and neocortex were immunohistochemically stained with antibodies against phosphorylated TDP‐43 and staged according to the TDP‐43 in AD staging scheme. TDP‐43 pathology was present in all groups: AD: 73.9%, DLB: 33.3%, Mx AD/DLB: 52.6% and controls: 17.9%. Prevalence of TDP‐43 pathology was significantly higher in AD and Mx AD/DLB compared to controls. In controls, higher age at death was associated with prevalence of TDP‐43 pathology and higher TDP‐43 in AD stage, suggesting that this type of TDP‐43 pathology may partly be an age‐associated phenomenon. Significantly higher prevalence of TDP‐43 pathology in the AD group indicates that AD pathology possibly triggers and aggravates TDP‐43 pathology. The validity of the TDP‐43 in AD staging scheme is not limited to AD and should be applied to assess TDP‐43 pathology in post mortem brains of aged individuals to further elucidate the role of TDP‐43 pathology in age associated neurodegeneration.
Keywords: ageing, Alzheimer's disease, dementia with Lewy bodies, hippocampal sclerosis, Lewy body diseases, mixed Alzheimer's disease and dementia with Lewy bodies, TDP‐43
Introduction
Transactive response DNA‐binding protein 43 (TDP‐43) is a ubiquitously expressed, highly conserved RNA‐ and DNA‐binding nuclear protein 32. Under pathological conditions TDP‐43 can be sequestered into the cytoplasm and cleaved into C‐terminal fragments that are abnormally hyperphosphorylated and subsequently aggregate forming intracellular inclusions. These inclusions are observed as neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs) and/or dystrophic neurites (DNs) and are collectively referred to as TDP‐43 pathology.
Although TDP‐43 pathology is a hallmark pathology associated with a subtype of fronto‐temporal lobar degeneration (FTLD)‐TDP 4 and motor neuron disease 30, it is also present in Alzheimer's disease (AD) 1. The neuropathological hallmark lesions of AD include intracytoplasmic neurofibrillary tangles (NFTs) and dendritic and axonal neuropil threads (NTs) that are composed of aggregated hyperphosphorylated microtubule associated tau (HP‐τ) 14, 26, extracellular depositions of amyloid‐β protein (Aβ) and neuritic plaques consisting of Aβ and HP‐τ in DNs 9. Prevalence rates of TDP‐43 pathology in AD vary between 29% and 72% 1, 2, 8, 19, 20, 21, 22, 38, 39, however, it is generally accepted that TDP‐43 pathology in AD is frequent 22 and contributes to the AD neurodegenerative process 21. The presence of TDP‐43 pathology in AD has previously been shown to modify the clinical and radiological phenotype: AD subjects with TDP‐43 pathology had more severe cognitive impairment 21, with specific deficits in episodic and working memory 38, 39 and language domains 22 and greater hippocampal atrophy, as seen on MRI 19, compared to AD subjects lacking TDP‐43 pathology.
The distribution of TDP‐43 pathology differs between AD and FTLD‐TDP, with the amygdala being the first and most frequently affected region in AD 12, 13, 20. The progression of TDP‐43 pathology in AD follows a stereotypical pattern of deposition that has been reported and validated by Josephs and colleagues 20 who proposed a TDP‐43 in AD staging scheme: TDP‐43 pathology initially manifests in the amygdala (stage I), followed by the entorhinal cortex and/or subiculum (stage II), dentate gyrus and/or occipitotemporal cortex (OTC) (stage III), inferior temporal cortex (ITC) (stage IV) and finally the mid‐frontal cortex and/or striatum (stage V). Here, the term TDP‐43 pathology refers only to TDP‐43 deposition in AD and not to the hallmark pathology of FTLD‐TDP and motor neuron disease.
TDP‐43 pathology has also been identified in dementia with Lewy bodies (DLB), which is neuropathologically characterized by the presence of α‐synuclein (α‐syn) as intracytoplasmic aggregates, that is, Lewy bodies (LB) and dendritic and axonal Lewy neurites (LN) 33; although different studies have reported highly divergent prevalence rates, ranging from 0% 27 to 56% 2. The neuroanatomical distribution of TDP‐43 pathology in DLB has been shown to be similar to that seen in AD, as it predominantly affects the amygdala and hippocampal structures 12.
