Abstract
Brain endothelial cells have unique properties in terms of barrier function, local molecular signaling, regulation of local cerebral blood flow (CBF) and interactions with other members of the neurovascular unit. In cerebral small vessel disease (arteriolosclerosis; SVD), the endothelial cells in small arteries survive, even when mural pathology is advanced and myocytes are severely depleted. Here, we review aspects of altered endothelial functions that have been implicated in SVD: local CBF dysregulation, endothelial activation and blood–brain barrier (BBB) dysfunction. Reduced CBF is reported in the diffuse white matter lesions that are a neuroradiological signature of SVD. This may reflect an underlying deficit in local CBF regulation (possibly via the nitric oxide/cGMP signaling pathway). While many laboratories have observed an association of symptomatic SVD with serum markers of endothelial activation, it is apparent that the origin of these circulating markers need not be brain endothelium. Our own neuropathology studies did not confirm local endothelial activation in small vessels exhibiting SVD. Local BBB failure has been proposed as a cause of SVD and associated parenchymal lesions. Some groups find that computational analyses of magnetic resonance imaging (MRI) scans, following systemic injection of a gadolinium‐based contrast agent, suggest that extravasation into brain parenchyma is heightened in people with SVD. Our recent histochemical studies of donated brain tissue, using immunolabeling for large plasma proteins [fibrinogen, immunoglobulin G (IgG)], do not support an association of SVD with recent plasma protein extravasation. It is possible that a trigger leakage episode, or a size‐selective loosening of the BBB, participates in SVD pathology.
Keywords: endothelial cells, human subcortical small vessel disease, vascular dementia
Introduction to Cerebral Endothelial Cells
The endothelium that lines the entire vascular tree is a thin, flat cellular monolayer. Endothelial cells are phenotypically plastic, sensitive to injury and central to several major vascular pathologies 32, 40, 56. All vascular endothelial layers share the following functions.
To allow ready diffusion of oxygen and CO2 across capillary walls.
To form a physical barrier, preventing escape of cells, platelets and plasma proteins from an intact vessel.
To prevent thrombus formation in healthy tissue and to promote thrombosis following tissue injury.
To act as a gatekeeper in the inflammatory process, signaling blood‐borne leukocytes to adhere, migrate and invade a localized region of tissue injury.
To lead vessel sprouting and revascularization, in rapid response to angiogenic stimuli, under the influence of cell signaling molecules, such as vascular endothelial growth factor (VEGF)‐A and angiopoietin.
In small contractile vessels, endothelial‐derived nitric oxide reduces myocyte contractile tone and hence augments vessel diameter and distal blood flow.
Other paracrine roles include release of the vasoconstrictor peptide endothelin‐1.
In addition to these properties common to all endothelia, brain endothelial cells have the following specialized functions.
The blood–brain barrier (BBB). This is a combination of exceptionally tight junctions between adjacent endothelial cells, combined with an array of transporter proteins in endothelial plasma membrane and other cellular players (notably astrocytic foot processes, pericytes and microglia) 1, 103. These mechanisms together exclude potentially harmful solutes from brain tissue and from the endothelial cytoplasm.
As a corollary of the BBB, metabolic fuel substrates for brain metabolism—most notably glucose—have to enter brain tissue entirely via transport proteins in the luminal membrane of endothelial cells.
Brain endothelial cells have a distinctive expression profile, relative to endothelia in other vascular beds 18, 66, 69.
Endothelia in brain small arteries (and to some degree capillaries) participate in the autoregulation of local cerebral blood flow (CBF), which is a special feature of the brain vasculature 6, 8.
Brain endothelial cells regulate, and interact with, other mural components (eg, basal lamina) 15.
What Is Small Vessel Disease (SVD)?
Cerebrovascular SVD comprises a number of distinct histopathological entities, including (i) “arteriolosclerosis”; (ii) “lipohyalinosis” (and the related condition of “fibrinoid necrosis”); and (iii) “small vessel atherosclerosis” 25, 49, 50, 68. There is an overlap in their clinical, radiological and etiopathogenic features, and the distinction (particularly in studies without autopsy follow‐up) is not always clear‐cut. In this review, we will focus on “arteriolosclerosis,” the form of SVD that correlates best with the diffuse radiological changes in white matter known as “leukoaraiosis” 48, 50, 68, 80. In the old literature, this was sometimes referred to, in its severe form, as Binswanger's disease 22, 101. Arteriolosclerosis is also associated with lacunar infarcts 17, 49, 68 and with vascular contributions to cognitive impairment, gait disturbance and dementia 19, 30, 82.
Arteriolosclerosis (also referred to as “hyaline arteriolosclerosis” or “simple small vessel disease”) is the most common form of SVD and has well‐known associations with aging, hypertension and diabetes 49, 50, 68. Genotyping studies have thus far revealed no strong genetic basis for sporadic SVD 44, including prospective tests for linkage with the genes encoding endothelin‐1 (ET‐1) and endothelial nitric oxide synthase (eNOS) 31, 36. For review, see 11. By contrast, CADASIL (cerebral autosomal‐dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is a monogenetic form of SVD (much less common than sporadic SVD). The causal gene for CADASIL is NOTCH3 and SVD pathology manifests in early adulthood. In adults, NOTCH3 expression is primarily within myocytes. Clinical studies of CADASIL support the notion of a relatively intact endothelium in the presence of SVD vasculopathy 62, 71, 72, 78.
