Abstract
Neuromyelitis optica (NMO) is characterized by severe optic neuritis and longitudinally extensive transverse myelitis. The discovery of an NMO‐specific autoantibody to the aquaporin‐4 (AQP4) water channel has improved knowledge of NMO pathogenesis. Many studies have focused on inflammatory and pathological biomarkers of NMO, including cytokines and chemokines. Increased concentrations of T helper (Th)17‐ and Th2‐related cytokines and chemokines may be essential factors for developing NMO inflammatory lesions. For example, interleukin‐6 could play important roles in NMO pathogenesis, as it is involved in the survival of plasmablasts that produce anti‐AQP4 antibody in peripheral circulation and in the enhancement of inflammation in the central nervous system. Therefore, assessment of these useful biomarkers may become a supportive criterion for diagnosing NMO. Significant advances in the understanding of NMO pathogenesis will lead to the development of novel treatment strategies. This review focuses on the current advances in NMO immunological research, particularly that of cytokines and chemokines.
Keywords: chemokine, cytokine, interleukin‐17, interleukin‐6, neuromyelitis optica, Th17
Introduction
Neuromyelitis optica (NMO) is an autoimmune inflammatory disorder of the central nervous system (CNS), clinically presenting with longitudinally extensive transverse myelitis (LETM) and optic neuritis 54. The discovery of the disease‐specific serum anti‐aquaporin‐4 (AQP4) antibody in NMO 25, 26 has dramatically changed the clinical definition of NMO, leading to recent advances in NMO research. The pathogenic role of anti‐AQP4 antibody was demonstrated in vivo by passive transfer experiments in animal models 7, 22, 34. Several lines of evidence differentiating between NMO and multiple sclerosis (MS) have accumulated based on pathology 30, 33, neuroimaging 16, immunological findings 38 and responses to immunotherapies 24, 29, 39. On the basis of these extensive data, NMO is now considered an anti‐AQP4 antibody‐mediated astrocytopathy distinct from demyelinating disorders as represented by MS 12. Besides anti‐AQP4 antibody, many additional biomarkers have proven useful for understanding the pathogenetic and immunological aspects of NMO 32, 36, 38. T and B cells may be implicated in the peripheral/CNS immune responses and pathogenesis of NMO, whereas various cytokines and chemokines have also been associated with the pathogenesis of NMO 38. Therefore, this review focuses on the current research on the roles of cytokines and chemokines in NMO pathogenesis and their therapeutic applications.
Cerebrospinal fluid (CSF) cytokines and chemokines in NMO patients
Many studies have analyzed CSF cytokine and chemokine levels in NMO patients (Table 1 ). Although some cytokines may increase nonspecifically because of CNS inflammation, several cytokines and chemokines are directly related to NMO pathogenesis. T helper (Th)17‐ and Th2‐related cytokines are upregulated in the CSF of NMO patients 38. CSF interleukin (IL)‐17 levels increase in patients with NMO 48 or opticospinal MS (OSMS; some of whom were considered to have NMO) 15, 37. Many studies have also shown increased CSF IL‐6 levels in patients with NMO. Presumably, NMO expresses Th17 and Th2 axes in CNS (Figure 1) differently from MS, which is primarily a Th1‐dominant disease. However, further studies are necessary to clarify the definite cytokine and chemokine profiles in NMO.
Table 1.
Cerebrospinal fluid (CSF) cytokine/chemokine levels in NMO patients
| CSF cytokines/chemokines | Axis | Change | Reference | Correlation |