Multiple pathological lesions are frequently seen in post‐mortem brains of nondemented and demented elderly subjects, and in the latter group mixed pathology is rather the rule than the exception 16, 34, 35. Mixed dementia, conversely, is less frequent and should neuropathologically only be diagnosed if criteria for more than one full‐blown disease are met as in mixed AD/DLB (Mx AD/DLB), which fulfills neuropathological criteria for both AD 26 and DLB 25. One study found TDP‐43 pathology in 31% of Mx AD/DLB 27. However, it is not known whether Mx AD/DLB cases show the same severity and topographical progression pattern of TDP‐43 pathology as “pure” AD cases, which exhibit no/minimal concomitant neurodegenerative or cerebrovascular pathology.
Ageing renders the brain vulnerable to pathological insults and accumulation of HP‐τ, Aβ and α‐syn pathology frequently occurs in normal aged individuals without compromising cognitive function 5, 15, 18. TDP‐43 pathology has been shown to occur in cognitively normal aged subjects with prevalence rates ranging between 10.5% and 36.4% 3, 28. TDP‐43 pathology increased with age in control cases, while it was absent in subjects below the age of 65 (11), suggesting that TDP‐43 pathology occurs partly as consequence of ageing.
It is indeed conceivable that both ageing and neurodegenerative processes are independently associated with the presence and severity of TDP‐43 pathology, but also may have a synergistic effect with advancing age rendering neurons vulnerable to TDP‐43 pathology that may be exacerbated by other concomitant neurodegenerative diseases. However, data from previous studies on TDP‐43 pathology in age‐associated neurodegeneration cannot be compared as these studies have employed different methodologies.
Here, we investigate and compare the prevalence, severity and topographical distribution of TDP‐43 pathology in subjects with AD, DLB, Mx AD/DLB and cognitively normal aged controls using a consistent staging scheme 20 and evaluate the relationship between stage of TDP‐43 pathology and age at death, the severity of HP‐τ, Aβ and α‐syn pathology, as well as clinical measures of cognition.
Materials and Methods
For this study we used a consecutive series of 119 human post‐mortem brains from demented and nondemented elderly (mean age 81.39 ± 9.6 years; male: 65, female: 54) in whom AD pathology was assessed according to the National Institute on Aging‐Alzheimer's Association (NIA‐AA) criteria 26. Of note, cases with familial or rare neurodegenerative diseases (eg, motor neuron disease, progressive supranuclear palsy) were excluded. Brain tissue was obtained at autopsy and stored within the Newcastle Brain Tissue Resource (NBTR) in accordance with Newcastle University Ethics Board (The Joint Ethics Committee of Newcastle and North Tyneside Health Authority, reference: 08/H0906/136). After autopsy the right hemisphere, brainstem and cerebellum were immersion fixed in 4% buffered aqueous formaldehyde solution for 6 weeks. Irrespective of clinical diagnoses, all brains underwent neuropathological assessment and were stratified by neuropathogical diagnosis. Fourty‐six cases fulfilled neuropathological criteria for high AD neuropathological change according to NIA‐AA criteria 26, 15 for DLB (inclusive of DLB and Parkinson's disease dementia clinical phenotypes) according to McKeith Lewy body criteria 25 [limbic/neocortical Lewy body disease; Braak Lewy body stage, 4 or higher 6], 19 for Mx AD/DLB [high AD neuropathologic change 26 and limbic/neocortical Lewy body disease 25] and 39 clinically nondemented cases showed no to moderate degrees of neurodegenerative pathology. The presence and type of cerebral amyloid angiopathy (CAA) 36 and presence of hippocampal sclerosis were noted. For neuropathological details see Table 1.
Table 1.
Characteristics of study cohort.