Arteriolosclerosis is characterized by smooth muscle loss and collagenization of small arteries and arterioles (approximately 40–150 μm outer diameter) 7, 49, 50, 51, 61. It can be considered the “smallest” of common cerebral small vessel diseases, in that somewhat larger‐diameter vessels are affected by lipohyalinosis (40–300 μm) and small vessel atherosclerosis (200–800 μm) 50.
In arteriolosclerosis, the vessels become “an elongated, tortuous and pauci‐cellular hyaline tube with a variable degree of stenosis” 49. The degree of stenosis can be measured by the sclerotic index [defined as (OD‐ID)/OD, where ID and OD represent vessel inner and outer diameters, respectively], which is considered an established measure of single vessel SVD vasculopathy 13, 29, 34, 63, 64. Although the endothelium appears microscopically intact in arteriolosclerosis, a number of studies suggest that endothelial function is disturbed, and indeed, that this disturbance may be primary in the pathogenesis of SVD.
Endothelial Cells in SVD
A long‐standing observation is that even in severe SVD, with almost complete loss of myocytes and other mural cells, the endothelial layer remains intact 13, 49 (see Figure 1). We and others have noted robust endothelial labeling with histological markers in cases with severe SVD. This includes endothelial labeling for CD34, CD31, factor VIII, glucose transporter 1 and Ulex lectin 13, 27, 29, 65, 100, 101. This paradoxical survival of the endothelium is also evident in patients with CADASIL 13.
Figure 1.
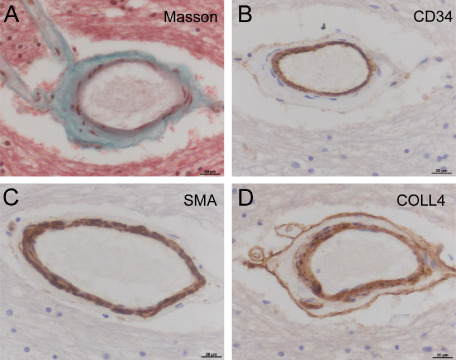
Small vessel disease vasculopathy (arteriolosclerosis) in a small artery of deep subcortical white matter. A. With Masson trichrome stain, fibrous connective tissue (green) is clearly seen in medial layer of the vessel wall. Counterstains show cytoplasm and parenchymal tissue (pink) and nuclei (black). B. Neighboring section, immunolabeled with endothelial marker CD34 (brown). Despite medial fibrosis and depletion of myocytes from the vessel wall, endothelial layer is intact. C. Myocytes within the medial layer are labeled with smooth muscle actin (SMA). D. “Double layer” labeling with collagen‐4 (COLL4). In B–D, the chromogen is diaminobenzidine (brown) and nuclear chromatin is counterstained with hematoxylin (blue). Deep frontal white matter, male aged 77 years; SVD severity score 6, see Ref. 82. Scale bars: 20 μm.
Dysregulation of CBF Occurs in SVD
Measurements of CBF in living subjects suggest a reduction in baseline CBF within deep white matter, and impaired CBF autoregulation, in people with symptomatic SVD 57, 67. Endothelial nitric oxide signaling is an important factor in local CBF regulation and has been used as a marker to demonstrate endothelial dysfunction and decreased vasodilation in response to external stimuli such as hypercapnia or salbutamol in patients with lacunar infarction, compared with controls [eg 16, 33 ]. For a detailed discussion, see a recent systematic review 59. As endothelial nitric oxide signaling is an important factor in local CBF regulation, this further suggests that endothelial dysfunction is a pathogenic factor in SVD.
Immunohistological labeling of a large population cohort of donated brains showed upregulation of hypoxia‐related markers within white matter lesions 24. These markers included HIF‐1α, VEGFR2 and neuroglobin. Evidence of vessel wall thickening was also apparent in these white matter lesions 24. This is an important evidence of the association between SVD vasculopathy and diffuse ischemic white matter lesions seen on magnetic resonance imaging (MRI) scans.
Several studies have used measurements in other vascular beds to report on endothelial changes in cerebral SVD. These include the skin 77, the kidney 29, 94, 95 and the sublingual vessel network 60. If cerebral SVD is indeed a manifestation of a generalized systemic change 87, then these approaches may well be revealing.
SVD may Be Associated with Endothelial Activation—But Where?
Endothelial activation refers to the change in the expression of surface markers within endothelia in response to a noxious stimulus or other challenges 28. Numerous laboratories worldwide have assayed serum biomarkers of endothelial activation in patients with SVD, in a range of clinical settings [eg 14, 21, 26, 36, 37, 38, 53, 58, 74, 90, 96 ]. These circulating markers of endothelial activation include intercellular adhesion molecule‐1 (ICAM‐1), soluble thrombomodulin (sTM), interleukin‐6 (IL‐6), plasminogen activator inhibitor‐1 (PAI‐1), von Willebrand factor and others. In a large prospective population study of type 2 diabetic patients, serum ICAM‐1 was positively associated with white matter lesion progression (a surrogate for SVD) over a 6‐year study period 58. This association survived correction for other risk factors and for baseline lesion load, and was considered strongly supportive of a role for endothelial activation in SVD. Serum biomarker studies have been recently reviewed 46, 47, 84.
