Clinical History
A 44‐year‐old man with a past medical history of arterial hypertension, hypercholesterolemia, cigarette smoking (45 pack‐years) and obesity (BMI 32.8) presented to our department with a 3‐month history of right‐sided facial numbness. Four weeks prior to admission he experienced a single episode of involuntary muscle movements on the left‐side of his body. His neurologic exam was normal and initial laboratory results including CBC and blood chemistry were within normal range. A magnetic resonance imaging (MRI) scan of the patient's brain (Figure 1) showed a 7.3 × 4.9 × 3.6 cm, right fronto‐parietal, extra‐axial space‐occupying lesion with lobulated contrast‐enhancement and mild perifocal edema. The superior sagittal sinus was slightly compressed and the overlying cranium was infiltrated. The patient underwent angio‐embolization of the lesion and two days later a right fronto‐temporo‐parietal craniectomy was performed. The tumor was resected subtotally, leaving a thin superficial infiltrative layer on eloquent cortex. The infiltrated cranium was reconstructed using polymethyl‐methacrylate (PMMA) cranioplasty and the resected dura was replaced by a neuropatch. Postoperatively, an MRI of the spine and a lumbar puncture did not show any evidence for disease dissemination. The patient had no neurological deficit and underwent adjuvant radiation therapy of the tumor bed (36 Gy) and 2‐years after diagnosis he was clinically and radiologically disease‐free.
Figure 1.
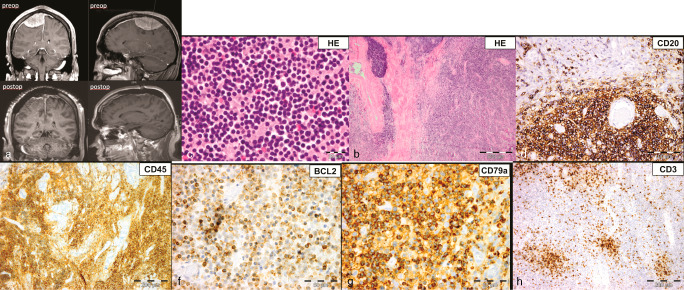
Microscopic Patholgy
Microscopic examination of the biopsy revealed fragments of dense connective tissue infiltrated by closely‐packed, medium‐sized, monomorphic lymphocytes and scattered plasma cells (Figures 1b and 1c). Occasional thrombosed vessels surrounded by necrotic tissue were seen. The lymphocytic infiltrates were positive for CD20 (Figure 1d), CD45 (Figure 1e), BCL2 (Figure 1f), and CD79a (Figure 1g) and negative for EMA, CD34, TDT and CD99. In addition, there were smaller collections of CD3 (Figure 1h) and CD23 positive lymphocytes, which were often perivascular, with rare cells staining for CD5 and CD10. Ki‐67 immunostaining showed a patchy distribution, labeling up to 30% (average, 10%) of the neoplastic cells. What is the diagnosis?
Diagnosis
Dura‐associated extranodal marginal zone B cell lymphoma (MALT lymphoma).
Discussion
Extranodal marginal zone B cell lymphomas of the mucosa‐associated lymphoid tissue (MALT) most often originate in the stomach, but sometimes occur in other locations such as the dura mater 7. While most primary central nervous system lymphomas (PCNSLs) are high grade diffuse large B cell non‐Hodgkin lymphomas, primary dural lymphomas (PDLs), a rare subtype of PCNSL, are usually extranodal MALT lymphomas and low‐grade lesions 5. PDLs tend to show a female predominance, usually occur in immunocompetent hosts and have a better prognosis than other forms of PCNSL. Due to these clinicopathological features, the diagnostic work‐up and the treatment strategy is entirely different from most PCNSLs. It is known that PDLs often mimic meningioma or even subdural hematoma on preoperative imaging 4, 8.
Histologically, dura‐associated extranodal marginal zone B cell lymphomas (MALT lymphomas) have similar morphologic and immunohistochemical features of their counterparts in other locations 3. Usually, small mature lymphocytes invade the dura in a perivascular fashion and immunolabel with CD20 and BCL2, while they are negative for CD5, CD10, CD23, and BCL6 3. Although not present in this case, some MALT lymphomas have rearrangements of the immunoglobulin heavy chain genes 6 or chromosomal aberrations involving the MALT1 gene. One is a translocation characteristic for gastric and pulmonary MALT lymphomas, t(11;18)(q21;q21), resulting in a API2‐MALT1 fusion transcript and the other is t(14;18)(q32;q21) with IgH‐MALT1 fusion, frequently seen in MALT lymphomas of the skin, salivary glands and ocular adnexa 2. Interestingly, in a large series of dural MALT lymphoma reported by Tu et al, fluorescent in situ hybridization (FISH) performed in 12 of 15 cases failed to reveal t(14;18) or t(11;18) translocations 8.
So far, standard treatment has not yet been established. In a systematic review of the literature by Beltran et al that included 91 patients, 70% had surgery, 73% had radiation therapy and 37% chemotherapy 1. Larger prospective studies are needed to determine optimal management.
References
- 1. Beltran BE, Kuritzky B, Quinones P, Morales D, Alva JC, Lu G, Goswami M, Miranda RN, Castillo JJ (2013) Extranodal marginal zone lymphoma of the cranial dura mater: Report of three cases and systematic review of the literature. Leuk Lymphoma 54: 2306–2309. [DOI] [PubMed] [Google Scholar]
- 2. Bhagavathi S, Greiner TC, Kazmi SA, Fu K, Sanger WG, Chan WC (2008) Extranodal marginal zone lymphoma of the dura mater with IgH/MALT1 translocation and review of literature. J Hematopathol 1:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavalli F, Isaacson PG, Gascoyne RD, Zucca E (2001) MALT Lymphomas. Hematology Am Soc Hematol Educ Program 1:241–258. [DOI] [PubMed] [Google Scholar]
- 4. Gocmen S, Gamsizkan M, Onguru O, Sefali M, Erdogan E (2010) Primary dural lymphoma mimicking a subdural hematoma. J Clin Neurosci 17:380–382. [DOI] [PubMed] [Google Scholar]
- 5. Iwamoto FM, Abrey LE (2006) Primary dural lymphomas: a review. Neurosurg Focus 21:E5. [DOI] [PubMed] [Google Scholar]
- 6. Miranda RN, Glantz LK, Myint MA, Levy N, Jackson CL, Rhodes CH, Glantz MJ, Medeiros LJ (1996) Stage IE non‐Hodgkin's lymphoma involving the dura: A clinicopathologic study of five cases. Arch Path Lab Med 120:254–260. [PubMed] [Google Scholar]
- 7. Olszewski AJ, Castillo JJ (2013) Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer 119:629–638. [DOI] [PubMed] [Google Scholar]
- 8. Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O'Neill BP, Yachnis AT, Burger PC, Scheithauer BW, Perry A (2005) Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low‐grade CNS lymphoma that mimics meningioma. J Clin Oncol 23:5718–5727. [DOI] [PubMed] [Google Scholar]