| AD | DLB | Mx AD/DLB | Controls | |
|---|---|---|---|---|
| Case n | 46 | 15 | 19 | 39 |
| Age at death (mean ± years) | 83.5 (± 8.4) | 81.0 (± 5.3) | 78.7 (± 9.3) | 80.4 (± 12) |
| Sex (M : F) | 23:23 | 9:6 | 13:6 | 20:19 |
| Braak NFT stage (5) | NFT stage 5, n = 3 NFT stage 6, n = 43 | NFT stage 1, n = 1 NFT stage 2, n = 1 NFT stage 3, n = 10 NFT stage 4, n = 3 | NFT stage 5, n = 2 NFT stage 6, n = 17 | NFT stage 0, n = 7 NFT stage 1, n = 7 NFT stage 2, n = 14 NFT stage 3, n = 10 NFT stage 4, n = 1 |
| Thal Abeta phase (37) | Phase 4, n = 5 Phase 5, n = 41 | Phase 0, n = 1 Phase 1, n = 1 Phase 2, n = 1 Phase 3, n = 5 Phase 4, n = 5 Phase 5, n = 2 | Phase 4, n = 4 Phase 5, n = 15 | Phase 0, n = 11 Phase 1, n = 13 Phase 2, n = 7 Phase 3, n = 4 Phase 4, n = 3 Phase 5, n = 1 |
| CERAD score (26) | B, n = 3 C, n = 43 | negative, n = 7 B, n = 8 | C, n = 19 | negative, n = 34 A, n = 4 B, n = 1 |
| Alzheimer's disease neuropathologic change (NIA‐AA) (26) | High, n = 46 | Not, n = 1 Low, n = 4 Intermediate, n = 10 | High, n = 19 | Not, n = 12 Low, n = 25 Intermediate, n = 2 |
| Cerebral amyloid angiopathy (36) | Absent, n = 1 Type 1, n = 21 Type 2, n = 24 | Absent, n = 5 Type 1, n = 0 Type 2, n = 10 | Absent, n = 1 Type 1, n = 6 Type 2, n = 12 | Absent, n = 21 Type 1, n = 3 Type 2, n = 15 |
| Braak LB stage (6) | LB stage 0, n = 41 LB stage 1, n = 1 LB stage 2, n = 2 LB stage 3, n = 1 LB stage 4, n = 1 | LB stage 4, n = 2 LB stage 5, n = 3 LB stage 6, n = 10 | LB stage 4, n = 3 LB stage 5, n = 2 LB stage 6, n = 14 | LB stage 0, n = 35 LB stage 1, n = 1 LB stage 2, n = 1 LB stage 3, n = 2 |
| McKeith criteria (25) | No LBD, n = 37 Brainstem, n = 2, Limbic, n = 7 | Limbic, n = 3 Neocortical, n = 12 | Limbic, n = 5 Neocortical, n = 14 | No LBD, n = 36 Brainstem, n = 2, Limbic, n = 1 |
| Hippocampal Sclerosis | Absent, n = 38 Present, n = 8 | Absent, n = 15 Present, n = 0 | Absent, n = 18 Present, n = 1 | Absent, n = 37 Present, n = 2 |
Abbreviations: AD = Alzheimer's disease; DLB = dementia with Lewy bodies; Mx AD/DLB = mixed Alzheimer's disease and dementia with Lewy bodies; Case n = case number; M = male; F = female; NFT = neurofibrillary tangle; Aβ = amyloid‐beta; LB = Lewy body.
During life, all dementia subjects underwent clinical assessments by board certified Old Age Psychiatrists or Neurologists and most were assessed in prospective research studies with repeated cognitive evaluation including Mini‐Mental State Examination (MMSE; AD: 37, DLB: 15, Mx AD/DLB: 14) 10. Aged control subjects were assessed clinically and the absence of dementia confirmed but did not have repeated cognitive evaluation. The rate of cognitive decline 31 was determined for dementia cases with more than one MMSE score available. Age of onset of cognitive decline was also recorded in 76 patients (AD: 40, DLB: 14, Mx AD/DLB 17, aged controls: 5).
Tissue preparation
6 μm paraffin‐embedded sections were taken from the amygdala, subiculum and entorhinal cortex (BA 36, 28), dentate gyrus of the posterior hippocampus, OTC (BA 36), ITC (BA 20), mid‐frontal cortex (BA 8, 9) and basal ganglia, that is, putamen, globus pallidus and caudate. Sections were mounted onto 4% 3‐aminopropyltriethoxysilane (APES)‐coated glass slides and immunohistochemistry was performed for phosphorylated TDP‐43 (antibody phospho‐TDP‐43 (pS0409/410‐2; dilution 1:10 000; Cosmo Bio Ltd, Bicester, UK). Prior to immunostaining, antigen retrieval was performed by microwaving slides in 0.01 mL EDTA for 10 minutes. Immunopositivity was detected using the Menarini X‐Cell‐Plus HRP Detection Kit (Menarini Diagnostics, Winnersh‐Wokingham, UK) with 3,3‐diaminobenzidine (DAB) as a chromagen and haematoxylin as a counter stain. Sections were subsequently dehydrated through a series of alcohols, cleared and mounted using DPX (CellPath, Powys, UK).
Assessment of TDP‐43 pathology
Neuropathological assessment of TDP‐43 pathology was performed according to the TDP‐43 in AD staging scheme 20. Briefly, TDP‐43 pathology was examined by assessing TDP‐43 immunoreactivity of NCIs, NIIs and DNs in sections of the amygdala, entorhinal cortex, subiculum, dentate gyrus, OTC, ITC, mid‐frontal cortex and basal ganglia. The severity of TDP‐43 pathology in the amygdala was graded on a five‐tiered scale (0, absent; 1, scant; 2, sparse; 3, moderate; 4, frequent). Classification of stages of TDP‐43 deposition was as follows: stage I, scant to sparse deposition in the amygdala; stage II, moderate to frequent deposition in the amygdala and entorhinal cortex/subiculum; stage III, deposition in the amygdala, entorhinal cortex/subiculum and dentate gyrus or OTC; stage IV, deposition in the amygdala, entorhinal cortex/subiculum, dentate gyrus/OTC and ITC; stage V, deposition in the amygdala, entorhinal cortex/subiculum, dentate gyrus/OTC, ITC and mid‐frontal cortex or basal ganglia. The TDP‐43 in AD stage for each case was determined by consensus between two assessors.