With these serum markers in mind, we tested for local expression of three markers (ICAM‐1, thrombomodulin and IL‐6) in vascular endothelium of small arteries of human deep gray matter, relevant to SVD 29. We examined donated brains from older people (age 65 years or older) with neuropathologically defined SVD 19 and compared these with aged control subjects where SVD was graded as “absent” or mild” (based on criteria related to vascular pathology and parenchymal effects of SVD) 19, 29. All sections were examined by at least two observers, blinded to clinical details and SVD grading, and sclerotic index was also measured in small arterial vessels within the size range of interest (20–200 μm outer diameter). We hypothesized that endothelial activation would be more evident in small arteries of SVD cases than in aged controls, and that this would be manifest as greater prevalence of endothelial ICAM‐1 and IL‐6, with depletion of thrombomodulin. In fact, we saw no association of ICAM‐1 or IL‐6 with SVD grade or sclerotic index, and far from being depleted, endothelial thrombomodulin of small arteries was augmented in SVD cases relative to aged controls 29.
These results indicated to us that cerebral endothelial activation is not a feature of SVD 29. Further, as thrombomodulin is a potent anticoagulant, it seemed unlikely that local thrombotic events are primary in SVD‐related stroke events. Thrombotic occlusion is not a recognized common feature of arteriolosclerosis 50.
Histopathological data indicating that local arterial endothelium is not activated in SVD 29 can be reconciled with serum biomarker data, suggesting an association with endothelial activation (listed previously). As concluded in a recent meta‐analysis 84, the recorded serum levels of endothelial markers may derive from any vascular bed (ie, not necessarily brain endothelium). The notion of heightened circulating markers of endothelial activation in patients with SVD is consistent with the hypothesis that cerebral SVD is part of a systemic vasculopathy in which the profile of endothelial activation differs between organs 87.
A related theme is the suggestion that SVD is associated with “neuroinflammation” 29, 74, 75. While cerebral tissue does not undergo conventional inflammation in SVD 85, activation of microglia is clearly a feature of the diffuse white matter parenchymal lesions associated with SVD 23, 24. We and others have used CD68‐labeled cells (clone PGM1) with ameboid morphology as an indicator of cerebral parenchymal injury. Clearly, there are subfamilies of microglial cells, differing in phenotype and pathophysiological role 70, 79, 85. These may provide useful clues to the pathogenic process of SVD vasculopathy and its parenchymal impact.
BBB impairment occurs in SVD—true or false?
The notion that local BBB impairment and arterial SVD are associated—and even causally linked—has been raised in many histopathology studies 2, 3, 55, 88 and was well‐articulated in an influential review in 2003 97. This is an attractive concept to explain the SVD‐related diffuse white matter lesions seen on clinical T2‐weighted MRI scans that resemble patterns of diffusion. Defective endothelial cells are central to this idea, with an imperfect BBB allowing harmful plasma components to escape the lumen, leading to myocyte damage in the artery wall and diffuse tissue damage in the surrounding parenchyma (with edema, ischemia and cytotoxicity all playing a role). This notion has been addressed in biochemical analyses of serum proteins in cerebrospinal fluid and is supported in some studies but not others [see a recent meta‐analysis 20 ]. Likewise, several radiological studies have examined partition of intravascular contrast agent into brain tissue, as a marker of BBB dysfunction, in patient groups with SVD, lacunar stroke and/or VCID 20, 86, 89, 98, 99. These radiological mapping methods require dedicated software algorithms, developed in several expert centers, to process the MRI scans before and after contrast injection. Some of these studies suggested an association of contrast agent extravasation with disease 86, 89, while other studies did not 20, 98, 99. Among neuropathology reports on brain tissue, labeled for extravasation of vascular markers, some have found a positive association between SVD severity and extravascular plasma proteins (eg, fibrinogen, albumin or immunoglobulin G (IgG)] 88, 91, while others found no such association 93, 100. We have recently examined a large collection of donated brains of older people (age 65 years or older) with minimal Alzheimer's disease pathology (Braak stage II or less) 12, 82. Within subcortical white matter and putaminal gray matter (two SVD‐prone anatomical regions), extravascular labeling of fibrinogen (in cells and extracellular tissue) was estimated in a blinded fashion, using machine‐based morphometry in parallel with categorical scoring by a neuropathologist. We found no association of fibrinogen with SVD severity, in either anatomical location 12.
