Clinical History
A 20 year‐old male complaining of headache, photophobia and altered mental status arrived to the ER from another hospital. His symptoms began a few months earlier with diarrhea and vomiting, daily fever up to 39°C, and weight loss. At that time, the neurological examination showed a Glasgow Coma Scale (GCS) score of 8 points and nuchal rigidity. A brain CT scan was normal, and ceftriaxone 2 gr per day, vancomycin 2 gr every 12 hours, ampicillin 2 gr every 4 hours, clarithromycin 500 mg every 12 hours, and acyclovir 800 mg every 5 hours were started. Because there was no improvement, two days later he was transferred to our hospital. At entry the heart rate was 53 beats per minute, breathing rate was 18 per minute with Cheyne‐Stokes respiration, blood pressure was 100/60 mmHg, and temperature was 38°C. The GCS score was of 6 points: 2 points for the ocular response, 1 point for the verbal response and 3 points for the motor the response. His head and gaze were persistently deviated to the right. He had bilateral papilledema, and his pupils were asymmetric, with the right pupil size of 2 mm and the left of 5 mm. Strength was abated, but when pain was elicited he acquired a posture of decerebration. The Hoffman and Babinski signs were produced on the right side. He had involuntary myoclonic jerks in the upper and lower extremities that lasted more than 5 minutes. Nuchal rigidity was present. He was intubated and put on mechanical ventilation. Dexamethasone was administrated for cerebral edema, levetiracetam and propofol for persistent epileptic seizures. Amphotericin B was added and the antibiotics were continued. Two days later mannitol, meropenem, rifampicin, pyrazinamide and ethambutol were included, however, the patient continued to be hypothermic and had severe slow heart rate and hypotension. He died 9 days later.
Laboratory and Neuroimage
CBC showed a total blood count of 7000 cells/mm3 (neutrophils of 87%, lymphocytes of 7.95% and monocytes of 4.8%), hemoglobin of 15.4 gr/dL and platelets of 124,000 cells/mm3. Serum glucose was 101 mg/dL, creatinine was 0.9 mg/L and sodium was 134 mg/dL. The rest of the laboratory studies including liver function tests were normal. An ELISA for HIV was negative.
A brain MRI showed enlargement of the ventricles with transependymal migration of CSF surrounding the ventricles symptomatic of acute hydrocephalus, effacement of sulci due to cerebral edema, bilateral extensive hyperintense images in the basal ganglia and surrounding parenchyma in the DWI suggesting ischemic lesions in both territories of the middle cerebral artery (MCA) (Figure 1a, 1b), and generalized dense leptomeningeal enhancement and arachnoiditis in the post‐contrast T1‐weighted images. An angioMRI showed a severely injured left MCA (Figure 1c).
Figure 1.
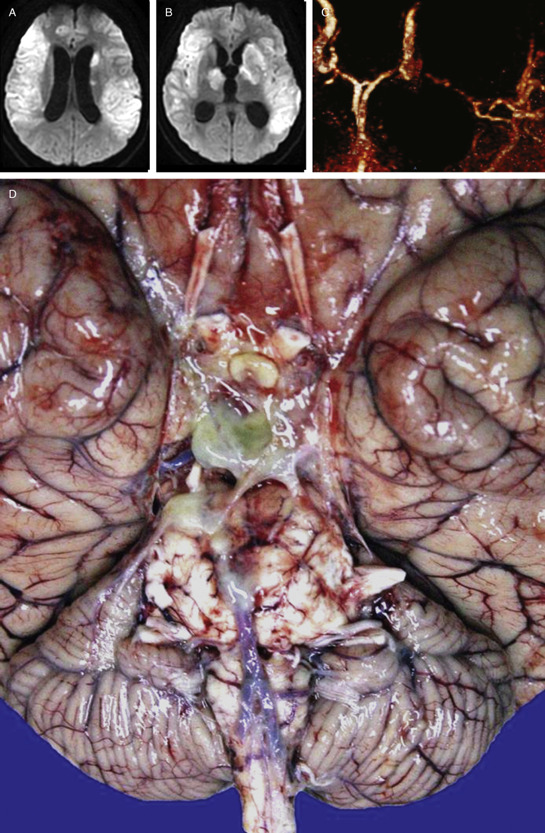
A lumbar puncture was performed with an opening pressure of 22 cmH20, and the CSF showed a pH of 7.47, glucose of 52 mm/dL, proteins of 111 mg/dL, and WBC of 32cells/mm3. Adenosine deaminase enzyme (ADA) in CSF was 9 UI. CSF PCR for M. tuberculosis, CMV, VSH and VHZ were negative, as well as the cryptococcus and pneumococcus antigen, and the CSF IgG for toxoplasmosis. A transcranial Doppler ultrasound demonstrated bilateral high velocities of the middle and anterior cerebral arteries suggestive of vasospasm. The EEG showed generalized theta rhythm.
Pathology
The total brain weight was 1450 g. An extensive green dense exudate was found in the basal subarachnoid cisterns of the brain (Figure 1d). Cerebral edema was found and both cerebellar amygdalas were displaced downward. In contrast to what was shown in the MRI, only one infarct was found at the left thalamus (Figure 2a). Microscopically, the brain had features of acute purulent meningitis, which were more prominent on the basal surface of the cerebrum, consisting of areas of inflammatory infiltrate composed of lymphocytes and plasmatic cells outspread with epithelioid histiocytes, alternated with extensive areas of necrosis (Figure 2b). The characteristic Langhans′ gigantic cells or caseous necrosis were not observed. The Ziehl‐Neelsen acid‐fast stain is shown in Figure 2c. Because there were so many, a PAS stain was performed specifically to rule‐out MAC complex, which was negative. The lumen of the MCA branches was clear, and there were no inflammatory infiltrates in any other vessel. What is your diagnosis?