Statistical analysis
The Statistical Package for Social Sciences software (SPSS ver. 21) was used for statistical evaluation. Variables were tested for normality using the Shapiro‐Wilk test and visual inspection of variable histograms. Our data were not normally distributed; therefore, nonparametric procedures were employed. Χ2 and Fisher's exact test were employed to assess differences in TDP‐43 prevalence (indicated by TDP‐43 pathology in the amygdala) between the disease groups. Spearman's (ρ) correlation coefficient (two tailed) was used to assess associations between TDP‐43 stage with age at death, Braak NFT stage, Thal Aβ phase and Braak Lewy body stage. Partial Spearman's (ρ′) correlation coefficient (one tailed) was used to assess associations between TDP‐43 stage with age at death (controlling for Braak NFT stage) and Braak NFT stage (controlling for age at death). Group effects on age were assessed using Kruskal‐Wallis and clinical measurements were assessed using a Mann‐Whitney U tests, respectively. P‐values below 0.05 were accepted as statistically significant.
Results
No significant differences in age at death were found between any of the disease groups. In the entire cohort, cases with TDP‐43 pathology were significantly older at death compared to cases without TDP‐43 pathology (P = 0.008). When stratified by neuropathopathological diagnosis, only the control group cases with TDP‐43 pathology were significantly older compared to cases without TDP‐43 pathology (P = 0.005), while no such differences were seen in AD (P = 0.117), DLB (P = 0.099), or Mx AD/DLB (P = 0.720) groups.
Prevalence of TDP‐43 pathology
TDP‐43 pathology (Figure 1) was observed in 34 AD cases (73.9%), 5 DLB cases (33.3%), 10 Mx AD/DLB cases (52.6%) and 7 aged controls (17.9%). The prevalence of TDP‐43 pathology was significantly higher in AD and Mx AD/DLB compared to aged controls (AD vs. aged controls, Χ2 = 24.28, P = 0.0001; Mx AD/DLB vs. aged controls, Χ2 = 5.838, P = 0.016) and in AD compared to DLB (Χ2 = 6.414, P = 0.011). However, no such significant differences were seen between AD and Mx AD/DLB (Χ2 = 1.9, P = 0.168), Mx AD/DLB and DLB (Χ2 = 0.604, P = 0.437) as well as DLB and aged controls (P = 0.279, Fisher's exact test).
Figure 1.
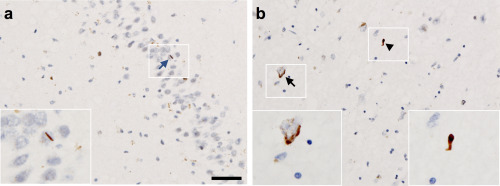
Photomicrograph illustrating types of TDP‐43 pathology observed in the study, taken from a mixed AD/DLB case. A. Highlights a neuronal intranuclear inclusion (NII)—blue arrow, seen in the dentate gyrus of the hippocampus (stage III). B. Demonstrates a neuronal cytoplasmic inclusion (NCI)—black arrow, and dystrophic neurite (DN)—black arrowhead seen in the amygdala (stage I). Scale bar in (A) represents 50 μm and is valid for (B).
Severity of TDP‐43 pathology
TDP‐43 in AD stages for each disease group are shown in Table 2. TDP‐43 pathology reached highest stages in AD cases; of 34 AD cases exhibiting TDP‐43 pathology, a considerable proportion reached higher stages IV and V (38.2% stage IV and 3% stage V) while 29.4% showed stage I, 17.6% stage II and 11.8% stage III. By contrast, one of five DLB cases with TDP‐43 pathology showed stage IV (20%), and the remaining four cases (80%) reached stage I only. Of the 10 Mx AD/DLB cases with TDP‐43 pathology, 20% were stage II, 20% were stage I, while 50% showed stage III and 10% stage IV. Finally, seven aged controls exhibited TDP‐43 pathology with 28.5% at stage I, 14.3% in stage II and III each and 42.9% at stage IV.
Table 2.