|---|---|---|---|---|
| IL‐17 | Th17 | ↑ (vs. MS, ONNDs) | 48 | CSF HMGB1 |
| IL‐6 | Th17 | ↑ (vs. MS, HC) | 9 | |
| ↑ (vs. MS, ONNDs) | 14 | EDSS and AQP4 ab positivity | ||
| ↑ (vs. MS) | 55 | Definite form > limited form | ||
| ↑ (vs. MS, ONNDs) | 41 | CSF cells, CSF proteins and AQP4 ab positivity | ||
| ↑ (vs. MS, ONNDs) | 38 44 | CSF cells, CSF GFAP, AQP4 ab, recovery from relapse and relapse duration | ||
| ↑ (vs. MS, ONNDs) | 43 | CSF HMGB1 | ||
| ↑ (vs. MS, ONNDs) | 49 | EDSS | ||
| ↑ (vs. MS, ONNDs) | 48 | CSF HMGB1 | ||
| IL‐1ra | Th17 | ↑ (vs. MS, ONNDs) | 38 | CSF cells and CSF GFAP |
| G‐CSF | Th17 | ↑ (vs. MS, ONNDs) | 38 | CSF cells and CSF GFAP |
| IL‐8 | Th17 | ↑ (vs. MS, ONNDs) | 38 | CSF cells, CSF GFAP and EDSS |
| → (vs. MS) | 55 | |||
| IL‐13 | Th2 | ↑ (vs. MS, ONNDs) | 38 | CSF cells and CSF GFAP |
| IL‐5 | Th2 | ↑ (vs. MS, HC) | 9 | |
| Eotaxin‐2, ‐3 | Th2 | ↑ (vs. MS, HC) | 9 | |
| Eotaxin | Th2 | → (vs. MS, ONNDs) | 38 | |
| → (vs. MS, ONNDs) | 31 | |||
| TARC | Th2 | ↑ (vs. ONNDs) | 31 | |
| IL‐10 | Treg | ↑ (vs. ONNDs) → (vs. MS) | 38 | CSF cells, AQP4 ab and CSF GFAP |
| → (vs. MS) | 55 | |||
| IL‐12 | Th1 | ↑ (vs. HC) | 9 | |
| → (vs. MS, ONNDs) | 38 | |||
| IL‐1β | Th1 | ↑ (vs. MS) | 55 | Definite form > limited form |
| → (vs. MS, ONNDs) | 38 | |||
| CXCL10 (IP‐10) | Th1 | ↑ (vs. ONNDs) | 31 | |
| ↑ (vs. ONNDs) | 38 | CSF cells and CSF GFAP | ||
| CXCL13 | B cell | ↑ (vs. MS, ONNDs) | 57 | ARR and EDSS |
| IFN‐γ, G‐CSF, IL‐17 | ↑ (vs. CMS, ONNDs) | 37, * | ||
|
IL‐17, MIP‐1β, IL‐1β, IL‐13, IL‐8, IL‐10, TNF‐α, IL‐5 |
↑ (vs. ONNDs) ↑ (vs. CMS) |
15, * |
IL‐8: EDSS, albumin quotient and length of spinal cord lesion IL‐17: albumin quotient and length of spinal cord lesion |
*Cytokine analyses were performed in opticospinal MS patients.
AQP4 ab = aquaporin‐4 antibody; ARR = annualized relapse rate; CMS = conventional multiple sclerosis; CXCL = (C‐X‐C motif) ligand; EDSS = Expanded Disability Status Scale; G‐CSF = granulocyte colony‐stimulating factor; GFAP = glial fibrillary acidic protein; HC = healthy controls; HMGB1 = high mobility group box 1; IFN‐γ = interferon‐gamma; IL = interleukin; IP‐10 = interferon gamma‐induced protein 10; MIP = macrophage inflammatory protein; MS = multiple sclerosis; NMO = neuromyelitis optica; ONNDs = other noninflammatory neurological disorders; TARC = thymus and activation‐regulated chemokine; Th = T helper; TNF‐α = tumor necrosis factor‐alpha; Treg = regulatory T cell. ↑ = upregulation; → = unchanged.
Figure 1.
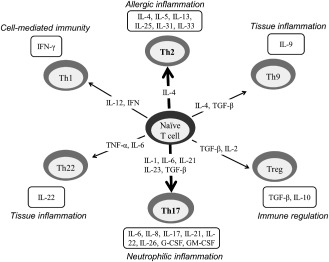
Differentiation pathways of naïve CD4+T cells. CD4+ T cells can differentiate into T helper (Th)1, Th2, Th9, Treg, Th17 or Th22 by the actions of differentiation cytokines. These T‐cell subsets promote different inflammatory responses based on their respective cytokine profiles, responses to chemokines and interactions with other cells. The Th17 and Th2 axes may be mainly upregulated in neuromyelitis optica. IL = interleukin; TGF = transformaing growth factor; TNF = tumor necrosis factor.
Th17‐related cytokines and chemokines
IL‐17 is involved in the development of autoimmune diseases and acts as a potent mediator in delayed‐type inflammatory reactions by increasing chemokine production in various tissues to recruit monocytes and neutrophils to the inflammation site. As described earlier, several reports have revealed elevated CSF IL‐17 levels in NMO 48 or OSMS patients 15, 37. Our own study could not confirm such an elevation, but levels of some Th17‐related cytokines and chemokines are reportedly increased in NMO patients 38.