Observations that are difficult to reconcile with a BBB‐SVD causal link are the findings that other pathological states where plasma extravasation clearly is a feature, such as multiple sclerosis and neuromyelitis optica, do not involve SVD‐like arteriopathy. In addition, examination of histopathological material suggests that many of the small vessels exhibiting extravascular labeling of cells and surrounding tissue are veins. In two well‐powered studies where quantitation was carried out in a blinded fashion, labeling of extravascular plasma proteins did not support an association of BBB dysfunction with SVD 12, 100 . A possible “trigger” event, where brief, local BBB “loosening” has a role in SVD pathogenesis, is difficult to exclude and to test.
BBB formation is not intrinsic to cerebral endothelial cells but is controlled by local cell signaling molecules, such as VEGF 4, 5 and transforming growth factor‐β (TGF‐β) 54. Recent elegant studies of transgenic mice that lack effective pericyte coverage of capillaries suggest an influence of pericyte‐endothelial cross talk on BBB function 9, 10. This influence of pericyte signaling on the BBB is borne out in a small study of human tissue comparing Alzheimer's disease cases with aged controls 81.
Dietary Factors, Endothelia and SVD
Several clinical studies hint at the influence of dietary factors in SVD. These include diabetic status 92, plasma glycemic state 33, 104 and plasma levels of the non‐essential amino acid homocysteine 37, 38, 43, 53. Moderately elevated plasma homocysteine concentrations (10–20 μmol/L) were associated with increasing leukoaraiosis severity 38, 43. This statistical association was lost after correction for circulating endothelial markers (ICAM‐1 and thrombomodulin), suggesting that the interaction between plasma homocysteine and white matter lesions may be endothelium‐dependent 38. How these nutritional factors relate to SVD vasculopathy, and its effects on brain parenchymal tissue, seem an area that may be fruitful for future research.
Conclusion
The endothelial cells that line the small arteries deep within our brains are integral to the SVD process, although much molecular detail remains to be understood. They may also be an important first point of contact for any preventive or therapeutic strategy.
Search strategy
For this review, we incorporated a PubMed search for publications in English whose title or abstract contained the following terms: endotheli* AND (brain or cerebr*) AND (small_vessel_disease or arteriolosclerosis or lipohyalinosis or fibrohyaline or hyalin*) (search date: August 13, 2014). This search retrieved 103 publications. Abstracts were viewed by two authors (AHH and ATO) and reviews, animal studies and irrelevant diseases were excluded, leaving n = 37 primary publications 14, 16, 21, 27, 29, 31, 33, 35, 36, 37, 38, 39, 41, 42, 43, 45, 52, 53, 58, 60, 62, 65, 71, 72, 73, 74, 75, 76, 77, 78, 83, 89, 90, 94, 95, 96, 102, 104. Bibliographies of these papers, and of related reviews, were also searched.
Acknowledgments
We thank our colleagues in St George's Healthcare NHS Trust Cellular Pathology Service and St George's Imaging Resource Facility. Our research receives financial support from Alzheimer's Society (UK; PG146/151), Alzheimer's Drug Discovery Foundation, Alzheimer's Research UK, St. George's Hospital Charity and The Neuroscience Research Foundation. We are very grateful to Professor Margaret M. Esiri for many helpful discussions.
References
- 1. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood‐brain barrier. Neurobiol Dis 37:13–25. [DOI] [PubMed] [Google Scholar]
- 2. Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H (1998) Blood‐brain barrier dysfunction in Binswanger's disease; an immunohistochemical study. Acta Neuropathol 95:78–84. [DOI] [PubMed] [Google Scholar]
- 3. Alafuzoff I, Adolfsson R, Grundke‐Iqbal I, Winblad B (1985) Perivascular deposits of serum proteins in cerebral cortex in vascular dementia. Acta Neuropathol 66:292–298. [DOI] [PubMed] [Google Scholar]
- 4. Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR (2009) VEGF‐mediated disruption of endothelial CLN‐5 promotes blood‐brain barrier breakdown. Proc Natl Acad Sci U S A 106:1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC et al (2006) IL‐1beta regulates blood‐brain barrier permeability via reactivation of the hypoxia‐angiogenesis program. J Immunol 177:5574–5584. [DOI] [PubMed] [Google Scholar]
- 6. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker AB (1941) Structure of the small cerebral arteries in hypertension. Am J Pathol 17:39–46. [PMC free article] [PubMed] [Google Scholar]
- 8. Baumbach GL, Heistad DD (1988) Cerebral circulation in chronic arterial hypertension. Hypertension 12:89–95. [DOI] [PubMed] [Google Scholar]