Prevalence and severity of TDP‐43 pathology.
| TDP‐43 in AD stage (21) | Negative | I | II | III | IV | V |
|---|---|---|---|---|---|---|
| AD n, (%) | 12 (26.1) | 10 (21.7) | 6 (13) | 4 (8.7) | 13 (28.3) | 1 (2.2) |
| DLB n, (%) | 10 (66.7) | 4 (26.7) | 0 | 0 | 1 (6.6) | 0 |
| Mx AD/DLB n, (%) | 9 (47.4) | 2 (10.5) | 2 (10.5) | 5 (26.4) | 1 (5.2) | 0 |
| Controls n, (%) | 32 (82.1) | 2 (5.1) | 1 (2.5) | 1 (2.5) | 3 (7.8) | 0 |
Abbreviations: AD = Alzheimer's disease; DLB = dementia with Lewy bodies; Mx AD/DLB = mixed Alzheimer's disease and demential with Lewy bodies.
Associations between TDP‐43 in AD stage, age and severity of AD and DLB pathology
We investigated whether age at death, Braak NFT stage, Thal Aβ phase or Braak LB stage was associated with the TDP‐43 in AD stages (absent—stage V). A higher TDP‐43 in AD stage correlated with both age at death (ρ = 0.448, P = 0.004) and Braak NFT stage (ρ = 0.370, P = 0.020) in the control group only, while no such correlations were seen in any of the disease groups (Table 3).
Table 3.
Correlations between TDP‐43 in AD stage with age and cortical pathology stages.
| AD | DLB | Mx AD/DLB | Controls | |
|---|---|---|---|---|
| TDP‐43 in AD stage (21) | TDP‐43 in AD stage | TDP‐43 in AD stage | TDP‐43 in AD stage | |
| Age at death | ρ = 0.175, P = 0.245 | ρ = −0.485, P = 0.067 | ρ = 0.099, P = 0.688 | ρ = 0.448, P = 0.004** |
| Braak NFT stage (8) | ρ = 0.139, P = 0.357 | ρ = 0.372, P = 0.172 | ρ = 0.100, P = 0.683 | ρ = 0.370, P = 0.020* |
| Thal Aβ phase (39) | ρ = 0.051, P = 0.735 | ρ = −0.139, P = 0.622 | ρ = −0.378, P = 0.111 | ρ = 0.145, P = 0.378 |
| Braak LB stage (7) | ρ = −0.069, P = 0.651 | ρ = 0.296, P = 0.304 | ρ = 0.189, P = 0.437 | ρ = −0.148, P = 0.376 |
Abbreviations: NFT = neurofibrillary tangle; Aβ = amyloid‐beta; LB = Lewy body; AD = Alzheimer's disease; DLB = dementia with Lewy bodies; Mx AD/DLB = mixed Alzheimer's disease and demential with Lewy bodies; ρ = Spearman's rank correlation coefficient; N/A = data not available.TDP‐43 stage in AD determined as negative to stage V.
*P < 0.05.
**P < 0.01.
We further investigated the correlations between TDP‐43 in AD stage with age at death and Braak NFT stage in the control group using partial Spearman's correlation analysis (ρ′). The correlation between TDP‐43 in AD stage and age at death remained statistically significant when controlled for Braak NFT stage (ρ′ = 0.297, P = 0.041), while no correlation was seen between TDP‐43 in AD and Braak NFT stages when the analysis was controlled for age at death (ρ = 0.125, P = 0.236).
In the seven control cases with TDP‐43 pathology the TDP‐43 in AD stage and AD neuropathologic change (NIA‐AA criteria) were stage IV and “low” (n = 3), stage III and “intermediate” (n = 1) stage II and “low” (n = 1), stage I and “low” (n = 1) and stage I and “no” (n = 1).
TDP‐43 pathology and clinical measures of cognitive impairment
In AD, DLB and Mx AD/DLB there was no significant difference between age of onset of cognitive decline (Figure 2A) as well as rate of cognitive decline between cases with and without TDP‐43 pathology (Figure 2B). However, AD cases with TDP‐43 pathology had significantly lower final MMSE scores compared to AD cases lacking TDP‐43 pathology (P = 0.041: Figure 2C), while no such significant differences were seen in DLB, Mx AD/DLB and aged controls.
Figure 2.