Elevated CSF IL‐6 levels in NMO have also been reported. IL‐6 is a proinflammatory cytokine with a wide variety of functions. Secreted by immunocytes and activated astrocytes 11, it promotes immunoglobulin (Ig) synthesis in activated B cells and differentiation of naïve T cells into Th17 cells or cytotoxic T cells 6, 23. Among the several CSF cytokines and chemokines elevated in NMO, IL‐6 shows the strongest correlation with clinical variables in NMO; these include CSF glial fibrillary acidic protein (GFAP) levels, CSF cell counts and anti‐AQP4 antibody titers 38. Içöz et al reported that patients with NMO have higher CSF IL‐6 levels than those with optic neuritis, relapsing–remitting MS or healthy control (HC). Further, CSF IL‐6 levels in NMO patients correlate with anti‐AQP4 antibody titers and the Expanded Disability Status Scale (EDSS) score 14. Wang et al found that CSF IL‐6 and soluble IL‐6 receptor levels are significantly higher in patients with NMO than in those with MS and other noninflammatory neurological disorders (ONNDs) 49. Yanagawa et al reported elevated CSF IL‐6 levels in patients with definite NMO compared with those with limited NMO (anti‐AQP4‐positive myelitis without optic neuritis) 55. The CSF/serum ratio of IL‐6 is significantly higher in NMO than in ONNDs, suggesting that IL‐6 is mainly produced in the CNS of NMO patients 38. Although IL–6‐producing cells in CNS have not yet been identified, activated or damaged, astrocytes by anti‐AQP4 antibody may produce IL‐6 in the CNS of NMO patients. Of note, high CSF IL‐6 levels have been found in 82.3% of NMO patients, but no such increase has been observed in MS patients 38. CSF IL‐6 levels are also markedly high not only during relapse, but also during the initial attacks in NMO patients 45. Interestingly, CSF IL‐6 levels can predict recovery from NMO relapses and relapse‐free duration 44. NMO patients who relapse with optic neuritis exhibit high CSF IL‐6 levels, similar to NMO patients who relapse with myelitis 38, 45; nevertheless, optic neuritis lesions are usually much smaller than myelitis lesions in NMO patients. These data suggest that CSF IL‐6 is not a product of NMO inflammation, but an important molecule in the pathology of this disease. We have recently shown that CSF IL‐6 levels correlate with CSF levels of high mobility group box 1 (HMGB1), a proinflammatory mediator 43, and with CSF‐soluble intercellular adhesion molecule 1 levels, one of the markers of blood–brain barrier disruption 42. Accumulated evidence suggests important roles for CSF IL‐6 in NMO pathogenesis; these could include CNS inflammation, astrocytic damage and blood–brain barrier disruption. In addition, CSF IL‐6 may serve as a biomarker to diagnose NMO and differentiate it from MS. It remains unclear whether astrocytic damage releases IL‐6, or IL‐6 directly contributes to astrocytic damage and CNS inflammation in NMO.
IL‐1ra is a member of the IL‐1 cytokine family. CSF IL‐1ra levels are significantly elevated in NMO compared with MS or ONNDs and correlate with CSF cells and CSF GFAP levels 38. Granulocyte colony‐stimulating factor (G‐CSF) stimulates survival, proliferation and differentiation of neutrophils. CSF G‐CSF levels are higher in NMO than in MS and ONNDs and correlate with CSF GFAP levels and CSF cell counts 38. Tanaka et al reported significantly elevated CSF G‐CSF levels in patients with OSMS (some of whom were considered to have NMO) compared with conventional MS (CMS) and ONNDs, and found correlations with the albumin quotient, length of spinal magnetic resonance image (MRI) lesions and EDSS score 37. IL‐8 is known as a neutrophil chemotactic factor. CSF IL‐8 levels are significantly elevated in NMO compared with MS and ONNDs, and correlate with the CSF GFAP levels, CSF cell counts and EDSS score 38. However, Yanagawa et al reported no difference between CSF IL‐8 levels in NMO and MS patients 55. Ishizu et al found that significantly elevated CSF IL‐8 levels in OSMS patients (some of whom were considered to have NMO) compared with CMS and ONNDs. These levels correlate with the albumin quotient, length of spinal MRI lesion and EDSS score 15. They speculated that the markedly increased IL‐8 in CSF may be relevant to neutrophil infiltration in CNS.
Th2‐related cytokines and chemokines
Although IL‐4, a representative Th2‐related cytokine, has not been elevated in CSF 38, other Th2‐related cytokines and chemokines are upregulated in the CSF of NMO patients. The effects of IL‐13 on immune cells are similar to those of IL‐4. CSF IL‐13 levels are elevated in NMO compared with MS or ONNDs, and their levels correlate with CSF cells and CSF GFAP levels 38. IL‐5 stimulates B‐cell growth and increases Ig secretion; it is also a key mediator in eosinophil activation. CSF IL‐5 levels are significantly higher in NMO patients than in MS patients or HC 9. Eotaxin is an eosinophil‐selective chemokine. CSF eotaxin levels in NMO patients are similar to those in MS or ONNDs patients 31, 38. However, Correale and Fiol reported significant increases in CSF eotaxin‐2 and eotaxin‐3 levels in NMO patients compared with MS patients or HC 9. The chemokine thymus and activation‐regulated chemokine (TARC) specifically binds and induces chemotaxis in T cells. CSF TARC levels are significantly higher in NMO than in ONNDs 31.