- 9. Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z et al (2012) Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bevan S, Markus HS (2013) Genetic profiles in ischaemic stroke. Curr Atheroscler Rep 15:342. [DOI] [PubMed] [Google Scholar]
- 12. Bridges LR, Andoh J, Lawrence AJ, Khoong CH, Poon WW, Esir MM, Markus HS, Hainsworth AH (2014) Blood‐brain barrier dysfunction and cerebral small vessel disease (arteriolosclerosis) in brains of older people. J Neuropathol Exp Neurol 73:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craggs LJ, Hagel C, Kuhlenbaeumer G, Borjesson‐Hanson A, Andersen O, Viitanen M et al (2013) Quantitative vascular pathology and phenotyping familial and sporadic cerebral small vessel diseases. Brain Pathol 23:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Leeuw FE, de Kleine M, Frijns CJ, Fijnheer R, van Gijn J, Kappelle LJ (2002) Endothelial cell activation is associated with cerebral white matter lesions in patients with cerebrovascular disease. Ann N Y Acad Sci 977:306–314. [DOI] [PubMed] [Google Scholar]
- 15. del Zoppo GJ, Milner R (2006) Integrin‐matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol 26:1966–1975. [DOI] [PubMed] [Google Scholar]
- 16. Deplanque D, Lavallee PC, Labreuche J, Gongora‐Rivera F, Jaramillo A, Brenner D et al (2013) Cerebral and extracerebral vasoreactivity in symptomatic lacunar stroke patients: a case‐control study. Int J Stroke 8:413–421. [DOI] [PubMed] [Google Scholar]
- 17. Dozono K, Ishii N, Nishihara Y, Horie A (1991) An autopsy study of the incidence of lacunes in relation to age, hypertension, and arteriosclerosis. Stroke 22:993–996. [DOI] [PubMed] [Google Scholar]
- 18. Enerson BE, Drewes LR (2006) The rat blood‐brain barrier transcriptome. J Cereb Blood Flow Metab 26:959–973. [DOI] [PubMed] [Google Scholar]
- 19. Esiri MM, Wilcock GK, Morris JH (1997) Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 63:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farrall AJ, Wardlaw JM (2009) Blood‐brain barrier: ageing and microvascular disease—systematic review and meta‐analysis. Neurobiol Aging 30:337–352. [DOI] [PubMed] [Google Scholar]
- 21. Fassbender K, Bertsch T, Mielke O, Muhlhauser F, Hennerici M (1999) Adhesion molecules in cerebrovascular diseases. Evidence for an inflammatory endothelial activation in cerebral large‐ and small‐vessel disease. Stroke 30:1647–1650. [DOI] [PubMed] [Google Scholar]
- 22. Feigin I, Popoff N (1963) Neuropathological changes late in cerebral edema: the relationship to trauma, hypertensive disease and Binswanger's encephalopathy. J Neuropathol Exp Neurol 22:500–511. [DOI] [PubMed] [Google Scholar]
- 23. Fernando MS, O'Brien JT, Perry RH, English P, Forster G, McMeekin W et al (2004) Comparison of the pathology of cerebral white matter with post‐mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol 30:385–395. [DOI] [PubMed] [Google Scholar]
- 24. Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R et al (2006) White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 37:1391–1398. [DOI] [PubMed] [Google Scholar]
- 25. Fisher CM (1968) The arterial lesions underlying lacunes. Acta Neuropathol 12:1–15. [DOI] [PubMed] [Google Scholar]
- 26. Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS et al (2008) Biomarkers of inflammation and MRI‐defined small vessel disease of the brain: the cardiovascular health study. Stroke 39:1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frischer JM, Pipp I, Stavrou I, Trattnig S, Hainfellner JA, Knosp E (2008) Cerebral cavernous malformations: congruency of histopathological features with the current clinical definition. J Neurol Neurosurg Psychiatry 79:783–788. [DOI] [PubMed] [Google Scholar]
- 28. Gallin JI, Snyderman R (1999) Inflammation: Basic Principles and Clinical Correlates, 3rd edn. Lippincott Williams & Wilkins: Philadelphia. [Google Scholar]
- 29. Giwa MO, Williams J, Elderfield K, Jiwa NS, Bridges LR, Kalaria RN et al (2012) Neuropathologic evidence of endothelial changes in cerebral small vessel disease. Neurology 78:167–174. [DOI] [PubMed] [Google Scholar]
- 30. Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C et al (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gormley K, Bevan S, Hassan A, Markus HS (2005) Polymorphisms in genes of the endothelin system and cerebral small‐vessel disease. Stroke 36:1656–1660. [DOI] [PubMed] [Google Scholar]
- 32. Goveia J, Stapor P, Carmeliet P (2014) Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med 6:1105–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gunarathne A, Patel JV, Kausar S, Gammon B, Hughes EA, Lip GY (2009) Glycemic status underlies increased arterial stiffness and impaired endothelial function in migrant South Asian stroke survivors compared to European Caucasians: pathophysiological insights from the West Birmingham Stroke Project. Stroke 40:2298–2306. [DOI] [PubMed] [Google Scholar]