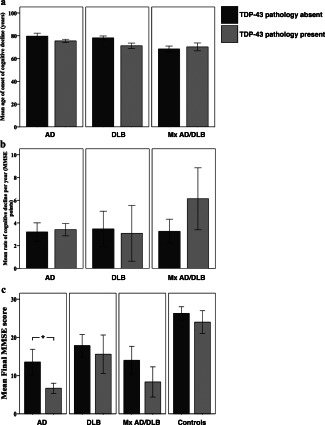
Age of onset of cognitive decline, rate of cognitive decline per year and final MMSE score in cases with and without TDP‐43 pathology. A. No significant differences were seen in age of onset between cases with and without TDP‐43 pathology in AD, DLB and Mx AD/DLB. B. No significant difference were seen in rate of cognitive decline per year between cases with and without TDP‐43 pathology in AD, DLB and Mx AD/DLB. C. Final MMSE score was significantly lower in AD cases with TDP‐43 pathology compared to AD cases without TDP‐43 pathology. No significant difference was seen in DLB, Mx AD/DLB or controls. AD, Alzheimer's disease, DLB, dementia with Lewy bodies; Mx AD/DLB, mixed AD/DLB; *, P < 0.05.
Additional pathological observations
CAA was observed in 45 AD cases (97.8%), 10 DLB cases (66.7%), 18 Mx AD/DLB cases (94.7%) and 18 aged control cases (46.2%) (Table 1). Hippocampal sclerosis was present in 11 cases (AD, 8; Mx AD/DLB, 1; aged control cases, 2) which all exhibited TDP‐43 pathology in the amygdala. TDP‐43 pathology was present in the hippocampus in ten cases (stage III), of which eight progressed to ITC (stage IV: AD, 6; aged control cases, 2). In general, 17.9% of cases exhibiting TDP‐43 pathology had hippocampal sclerosis.
Discussion
Here, we investigated TDP‐43 pathology in a cohort of AD, DLB, Mx AD/DLB and cognitively normal aged controls using the TDP‐43 in AD staging scheme. This is the first study to record the prevalence of TDP‐43 pathology in AD, DLB, Mx AD/DLB and aged control cases using a consistent staging scheme, that is, TDP‐43 in AD staging scheme 20. We have confirmed that TDP‐43 pathology is not exclusively found in AD, but is found across the spectrum of age‐related neurodegenerative diseases and in aged controls, where its spread follows the same topographical pattern that has been described for TDP‐43 pathology in AD. Our findings suggest that age has an independent role in the onset and development of TDP‐43 pathology. However, development of TDP‐43 pathology may also be exacerbated by the presence of AD pathology, as indicated by the higher prevalence of TDP‐43 pathology in AD and Mx AD/DLB.
Previously reported prevalence rates of TDP‐43 pathology in neurodegenerative diseases range from 29% to 72% in AD 1, 2, 8, 19, 20, 21, 22, 38, 39, from 0% to 56% in DLB 2, 12, 27, 38, 39 and from 10.5% to 36.4% in controls 3, 11, 27, 28, 37, 38, while only one study has been conducted in Mx AD/DLB showing a respective prevalence rate of 31% 27. While our data support previous studies, our reported TDP‐43 pathology prevalence rates for AD, DLB and aged controls are comparatively higher. We suggest that previously reported lower prevalence rates may be partly caused by inadequate sampling as some studies did not assess TDP‐43 pathology in the amygdala 8, 27, which has been shown to be the first and most severely affected region 12, 13, 20. Furthermore, rather than using antibodies specific for phosphorylated TDP‐43, some studies 12, 27 used antibodies that target the nonphosphorylated epitope and such an approach has been suggested to result in an underestimation of TDP‐43 pathology 38, 39.
Concomitant TDP‐43 pathology has been shown to modify the clinical phenotype of AD since AD subjects with TDP‐43 pathology had more severe cognitive impairment compared to AD subjects lacking TDP‐43 pathology 21. In our cohort, MMSE scores were lower in AD subjects with TDP‐43 pathology compared to those lacking TDP‐43 pathology, which is in agreement with findings from Josephs and colleagues 19. It is unclear whether the onset and evolution of TDP‐43 pathology in AD is associated with the fundamental neurodegenerative process of AD, or merely reflects age associated changes and has to be regarded as part of cerebral multimorbidity, which is frequently seen in aged human brains 15, 17, 23, 24, 34.