Th1‐related cytokines and chemokines
Interferon‐gamma (IFN‐γ), a representative Th1‐related cytokine, has not been elevated in the CSF of NMO patients 38. (C‐X‐C motif) ligand (CXCL10) (interferon gamma‐induced protein 10) is secreted by several cells in response to IFN‐γ. CSF CXCL10 levels are significantly higher in NMO than in ONNDs 31, 38, and correlate with CSF GFAP levels and CSF cell counts 38. IL‐12 is involved in the differentiation of naïve T cells into Th1 cells and plays an important role in the activities of natural killer cells and T lymphocytes. CSF IL‐12 levels are significantly elevated in NMO patients compared with HC 9. However, some studies report no differences in CSF IL‐12 levels between NMO, MS or ONNDs 38. IL‐1β is an important mediator of the inflammatory response. CSF IL‐1β levels are elevated in patients with definite NMO compared with those with limited NMO 55. However, no differences have been found between NMO, MS or ONNDs patients 38.
Other cytokines and chemokines
IL‐10, a regulatory T (Treg)‐related cytokine with pleiotropic effects in immunoregulation and inflammation, is capable of inhibiting proinflammatory cytokine synthesis. CSF IL‐10 levels are significantly elevated in NMO compared with ONNDs 38, but no difference is observed in MS patients 38, 55. CSF IL‐10 levels correlate with CSF GFAP levels, CSF cell counts and anti‐AQP4 antibody titers 38.
CXCL13 is selectively chemotactic for B cells. CSF CXCL13 levels are significantly higher in NMO than in MS or ONNDs and correlate with the annualized relapse rate and EDSS score 57. Alvarez et al reported elevated CSF CXCL13 levels in NMO and MS patients compared with ONNDs, which correlate with CSF cell counts in NMO patients 1.
Serum/plasma cytokines and chemokines in NMO patients
Serum/plasma cytokine and chemokine levels in NMO patients are summarized in Table 2. As with CSF analyses, Th17‐ and Th2‐related cytokines and chemokines are predominantly upregulated in the serum/plasma of NMO patients (Figure 1).
Table 2.
Serum cytokine/chemokine levels in NMO patients
| Serum cytokines/chemokines | Axis | Change | Reference | Correlation |
|---|---|---|---|---|
| IL‐17 | Th17 | ↑ (vs. HC) | 51 | |
| ↑ (vs. HC) | 52 | HMGB1 | ||
| ↑ (vs. HC) | 27 | |||
| ↑ (vs. MS, HC) | 50 | |||
| ↑ (vs. NMO without LSCL) | 13 | Length of spinal cord lesion | ||
| IL‐6 | Th17 | ↑ (vs. HC) | 28 | EDSS |
| ↑ (vs. MS, HC) | 9 | |||
| ↑ (vs. MS, ONNDs) | 14 | |||
| ↑ (vs. ONNDs) | 38 | |||
| ↑ (vs. HC) | 47 | IL‐32 | ||
| ↑ (vs. HC) | 46 | |||
| ↑ (vs. HC) | 51 | |||
| IL‐23 | Th17 | ↑ (vs. HC) | 27 | |
| ↑ (vs. HC) | 50 | |||
| ↑ (vs. HC) | 28 | |||
| IL‐21 | Th17 | ↑ (vs. HC) | 50 | |
| ↑ (vs. HC) | 28 | EDSS | ||
| IL‐4 | Th2 | ↑ (vs. HC) | 2 | |
| ↑ (vs. MS, HC) | 51 | |||
| IL‐10 | Treg | ↑ (vs. MS, HC) | 51 | |
| IL‐2 | Treg | ↑ (vs. MS, HC) | 51 | |
| ↓ (vs. HC) | 28 | |||
| IFN‐γ | Th1 | ↑ (vs. HC) | 52 | HMGB1 |
| ↑ (vs. MS, HC) | 51 | |||
| ↓ (vs. HC) | 28 | |||
| TNF‐α | Th1 | ↑ (vs. HC) | 52 | HMGB1 |
| ↑ (vs. HC) | 51 | |||
| IL‐32 | ↑ (vs. MS, HC) | 47 | IL‐6, EDSS |
EDSS = Expanded Disability Status Scale; HC = healthy controls; HMGB1 = high mobility group box 1; IFN‐γ = interferon‐gamma; IL = interleukin; LSCL = long spinal cord lesions greater than three vertebral segments; MS = multiple sclerosis; NMO = neuromyelitis optica; ONNDs = other noninflammatory neurological disorders; Th = T helper; TNF‐α = tumor necrosis factor‐alpha; Treg = regulatory T cell. ↑ = upregulation; ↓ = downregulation.