- 34. Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE et al (2006) National Institute of Neurological Disorders and Stroke‐Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 35. Han JH, Wong KS, Wang YY, Fu JH, Ding D, Hong Z (2009) Plasma level of sICAM‐1 is associated with the extent of white matter lesion among asymptomatic elderly subjects. Clin Neurol Neurosurg 111:847–851. [DOI] [PubMed] [Google Scholar]
- 36. Hassan A, Gormley K, O'Sullivan M, Knight J, Sham P, Vallance P et al (2004) Endothelial nitric oxide gene haplotypes and risk of cerebral small‐vessel disease. Stroke 35:654–659. [DOI] [PubMed] [Google Scholar]
- 37. Hassan A, Hunt BJ, O'Sullivan M, Parmar K, Bamford JM, Briley D et al (2003) Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 126:424–432. [DOI] [PubMed] [Google Scholar]
- 38. Hassan A, Hunt BJ, O'Sullivan M, Bell R, D'Souza R, Jeffery S et al (2004) Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain 127:212–219. [DOI] [PubMed] [Google Scholar]
- 39. Hegt VN (1976) Distribution and variation of fibrinolytic activity in the walls of human arteries and veins. Haemostasis 5:355–372. [DOI] [PubMed] [Google Scholar]
- 40. Hooper WC, Catravas JD, Heistad DD, Sessa WC, Mensah GA (2007) Vascular endothelium summary statement I: health promotion and chronic disease prevention. Vascul Pharmacol 46:315–317. [DOI] [PubMed] [Google Scholar]
- 41. Jimenez JJ, Jy W, Mauro LM, Horstman LL, Fontana V, Ahn YS (2008) Transendothelial migration of leukocytes is promoted by plasma from a subgroup of immune thrombocytopenic purpura patients with small‐vessel ischemic brain disease. Am J Hematol 83:206–211. [DOI] [PubMed] [Google Scholar]
- 42. Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R (1999) Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 30:1396–1401. [DOI] [PubMed] [Google Scholar]
- 43. Khan U, Hassan A, Vallance P, Markus HS (2007) Asymmetric dimethylarginine in cerebral small vessel disease. Stroke 38:411–413. [DOI] [PubMed] [Google Scholar]
- 44. Kilarski LL, Achterberg S, Devan WJ, Traylor M, Malik R, Lindgren A et al (2014) Meta‐analysis in more than 17,900 cases of ischemic stroke reveals a novel association at 12q24.12. Neurology 83:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kimura A, Sakurai T, Yamada M, Koumura A, Hayashi Y, Tanaka Y et al (2012) Anti‐endothelial cell antibodies in patients with cerebral small vessel disease. Curr Neurovasc Res 9:296–301. [DOI] [PubMed] [Google Scholar]
- 46. Knottnerus IL, Govers‐Riemslag JW, Hamulyak K, Rouhl RP, Staals J, Spronk HM et al (2010) Endothelial activation in lacunar stroke subtypes. Stroke 41:1617–1622. [DOI] [PubMed] [Google Scholar]
- 47. Knottnerus IL, ten Cate H, Lodder J, Kessels F, van Oostenbrugge RJ (2009) Endothelial dysfunction in lacunar stroke: a systematic review. Cerebrovasc Dis 27:519–526. [DOI] [PubMed] [Google Scholar]
- 48. Lammie GA (2000) Pathology of small vessel stroke. Br Med Bull 56:296–306. [DOI] [PubMed] [Google Scholar]
- 49. Lammie GA (2002) Hypertensive cerebral small vessel disease and stroke. Brain Pathol 12:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lammie GA (2005) Small vessel disease. In: Cerebrovascular Disease: Pathology and Genetics. Kalimo H (ed.), pp. 85–91. ISN Neuropathology Press: Basle. [Google Scholar]
- 51. Lammie GA, Brannan F, Slattery J, Warlow C (1997) Nonhypertensive cerebral small‐vessel disease. An autopsy study. Stroke 28:2222–2229. [DOI] [PubMed] [Google Scholar]
- 52. Lavallee PC, Labreuche J, Gongora‐Rivera F, Jaramillo A, Brenner D, Klein IF et al (2009) Placebo‐controlled trial of high‐dose atorvastatin in patients with severe cerebral small vessel disease. Stroke 40:1721–1728. [DOI] [PubMed] [Google Scholar]
- 53. Lavallee PC, Labreuche J, Faille D, Huisse MG, Nicaise‐Roland P, Dehoux M et al (2013) Circulating markers of endothelial dysfunction and platelet activation in patients with severe symptomatic cerebral small vessel disease. Cerebrovasc Dis 36:131–138. [DOI] [PubMed] [Google Scholar]
- 54. Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F et al (2011) Endothelial Smad4 maintains cerebrovascular integrity by activating N‐cadherin through cooperation with Notch. Dev Cell 20:291–302. [DOI] [PubMed] [Google Scholar]
- 55. Ma KC, Olsson Y (1993) Structural and vascular permeability abnormalities associated with lacunes of the human brain. Acta Neurol Scand 88:100–107. [DOI] [PubMed] [Google Scholar]
- 56. Marcelo KL, Goldie LC, Hirschi KK (2013) Regulation of endothelial cell differentiation and specification. Circ Res 112:1272–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Markus HS, Lythgoe DJ, Ostegaard L, O'Sullivan M, Williams SC (2000) Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J Neurol Neurosurg Psychiatry 69:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R (2005) Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke 36:1410–1414. [DOI] [PubMed] [Google Scholar]