Figure 2.
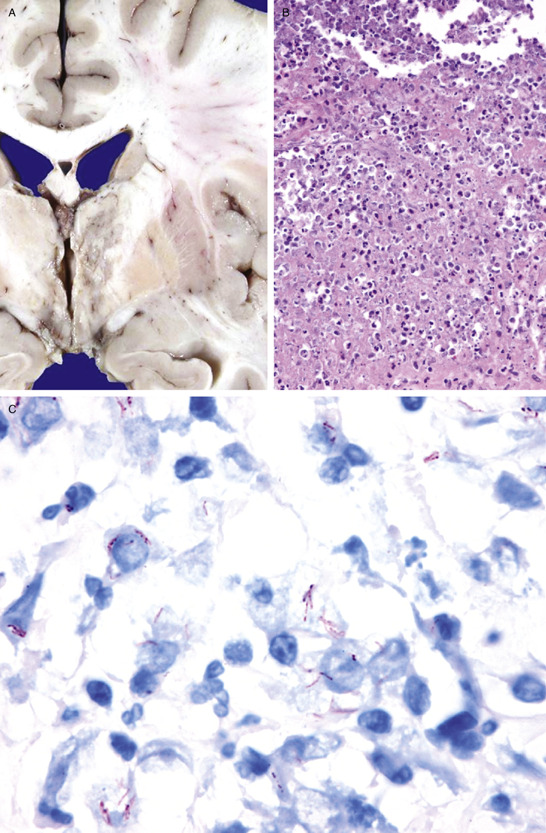
Diagnosis
Tuberculous meningitis.
Discussion
Tuberculosis (TB) is an infectious disease that continues to be a significant public health concern 1. Mycobacterium tuberculosis is the organism that is the causative agent for tuberculosis. In most patients, TB involving the leptomeninges is thought to spread by hematogenous dissemination from a primary source outside the CNS such as the lung or gastrointestinal tract. In the brain, a meningeal pattern of spread can occur, and the cerebrospinal fluid typically shows a high protein, low glucose and lymphocytosis. The most common finding in the image studies is communicating hydrocephalus, and it may be the only clue for diagnosis. Hydrocephalus may result from blockage of the basal subarachnoid cisterns by the dense basal exudate, or narrowing of the aqueduct and third ventricles by tuberculomas.
The base of the brain is often involved, so the proximal ends of the basal arteries are involved. The extension of the inflammatory exudate along the perforating blood vessels into the brain substance causes vascular damage, which may lead to spasm or thrombosis of the vessels, with resulting ischemia or infarction, most frequently seen at the basal ganglia and internal capsule. Although a basal ganglia infarct was found in this patient, the extensive ischemic lesions seen in the MRI were not found in the pathologic examination. Furthermore, vessels examination did not show the extensive vasculitis or thrombosis that we suspected. Despite the undisputed improvements in cerebral imaging with MRI, the consistency between the radiological and pathological findings is still poor. The pivotal event of ischemia is the decrease of cerebral blood flow (CBF), and flow thresholds may predict the tissue fate in the acute phase. CBF threshold that defines critical ischemia leading to irreversible infarction ranges from 8 to 12 ml/100 g per minute 5. Tissues experiencing perfusion values below this threshold have high probability to turn into irreversible infarction unless perfusion is quickly restored. The CBF values >20 mL/100 g per minute on the other hand, do not lead to a functional impairment of neuronal tissue and are termed as oligemia or normoperfusion. Thus, we assume that even with the dramatic brain images we showed, such low CBF was not achieved in our patient. This is relevant in the setting that many patients with infectious infarcts show great improvement and no neurological sequels after the infection is resolved, and it should be considered when doubt exists, as in this case, of pursuing more aggressive approaches as neurosurgery for treating the hydrocephalus.
Other interesting aspect of this case was the thick, yellowish, pus‐like exudate in the basal surface of the brain, and the absence of epithelioid cell granulomas with caseous necrosis in the meninges or brain parenchyma. This appearing‐like suppurative lesion could have been misdiagnosed pathologically as acute purulent meningitis caused by pyogenic bacteria, fortunately, numerous acid‐fast bacilli were found, but they could have been obviated if special stains would have not been used. Purulent meningitis has only been reported in 1.3% of the cases of meningeal tuberculosis 2, 3. While the typical granulomatous reaction is typical of tuberculosis, the diagnosis of tuberculosis meningitis may be established solely with the demonstration of tubercle bacilli 4. These type of “non‐reactive tuberculous lesions” have been previously associated with severely immune compromised patients 4. The case demonstrates the variability that exists not only of the clinical but also of the pathological picture seen in patients with mycobacterium tuberculosis infection.
References
- 1. Khoo JLS, Lau KY, Cheung CM, Tsoi TH (2003) Central Nervous System Tuberculosis. J HK Coll Radiol 6:217–228. [Google Scholar]
- 2. Kornetova NV. Variants of meningeal tuberculosis in adults (). Probl Tuberk 12:26–28. [PubMed] [Google Scholar]
- 3. Prakash B, Mehta R, Gondal S, Kumar S, Malhotra V (1989) Tuberculous Abscess of the Brain Stem. Surg Neurol 32:445–448. [DOI] [PubMed] [Google Scholar]
- 4. Shintaku M, Sasaki M, Sensaki H, Morri S, Marouka M, Ueda Y (1991) An Autopsy Case of Purulent Mycobacterial Meningitis in AIDS. Acta Pathologica Japonica 41(12):895–899. [DOI] [PubMed] [Google Scholar]
- 5. Sobesky J, Refinin G (2012) The mismatch concept in acute stroke: lessons learned from PET and MRI. Journal of Cerebral Blood Flow & Metabolism 32:1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]