Serum/plasma IL‐17 levels increase in NMO patients compared with HC or MS patients 27, 50, 51, 52. Plasma IL‐17 levels correlate with plasma HMGB1 levels 52. NMO patients with LETM (more than three vertebral segments) have higher serum IL‐17 levels than NMO patients without LETM 13. NMO patients in the relapse phase have significantly higher serum IL‐6 levels than ONND patients 38. Içöz et al also reported that patients with NMO, particularly those who are anti‐AQP4 antibody positive, have higher serum IL‐6 levels than those with optic neuritis, relapsing–remitting MS or HC 14. Wang et al found that plasma IL‐6 levels are higher in NMO patients than in HC and are positively correlated with IL‐32 levels 47. AQP4‐specific T‐cell responses are amplified in NMO patients and exhibit a Th17 bias, and intracellular IL‐6 production increases after lipopolysaccharide stimulation in monocytes from NMO patients 46. The number of anti‐myelin oligodendrocyte glycoprotein IL‐6‐ and IL‐12‐secreting cells in the peripheral blood and CSF of NMO patients is higher than that of MS, ONNDs or HC 9. The release of IL‐6, IL‐21 and IL‐23 from activated peripheral blood mononuclear cells is significantly higher in NMO patients than in controls, and IL‐6 and IL‐21 levels positively correlate with the EDSS score in NMO patients 28. Although the role of IL‐6 in peripheral blood is unclear, Chihara et al recently reported that the population of plasmablasts exhibiting the CD19intCD27highCD38highCD180− phenotype selectively increases in the peripheral blood of NMO patients, and that these plasmablasts are major producers of anti‐AQP4 antibodies 8. IL‐6 enhances plasmablast survival and anti‐AQP4 antibody production in these cells, whereas anti‐IL‐6 receptor antibody lessens their survival. IL‐6 in the peripheral blood of NMO patients is implicated in the peripheral immune response and anti‐AQP4 antibody production. Serum IL‐23 and IL‐21 levels are also elevated in NMO patients compared with HC 27, 50.
The Th2 cytokine IL‐4 is upregulated in the serum of NMO patients compared with HC and MS patients 2, 51. Other Th2‐related cytokines and chemokines have not been analyzed.
Studies of Treg‐related cytokines show that IL‐10 and IL‐2 levels increase significantly in NMO patients compared with MS patients and HC 51, but Linhares et al reported that IL‐2 levels decrease significantly in NMO patients compared with controls 28.
The levels of the Th1‐related cytokines IFN‐γ and tumor necrosis factor‐alpha (TNF‐α) increase in NMO patients compared with HC and MS patients 51, 52, and are correlated with plasma HMGB1 levels 52.
Chemokine receptor expression on peripheral blood T cells in NMO patients
CD8+CXCR3+T cells might affect the pathogenesis of both NMO and MS, and could be an important marker of disease activity. The CD8+CXCR3+/CD8+CCR4+ ratio, which reflects immune and inflammatory activities, is higher in NMO than in MS patients 35. Th1 dominance of chemokine receptors on blood T cells and the correlation between CXCR3+ T cells and disease activity have been confirmed by analyzing chemokine receptors on peripheral blood lymphocytes during the relapse phase in MS patients. However, such deviations in the Th1/Th2 balance have not been observed in NMO patients 40.
Pathogenic role of cytokines and chemokines
IL‐6 infusion into the spinal subarachnoid space of rats induces progressive weakness with CNS inflammation, axonal degeneration and myelin loss 18. CSF IL‐6 is mainly produced by astrocytes in transverse myelitis patients, and its levels correlate with astrocytic expression and disease severity 18. IFN‐β treatment is effective in reducing experimental autoimmune encephalomyelitis (EAE) symptoms induced by Th1 cells, but exacerbates disease induced by Th17 cells 4. The Th17 EAE model represents several aspects of NMO, suggesting that Th17 cells may play a pathogenic role in NMO pathogenesis. Ex vivo experiments performed on murine spinal cords have revealed that slices exposed to NMO IgG and human complement exhibit NMO‐like lesions. These lesions increase in severity with the addition of neutrophils, natural killer cells, macrophages or cytokines (such as TNF‐α, IL‐6, IL‐1β or IFN‐γ), implicating specific immune cells and cytokines may amplify tissue damage in NMO 56.
Therapeutic implications of cytokine blockade in NMO patients
Low‐dose oral corticosteroids, azathioprine, mitoxantrone, cyclophosphamide, mycophenolate mofetil and rituximab are used as maintenance treatments to prevent NMO relapses 10, 17, 20, 21, 53. Novel treatments using the IL‐6 pathway blocker tocilizumab, a recombinant humanized monoclonal antibody against the IL‐6 receptor, may be useful for suppressing relapses in NMO patients who cannot tolerate standard immunosuppression therapy 3, 5, 19. Treatment with tocilizumab rapidly reduces the number of elevated plasmablasts and anti‐AQP4 antibody titers in NMO patients. Furthermore, neuropathic pain and disability scores improve gradually 3. Patients with highly active anti‐AQP4 antibody‐positive NMO, in whom numerous immunosuppressive interventions had failed, exhibited improved EDSS scores and annualized relapse rates after initiating tocilizumab. Tocilizumab significantly reduces CSF IL‐6 levels, signal transducer and activation of transcription 3 (STAT3) activation 19. Three female patients with anti‐AQP4 antibody‐positive NMO, who were resistant to rituximab treatment, exhibited a decrease in the median annualized relapse rate from 3.0 to 0.6 after treatment with tocilizumab 5. IL‐6 receptor‐blocking therapy can be effective against NMO even in patients who fail to respond to conventional therapy. This direct clinical evidence suggests that IL‐6 may be a critical molecule in NMO immunopathogenesis. In the future, other cytokine‐blocking therapies may also be applied clinically.