- 59. Markus HS, Allan CL, Ebmeier KP (2014) Cerebral hemodynamics in cerebral small vessel disease. In: Cerebral Small Vessel Disease. Pantoni L, Gorelick PB (eds), pp. 180–191. Cambridge University Press: Cambridge. [Google Scholar]
- 60. Martens RJ, Vink H, van Oostenbrugge RJ, Staals J (2013) Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis 35:451–454. [DOI] [PubMed] [Google Scholar]
- 61. Masawa N, Yoshida Y, Yamada T, Joshita T, Sato S, Mihara B (1994) Morphometry of structural preservation of tunica media in aged and hypertensive human intracerebral arteries. Stroke 25:122–127. [DOI] [PubMed] [Google Scholar]
- 62. Meng H, Zhang X, Lee SJ, Wang MM (2013) Von Willebrand factor inhibits mature smooth muscle gene expression through impairment of Notch signaling. PLoS ONE 8:e75808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, Kalimo H (2004) Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol 14:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miao Q, Paloneva T, Tuisku S, Roine S, Poyhonen M, Viitanen M, Kalimo H (2006) Arterioles of the lenticular nucleus in CADASIL. Stroke 37:2242–2247. [DOI] [PubMed] [Google Scholar]
- 65. Neltner JH, Abner EL, Baker S, Schmitt FA, Kryscio RJ, Jicha GA et al (2014) Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 137:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ohtsuki S, Hirayama M, Ito S, Uchida Y, Tachikawa M, Terasaki T (2014) Quantitative targeted proteomics for understanding the blood‐brain barrier: towards pharmacoproteomics. Expert Rev Proteomics 11:303–313. [DOI] [PubMed] [Google Scholar]
- 67. O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS (2002) Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 59:321–326. [DOI] [PubMed] [Google Scholar]
- 68. Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9:689–701. [DOI] [PubMed] [Google Scholar]
- 69. Pardridge WM (2005) Molecular biology of the blood‐brain barrier. Mol Biotechnol 30:57–70. [DOI] [PubMed] [Google Scholar]
- 70. Perry VH, Holmes C (2014) Microglial priming in neurodegenerative disease. Nat Rev Neurol 10:217–224. [DOI] [PubMed] [Google Scholar]
- 71. Peters N, Freilinger T, Opherk C, Pfefferkorn T, Dichgans M (2007) Effects of short term atorvastatin treatment on cerebral hemodynamics in CADASIL. J Neurol Sci 260:100–105. [DOI] [PubMed] [Google Scholar]
- 72. Peters N, Freilinger T, Opherk C, Pfefferkorn T, Dichgans M (2008) Enhanced L‐arginine‐induced vasoreactivity suggests endothelial dysfunction in CADASIL. J Neurol 255:1203–1208. [DOI] [PubMed] [Google Scholar]
- 73. Rouhl RP, van Oostenbrugge RJ, Damoiseaux JG, Brus‐Palmans LL, Theunissen RO, Knottnerus IL et al (2009) Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr Neurovasc Res 6:32–41. [DOI] [PubMed] [Google Scholar]
- 74. Rouhl RP, van Oostenbrugge RJ, Theunissen RO, Knottnerus IL, Staals J, Henskens LH et al (2010) Autoantibodies against oxidized low‐density lipoprotein in cerebral small vessel disease. Stroke 41:2687–2689. [DOI] [PubMed] [Google Scholar]
- 75. Rouhl RP, Damoiseaux JG, Lodder J, Theunissen RO, Knottnerus IL, Staals J et al (2012) Vascular inflammation in cerebral small vessel disease. Neurobiol Aging 33:1800–1806. [DOI] [PubMed] [Google Scholar]
- 76. Rouhl RP, Mertens AE, van Oostenbrugge RJ, Damoiseaux JG, Brus‐Palmans LL, Henskens LH et al (2012) Angiogenic T‐cells and putative endothelial progenitor cells in hypertension‐related cerebral small vessel disease. Stroke 43:256–258. [DOI] [PubMed] [Google Scholar]
- 77. Ruchoux MM, Brulin P, Leteurtre E, Maurage CA (2000) Skin biopsy value and leukoaraiosis. Ann N Y Acad Sci 903:285–292. [DOI] [PubMed] [Google Scholar]
- 78. Rufa A, Blardi P, De Lalla A, Cevenini G, De SN, Zicari E et al (2008) Plasma levels of asymmetric dimethylarginine in cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy. Cerebrovasc Dis 26:636–640. [DOI] [PubMed] [Google Scholar]
- 79. Salter MW, Beggs S (2014) Sublime microglia: expanding roles for the guardians of the CNS. Cell 158:15–24. [DOI] [PubMed] [Google Scholar]
- 80. Schmidt R, Schmidt H, Haybaeck J, Loitfelder M, Weis S, Cavalieri M et al (2011) Heterogeneity in age‐related white matter changes. Acta Neuropathol 122:171–185. [DOI] [PubMed] [Google Scholar]
- 81. Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013) Deficiency in mural vascular cells coincides with blood‐brain barrier disruption in Alzheimer's disease. Brain Pathol 23:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smallwood A, Oulhaj A, Joachim C, Christie S, Sloan C, Smith AD, Esiri M (2012) Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol 38:337–343. [DOI] [PubMed] [Google Scholar]