In our study the prevalence of TDP‐43 pathology in AD was almost 74%, which was significantly higher than in DLB and controls. Moreover, AD subjects showed highest stages of TDP‐43 pathology 20. However, there was no significant difference regarding the prevalence of TDP‐43 pathology between AD and Mx AD/DLB and the latter had more advanced TDP‐43 pathology than DLB. This may suggest a synergistic relationship between classical AD pathology, (ie, HP‐τ and Aβ) and TDP‐43 pathology as the combination of AD and DLB pathology (ie, α‐syn)—but not DLB alone—is associated with an increase in both prevalence and severity of TDP‐43 pathology. HP‐τ has previously been suggested as a possible risk factor for the development of TDP‐43 pathology as a small number of NFTs have been shown to colocalise with cytoplasmic TDP‐43 inclusions and were associated with reduced nuclear TDP‐43 immunopositivity 27. We were unable to determine associations between TDP‐43 in AD stage and Braak NFT stage in AD cases, likely due to a ceiling effect as all AD cases were classified as Braak NFT stage V/VI. Braak NFT stages are based on semi‐quantitative assessment and, therefore, quantitative methods could potentially help to clarify if the development of TDP‐43 pathology is directly influenced by HP‐τ pathology.
Although we found a correlation between TDP‐43 in AD stages and Braak NFT stages in aged controls this was not significant when controlled for age of death. Also, subjects with TDP‐43 pathology were significantly older than those without TDP‐43 pathology. However, when stratified by neuropathological diagnosis, only the difference in control subjects remained significant, suggesting that TDP‐43 pathology is associated with increasing age and can occur independently of concomitant AD pathology. A neuropathological study by Davidson and colleagues showed that the prevalence of TDP‐43 pathology was significantly higher in sporadic late onset AD (over 65 years of age) compared to sporadic early onset AD (under 65 years of age with no associated genetic mutations) 8, indicating an influence of age on the development of TDP‐43 pathology in AD. Furthermore, in our study no independent association between AD pathology and TDP‐43 pathology was seen in control cases, while age was positively correlated with TDP‐43 pathology. Conversely, we found a high prevalence and severity of TDP‐43 pathology in AD and Mx AD/DLB and therefore, we suggest that while age plays an important role in the onset of TDP‐43 pathology it may be exacerbated by concomitant AD pathology.
Of the cases exhibiting hippocampal sclerosis, 91% were positive for TDP‐43 in the hippocampus in agreement with previous studies indicating an association between the presence of hippocampal sclerosis and TDP‐43 deposition 1, 8, 19, 29. Although hippocampal sclerosis likely influences cognitive decline and cortical atrophy when present in AD 7, 29, an association between TDP‐43 pathology with cognitive decline, memory impairment and medial temporal atrophy have been shown to occur independent of hippocampal sclerosis 21. The presence of TDP‐43 without hippocampal sclerosis was evident in our cohort as only 17.9% of cases that exhibited TDP‐43 pathology had co‐existing hippocampal sclerosis, indicating the development of TDP‐43 pathology can be independent of hippocampal sclerosis.
In conclusion we employed a consistent methodology to evaluate the prevalence of TDP‐43 pathology in AD, DLB, Mx AD/DLB and aged controls. We confirmed that TDP‐43 pathology is not exclusively seen in AD, but is also present in DLB, Mx AD/DLB and aged controls, where its topographical progression follows the pattern described for TDP‐43 pathology in AD 20. In addition, our data suggest that TDP‐43 pathology is associated with age and exacerbated by the presence of concomitant AD pathology. Further studies are warranted to elucidate the interactions between AD and TDP‐43 pathology and to clarify the implications of additional TDP‐43 pathology for patient stratification, the development of biomarkers and novel therapies against age associated neurodegeneration.
Author Contributions
Study concept and design was conceived by K.E.M, L.W, D.E and J.A. Data acquisition, collection, analysis and interpretation were carried out by K.E.M, L.W and D.E. Clinical data was collected by A.J.T and I.G.M. K.E.M drafted the manuscript with critical revisions from L.W, D.E, A.T, I.M and J.A. All authors read and approved the final manuscript.
Funding
Part of the research was funded by the National Institute for Health Research (NIHR), Newcastle Biomedical Research Centre for Ageing, and Age‐related based at Newcastle Upon Tyne Hospitals National Health Service (NHS) Foundation Trust and Newcastle University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. K.E.M is currently funded by the Alzheimer's Society. L.W is currently funded by the NIHR Newcastle Biomedical Research Unit, and D.E from the Yvonne Emily Mairy bequest. Tissue for this study was provided by the Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK Medical Research Council (grant number G0400074) and by Brains for Dementia research, a joint venture between Alzheimer's Society and Alzheimer's Research UK.
Conflict of Interest
The authors declare they have no conflict of interest.
Acknowledgments
We are grateful to the individuals and their families who kindly donated their brains to the Newcastle Brain Tissue Resource. We thank Mrs Lynne Ramsay, Mrs Debbie Lett and Mrs Mary Johnson for their excellent technical support, and Dr Sean Colloby for statistical advice.