Conclusions
A growing number of recent immunological studies have supported the important role of cytokines and chemokines in NMO pathogenesis. Although many cytokines and chemokines are upregulated in both the peripheral and CNS of NMO patients, Th17‐ and Th2‐related cytokines and chemokines, particularly Th17‐related cytokines, may be key players in NMO inflammation. IL‐6 in the peripheral blood is implicated in anti‐AQP4 antibody production in NMO patients, and IL‐6 in CSF plays important roles in CNS inflammation, astrocytic damage and blood–brain barrier disruption. Thus, IL‐6‐blocking therapy with tocilizumab may be a promising treatment option for NMO patients. New treatments need to be developed to prevent severe relapses in these patients. A better understanding of the role of cytokines and chemokines in NMO pathogenesis is critical to developing effective treatments.
Acknowledgments
Financial Disclosure: None reported.
Funding/Support: This study was supported, in part, by the Ministry of Education, Science and Technology (Akiyuki Uzawa, grant number 24790873).
References
- 1. Alvarez E, Piccio L, Mikesell RJ, Klawiter EC, Parks BJ, Naismith RT, Cross AH (2013) CXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditions. Mult Scler 19:1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alves‐Leon SV, Pimentel ML, Sant'Anna G, Malfetano FR, Estrada CD, Quirico‐Santos T (2008) Immune system markers of neuroinflammation in patients with clinical diagnose of neuromyelitis optica. Arq Neuropsiquiatr 66:678–684. [DOI] [PubMed] [Google Scholar]
- 3. Araki M, Aranami T, Matsuoka T, Nakamura M, Miyake S, Yamamura T (2013) Clinical improvement in a patient with neuromyelitis optica following therapy with the anti‐IL‐6 receptor monoclonal antibody tocilizumab. Mod Rheumatol 23:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P et al (2010) T helper type 1 and 17 cells determine efficacy of interferon‐beta in multiple sclerosis and experimental encephalomyelitis. Nat Med 16:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ayzenberg I, Kleiter I, Schröder A, Hellwig K, Chan A, Yamamura T, Gold R (2013) Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti‐CD20 therapy. JAMA Neurol 70:394–397. [DOI] [PubMed] [Google Scholar]
- 6. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 441:235–238. [DOI] [PubMed] [Google Scholar]
- 7. Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M et al (2009) Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo . Ann Neurol 66:630–643. [DOI] [PubMed] [Google Scholar]
- 8. Chihara N, Aranami T, Sato W, Miyazaki Y, Miyake S, Okamoto T et al (2011) Interleukin 6 signaling promotes anti‐aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci U S A 108:3701–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Correale J, Fiol M (2004) Activation of humoral immunity and eosinophils in neuromyelitis optica. Neurology 63:2363–2370. [DOI] [PubMed] [Google Scholar]
- 10. Costanzi C, Matiello M, Lucchinetti CF, Weinshenker BG, Pittock SJ, Mandrekar J et al (2011) Azathioprine: tolerability, efficacy, and predictor of benefit in neuromyelitis optica. Neurology 77:659–666. [DOI] [PubMed] [Google Scholar]
- 11. Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145. [DOI] [PubMed] [Google Scholar]
- 12. Fujihara K, Misu T, Nakashima I, Takahashi T, Bradl M, Lassmann H et al (2012) Neuromyelitis optica should be classified as an astrocytopathic disease rather than a demyelinating disease. Clin Exp Neuroimmunol 3:58–73. [Google Scholar]
- 13. Herges K, de Jong BA, Kolkowitz I, Dunn C, Mandelbaum G, Ko RM et al (2012) Protective effect of an elastase inhibitor in a neuromyelitis optica‐like disease driven by a peptide of myelin oligodendroglial glycoprotein. Mult Scler 18:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Içöz S, Tüzün E, Kürtüncü M, Durmuş H, Mutlu M, Eraksoy M, Akman‐Demir G (2010) Enhanced IL‐6 production in aquaporin‐4 antibody positive neuromyelitis optica patients. Int J Neurosci 120:71–75. [DOI] [PubMed] [Google Scholar]
- 15. Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y et al (2005) Intrathecal activation of the IL‐17/IL‐8 axis in opticospinal multiple sclerosis. Brain 128:988–1002. [DOI] [PubMed] [Google Scholar]