- 83. Staals J, Henskens LH, Delanghe JR, van Oostenbrugge RJ, Kessels AG, Kroon AA et al (2010) Haptoglobin phenotype correlates with the extent of cerebral deep white matter lesions in hypertensive patients. Curr Neurovasc Res 7:1–5. [DOI] [PubMed] [Google Scholar]
- 84. Stevenson SF, Doubal FN, Shuler K, Wardlaw JM (2010) A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke 41:e434–e442. [DOI] [PubMed] [Google Scholar]
- 85. Streit WJ, Xue QS (2014) Human CNS immune senescence and neurodegeneration. Curr Opin Immunol 29C:93–96. [DOI] [PubMed] [Google Scholar]
- 86. Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J et al (2011) Blood‐brain barrier permeability abnormalities in vascular cognitive impairment. Stroke 42:2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thompson CS, Hakim AM (2009) Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke 40:e322–e330. [DOI] [PubMed] [Google Scholar]
- 88. Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, Kimura J (1996) Alterations of the blood‐brain barrier and glial cells in white‐matter lesions in cerebrovascular and Alzheimer's disease patients. Stroke 27:2069–2074. [DOI] [PubMed] [Google Scholar]
- 89. Topakian R, Barrick TR, Howe FA, Markus HS (2010) Blood‐brain barrier permeability is increased in normal‐appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 81:192–197. [DOI] [PubMed] [Google Scholar]
- 90. Umemura T, Kawamura T, Umegaki H, Mashita S, Kanai A, Sakakibara T et al (2011) Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small‐vessel disease and cognitive impairment: a 6‐year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 82:1186–1194. [DOI] [PubMed] [Google Scholar]
- 91. Utter S, Tamboli IY, Walter J, Upadhaya AR, Birkenmeier G, Pietrzik CU et al (2008) Cerebral small vessel disease‐induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta‐protein in perivascular astrocytes. J Neuropathol Exp Neurol 67:842–856. [DOI] [PubMed] [Google Scholar]
- 92. Verdelho A, Madureira S, Moleiro C, Ferro JM, Santos CO, Erkinjuntti T et al (2010) White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology 75:160–167. [DOI] [PubMed] [Google Scholar]
- 93. Viggars AP, Wharton SB, Simpson JE, Matthews FE, Brayne C, Savva GM et al (2011) Alterations in the blood brain barrier in ageing cerebral cortex in relationship to Alzheimer‐type pathology: a study in the MRC‐CFAS population neuropathology cohort. Neurosci Lett 505:25–30. [DOI] [PubMed] [Google Scholar]
- 94. Wada M, Nagasawa H, Iseki C, Takahashi Y, Sato H, Arawaka S et al (2008) Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross‐sectional study in community‐based Japanese elderly. J Neurol Sci 272:36–42. [DOI] [PubMed] [Google Scholar]
- 95. Wada M, Nagasawa H, Kurita K, Koyama S, Arawaka S, Kawanami T et al (2007) Microalbuminuria is a risk factor for cerebral small vessel disease in community‐based elderly subjects. J Neurol Sci 255:27–34. [DOI] [PubMed] [Google Scholar]
- 96. Wada M, Takahashi Y, Iseki C, Kawanami T, Daimon M, Kato T (2011) Plasma fibrinogen, global cognitive function, and cerebral small vessel disease: results of a cross‐sectional study in community‐dwelling Japanese elderly. Intern Med 50:999–1007. [DOI] [PubMed] [Google Scholar]
- 97. Wardlaw JM, Sandercock PA, Dennis MS, Starr J (2003) Is breakdown of the blood‐brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 34:806–812. [DOI] [PubMed] [Google Scholar]
- 98. Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz MS et al (2009) Lacunar stroke is associated with diffuse blood‐brain barrier dysfunction. Ann Neurol 65:194–202. [DOI] [PubMed] [Google Scholar]
- 99. Wardlaw JM, Doubal FN, Valdes‐Hernandez M, Wang X, Chappell FM, Shuler K et al (2013) Blood‐brain barrier permeability and long‐term clinical and imaging outcomes in cerebral small vessel disease. Stroke 44:525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Young VG, Halliday GM, Kril JJ (2008) Neuropathologic correlates of white matter hyperintensities. Neurology 71:804–811. [DOI] [PubMed] [Google Scholar]
- 101. Zhang WW, Olsson Y (1997) The angiopathy of subcortical arteriosclerotic encephalopathy (Binswanger's disease): immunohistochemical studies using markers for components of extracellular matrix, smooth muscle actin and endothelial cells. Acta Neuropathol 93:219–224. [DOI] [PubMed] [Google Scholar]
- 102. Zhang WW, Lempessi H, Olsson Y (1998) Amyloid angiopathy of the human brain: immunohistochemical studies using markers for components of extracellular matrix, smooth muscle actin and endothelial cells. Acta Neuropathol 96:558–563. [DOI] [PubMed] [Google Scholar]
- 103. Zlokovic BV (2008) The blood‐brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. [DOI] [PubMed] [Google Scholar]
- 104. Zunker P, Schick A, Buschmann HC, Georgiadis D, Nabavi DG, Edelmann M, Ringelstein EB (1996) Hyperinsulinism and cerebral microangiopathy. Stroke 27:219–223. [DOI] [PubMed] [Google Scholar]