References
- 1. Amador‐Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R et al (2007) TDP‐43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol 61:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K et al (2009) Phosphorylated TDP‐43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol 117:125–136. [DOI] [PubMed] [Google Scholar]
- 3. Arnold SJ, Dugger BN, Beach TG (2013) TDP‐43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 126:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bigio EH (2011) TDP‐43 variants of frontotemporal lobar degeneration. J Mol Neurosci 45:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braak H, Braak E (1991) Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 82:239–259. [DOI] [PubMed] [Google Scholar]
- 6. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24:197–211. [DOI] [PubMed] [Google Scholar]
- 7. Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT (2014) Hippocampal sclerosis of aging is a key Alzheimer's disease mimic: clinical‐pathologic correlations and comparisons with both Alzheimer's disease and non‐tauopathic frontotemporal lobar degeneration. J Alzheimers Dis 39:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidson YS, Raby S, Foulds PG, Robinson A, Thompson JC, Sikkink S et al (2011) TDP‐43 pathological changes in early onset familial and sporadic Alzheimer's disease, late onset Alzheimer's disease and Down's syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol 122:703–713. [DOI] [PubMed] [Google Scholar]
- 9. Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36. [DOI] [PubMed] [Google Scholar]
- 10. Folstein MF, Folstein SE, McHugh PR (1975) “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- 11. Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK et al (2010) Pathological 43‐kDa transactivation response DNA‐binding protein in older adults with and without severe mental illness. Arch Neurol 67:1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K et al (2007) Concurrence of TDP‐43, tau and alpha‐synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res 1184:284–294. [DOI] [PubMed] [Google Scholar]
- 13. Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE (2008) Temporal lobar predominance of TDP‐43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 116:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National Institute on Aging‐Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ince PG (2001) Pathological correlates of late‐onset dementia in a multicentre, community‐based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 357:169–175. [DOI] [PubMed] [Google Scholar]
- 16. Jellinger KA (2007) The enigma of mixed dementia. Alzheimers Dement 3:40–53. [DOI] [PubMed] [Google Scholar]
- 17. Jellinger KA, Attems J (2007) Neuropathological evaluation of mixed dementia. J Neurol Sci 257:80–87. [DOI] [PubMed] [Google Scholar]
- 18. Jellinger KA, Attems J (2012) Neuropathology and general autopsy findings in nondemented aged subjects. Clin Neuropathol 31:87–98. [DOI] [PubMed] [Google Scholar]
- 19. Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M et al (2008) Abnormal TDP‐43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 70:1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR et al (2014) Staging TDP‐43 pathology in Alzheimer's disease. Acta Neuropathol 127:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM et al (2014) TDP‐43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol 127:811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM et al (2015) TAR DNA‐binding protein 43 and pathological subtype of Alzheimer's disease impact clinical features. Ann Neurol 78:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kovacs GG, Alafuzoff I, Al‐Sarraj S, Arzberger T, Bogdanovic N, Capellari S et al (2008) Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord 26:343–350. [DOI] [PubMed] [Google Scholar]
- 24. Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C et al (2013) Non‐Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community‐based autopsy series. Acta Neuropathol 126:365–384. [DOI] [PubMed] [Google Scholar]
- 25. McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 26. Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2012) National Institute on Aging‐Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakashima‐Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE et al (2007) Co‐morbidity of TDP‐43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229. [DOI] [PubMed] [Google Scholar]
- 28. Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM et al (2016) Higher prevalence of TDP‐43 proteinopathy in cognitively normal Asians: a clinicopathological study on a multiethnic sample. Brain Pathol 26:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E et al (2011) Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 134:1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al (2006) Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. [DOI] [PubMed] [Google Scholar]
- 31. Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ (1998) Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology 51:351–357. [DOI] [PubMed] [Google Scholar]
- 32. Ou SH, Wu F, Harrich D, Garcia‐Martinez LF, Gaynor RB (1995) Cloning and characterization of a novel cellular protein, TDP‐43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol 69:3584–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pollanen MS, Dickson DW, Bergeron C (1993) Pathology and biology of the Lewy body. J Neuropathol Exp Neurol 52:183–191. [DOI] [PubMed] [Google Scholar]
- 34. Rahimi J, Kovacs GG (2014) Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology 69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 36. Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H (2002) Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol 61:282–293. [DOI] [PubMed] [Google Scholar]
- 37.Thal, D.R., Rub, U., Orantes, M., Braak, H. (2002) Phases of A beta‐deposition in the human brain and its relevance for the development of AD. Neurology 12:1791–800. [DOI] [PubMed] [Google Scholar]
- 38. Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A et al (2015) Incidence and extent of TDP‐43 accumulation in aging human brain. Acta Neuropathol Commun 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA (2013) TDP‐43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 70:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]