- 16. Ito S, Mori M, Makino T, Hayakawa S, Kuwabara S (2009) “Cloud‐like enhancement” is a magnetic resonance imaging abnormality specific to neuromyelitis optica. Ann Neurol 66:425–428. [DOI] [PubMed] [Google Scholar]
- 17. Jacob A, Matiello M, Weinshenker BG, Wingerchuk DM, Lucchinetti C, Shuster E et al (2009) Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol 66:1128–1133. [DOI] [PubMed] [Google Scholar]
- 18. Kaplin AI, Deshpande DM, Scott E, Krishnan C, Carmen JS, Shats I et al (2005) IL‐6 induces regionally selective spinal cord injury in patients with the neuroinflammatory disorder transverse myelitis. J Clin Invest 115:2731–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kieseier BC, Stüve O, Dehmel T, Goebels N, Leussink VI, Mausberg AK et al (2013) Disease amelioration with tocilizumab in a treatment‐resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol 70:390–393. [DOI] [PubMed] [Google Scholar]
- 20. Kim SH, Kim W, Li XF, Jung IJ, Kim HJ (2011) Repeated treatment with rituximab based on the assessment of peripheral circulating B cells in patients with relapsing neuromyeltis optica. Arch Neurol 68:1412–1420. [DOI] [PubMed] [Google Scholar]
- 21. Kim SH, Kim W, Park MS, Sohn EH, Li XF, Kim HJ (2011) Efficacy and safety of mithoxantrone in patients with highly relapsing neuromyelitis optica. Arch Neurol 68:473–479. [DOI] [PubMed] [Google Scholar]
- 22. Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, Okuno T et al (2009) Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun 386:623–627. [DOI] [PubMed] [Google Scholar]
- 23. Kishimoto T (2005) Interleukin‐6: from basic science to medicine—40 years in immunology. Annu Rev Immunol 23:1–21. [DOI] [PubMed] [Google Scholar]
- 24. Kleiter I, Hellwig K, Berthele A, Kümpfel T, Linker RA, Hartung HP, et al, for the Neuromyelitis Optica Study Group (2012) Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol 69:239–245. [DOI] [PubMed] [Google Scholar]
- 25. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005) IgG marker of optic‐spinal multiple sclerosis binds to the aquaporin‐4 water channel. J Exp Med 202:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K et al (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Wang H, Long Y, Lu Z, Hu X (2011) Increased memory Th17 cells in patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol 234:155–160. [DOI] [PubMed] [Google Scholar]
- 28. Linhares UC, Schiavoni PB, Barros PO, Kasahara TM, Teixeira B, Ferreira TB et al (2013) The ex vivo production of IL‐6 and IL‐21 by CD4(+) T cells is directly associated with neurological disability in neuromyelitis optica patients. J Clin Immunol 33:179–189. [DOI] [PubMed] [Google Scholar]
- 29. Min JH, Kim BJ, Lee KH (2012) Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler 18:113–115. [DOI] [PubMed] [Google Scholar]
- 30. Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S et al (2007) Loss of aquaporin‐4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130:1224–1234. [DOI] [PubMed] [Google Scholar]
- 31. Narikawa K, Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y (2004) CSF chemokine levels in relapsing neuromyelitis optica and multiple sclerosis. J Neuroimmunol 149:182–186. [DOI] [PubMed] [Google Scholar]
- 32. Okada K, Matsushita T, Kira J, Tsuji S (2010) B‐cell activating factor of the TNF family is upregulated in neuromyelitis optica. Neurology 74:177–178. [DOI] [PubMed] [Google Scholar]
- 33. Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W et al (2007) Pattern‐specific loss of aquaporin‐4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130:1194–1205. [DOI] [PubMed] [Google Scholar]
- 34. Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC (2010) Intra‐cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimizu Y, Ota K, Kubo S, Kabasawa C, Kobayashi M, Ohashi T, Uchiyama S (2011) Association of Th1/Th2‐related chemokine receptors in peripheral T cells with disease activity in patients with multiple sclerosis and neuromyelitis optica. Eur Neurol 66:91–97. [DOI] [PubMed] [Google Scholar]
- 36. Takano R, Misu T, Takahashi T, Sato S, Fujihara K, Itoyama Y (2010) Astrocytic damage is far more severe than demyelination in NMO: a clinical CSF biomarker study. Neurology 75:208–216. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka M, Matsushita T, Tateishi T, Ochi H, Kawano Y, Mei FJ et al (2008) Distinct CSF cytokine/chemokine profiles in atopic myelitis and other causes of myelitis. Neurology 71:974–981. [DOI] [PubMed] [Google Scholar]
- 38. Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S et al (2010) Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin‐6. Mult Scler 16:1443–1452. [DOI] [PubMed] [Google Scholar]
- 39. Uzawa A, Mori M, Hayakawa S, Masuda S, Kuwabara S (2010) Different responses to interferon beta‐1b treatment in patients with neuromyelitis optica and multiple sclerosis. Eur J Neurol 17:672–676. [DOI] [PubMed] [Google Scholar]
- 40. Uzawa A, Mori M, Hayakawa S, Masuda S, Nomura F, Kuwabara S (2010) Expression of chemokine receptors on peripheral blood lymphocytes in multiple sclerosis and neuromyelitis optica. BMC Neurol 10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uzawa A, Mori M, Ito M, Uchida T, Hayakawa S, Masuda S, Kuwabara S (2009) Markedly increased CSF interleukin‐6 levels in neuromyelitis optica, but not in multiple sclerosis. J Neurol 256:2082–2084. [DOI] [PubMed] [Google Scholar]
- 42. Uzawa A, Mori M, Masuda S, Kuwabara S (2011) Markedly elevated soluble intercellular adhesion molecule 1, soluble vascular cell adhesion molecule 1 levels, and blood–brain barrier breakdown in neuromyelitis optica. Arch Neurol 68:913–917. [DOI] [PubMed] [Google Scholar]
- 43. Uzawa A, Mori M, Masuda S, Muto M, Kuwabara S (2013) CSF high‐mobility group box 1 is associated with intrathecal inflammation and astrocytic damage in neuromyelitis optica. J Neurol Neurosurg Psychiatry 84:517–522. [DOI] [PubMed] [Google Scholar]
- 44. Uzawa A, Mori M, Sato Y, Masuda S, Kuwabara S (2012) CSF interleukin‐6 level predicts recovery from neuromyelitis optica relapse. J Neurol Neurosurg Psychiatry 83:339–340. [DOI] [PubMed] [Google Scholar]
- 45. Uzawa A, Mori M, Sawai S, Masuda S, Muto M, Uchida T et al (2013) Cerebrospinal fluid interleukin‐6 and glial fibrillary acidic protein levels are increased during initial neuromyelitis optica attacks. Clin Chim Acta 421:181–183. [DOI] [PubMed] [Google Scholar]
- 46. Varrin‐Doyer M, Spencer CM, Schulze‐Topphoff U, Nelson PA, Stroud RM, Cree BA, Zamvil SS (2012) Aquaporin 4‐specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol 72:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang H, Wang K, Wang C, Xu F, Qiu W, Hu X (2013) Increased plasma interleukin‐32 expression in patients with neuromyelitis optica. J Clin Immunol 33:666–670. [DOI] [PubMed] [Google Scholar]
- 48. Wang H, Wang K, Wang C, Xu F, Zhong X, Qiu W, Hu X (2013) Cerebrospinal fluid high‐mobility group box protein 1 in neuromyelitis optica and multiple sclerosis. Neuroimmunomodulation 20:113–118. [DOI] [PubMed] [Google Scholar]
- 49. Wang H, Wang K, Zhong X, Dai Y, Qiu W, Wu A, Hu X (2012) Notable increased cerebrospinal fluid levels of soluble interleukin‐6 receptors in neuromyelitis optica. Neuroimmunomodulation 19:304–308. [DOI] [PubMed] [Google Scholar]
- 50. Wang HH, Dai YQ, Qiu W, Lu ZQ, Peng FH, Wang YG et al (2011) Interleukin‐17‐secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci 18:1313–1317. [DOI] [PubMed] [Google Scholar]
- 51. Wang KC, Lee CL, Chen SY, Chen JC, Yang CW, Chen SJ, Tsai CP (2013) Distinct serum cytokine profiles in neuromyelitis optica and multiple sclerosis. J Interferon Cytokine Res 33:58–64. [DOI] [PubMed] [Google Scholar]
- 52. Wang KC, Tsai CP, Lee CL, Chen SY, Chin LT, Chen SJ (2012) Elevated plasma high‐mobility group box 1 protein is a potential marker for neuromyelitis optica. Neuroscience 226:510–516. [DOI] [PubMed] [Google Scholar]
- 53. Watanabe S, Misu T, Miyazawa I, Nakashima I, Shiga Y, Fujihara K, Itoyama Y (2007) Low‐dose corticosteroids reduce relapses in neuromyelitis optica: a retrospective analysis. Mult Scler 13:968–974. [DOI] [PubMed] [Google Scholar]
- 54. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 55. Yanagawa K, Kawachi I, Toyoshima Y, Yokoseki A, Arakawa M, Hasegawa A et al (2009) Pathologic and immunologic profiles of a limited form of neuromyelitis optica with myelitis. Neurology 73:1628–1637. [DOI] [PubMed] [Google Scholar]
- 56. Zhang H, Bennett JL, Verkman AS (2011) Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol 70:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhong X, Wang H, Dai Y, Wu A, Bao J, Xu W et al (2011) Cerebrospinal fluid levels of CXCL13 are elevated in neuromyelitis optica. J Neuroimmunol 240–241:104–108. [DOI] [PubMed] [Google Scholar]


