Abstract
Primary melanocytic tumors of the central nervous system (CNS) represent a spectrum of rare tumors. They can be benign or malignant and occur in adults as well as in children, the latter often in the context of neurocutaneous melanosis. Until recently, the genetic alterations in these tumors were largely unknown. This is in contrast with cutaneous and uveal melanomas, which are known to harbor distinct oncogenic mutations that can be used as targets for treatment with small‐molecule inhibitors in the advanced setting. Recently, novel insights in the molecular alterations underlying primary melanocytic tumors of the CNS were obtained, including different oncogenic mutations in tumors in adult patients (especially GNAQ, GNA11) vs. children (especially NRAS). In this review, the focus is on molecular characteristics of primary melanocytic tumors of the CNS. We summarize what is known about their genetic alterations and discuss implications for pathogenesis and differential diagnosis with other pigmented tumors in or around the CNS. Finally, new therapeutic options with targeted therapy are discussed.
Keywords: brain, central nervous system, GNA11, GNAQ, leptomeningeal melanocytic neoplasms, melanocytoma, melanoma, melanotic schwannoma, metastasis, molecular, mosaic RASopathy, neurocutaneous melanosis, NRAS
Introduction
Primary melanocytic tumors of the central nervous system (CNS) represent a spectrum of rare neoplasms including benign and malignant tumors. They can be circumscribed tumors or diffuse lesions that expand along the leptomeninges. Both adults and children can be affected, the latter often in the context of neurocutaneous melanosis (NCM) 17.
Like other melanocytic neoplasms, primary melanocytic tumors of the CNS are derived from melanocytes that originate from the neural crest early during embryogenesis 80. Precursors of melanocytes, so‐called melanoblasts, migrate during embryonic development mainly via the dorsolateral route and travel to the skin in the first trimester of gestation 34, 63, 94. Most melanoblasts first reach the dermis and subsequently the epidermis that starts to become populated around week 7 in the human embryo 57, 94. Smaller numbers of melanoblasts travel to mucosal surfaces of for example, the aerodigestive and urogenital tract, to the inner ear, the uvea and to the leptomeninges (Figure 1A) 93.
Figure 1.
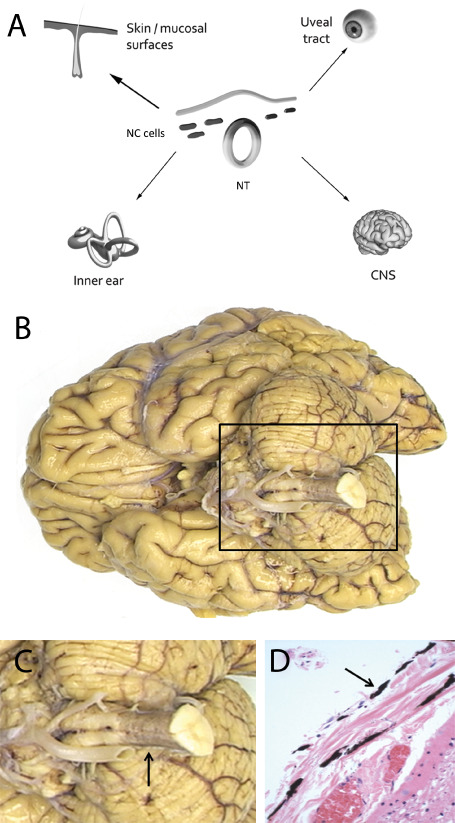
Origin and morphology of leptomeningeal melanocytes. A. Melanoblasts, the precursors of melanocytes, are derived from the neural crest early during embryogenesis and undergo migration to reach their destination in cutaneous and extra‐cutaneous locations, the latter including mucosal surfaces, uveal tract, inner ear and leptomeninges (arrows). At the center, an incomplete cross‐section of a human embryo is depicted showing neural crest cells that detach from the tips of the neural folds just before or shortly after they fuse to give rise to the neural tube. These multipotent NC cells, represented by black dots, yield melanoblasts that become spatially and temporally segregated from other NC‐derived cell types. B,C. Melanocytes can be found especially around the upper part of the cervical spinal cord and may cause a grayish hue (arrow in panel C). D. Microscopically, these leptomeningeal melanocytes are slender, elongated, and darkly pigmented cells (arrow). NC = neural crest cells; NT = neural tube.
Melanocytic neoplasms can therefore arise in cutaneous as well as in extra‐cutaneous localizations, although the latter is much less frequent. The primary function of melanin pigment is protection against the adverse effects of sunlight, but its function in ultraviolet‐protected locations is less clear 93. It has been suggested that leptomeningeal melanocytes capture toxic cations and free radical species from the blood circulation 129.
The highest concentration of melanocytes in the leptomeninges is usually found at the ventro‐lateral surfaces of the medulla oblongata and around the upper part of the spinal cord 47. Macroscopically, they may cause a grayish hue of the leptomeninges (Figure 1B–D).
As primary melanocytic tumors of the CNS are thought to arise from leptomeningeal melanocytes, we will refer to them as primary leptomeningeal melanocytic neoplasms (LMNs). The first report of such tumors dates from 1859 by Virchow in which he described autopsy findings of a male adult with diffuse leptomeningeal melanocytosis 135. Since that time, their diagnosis, especially the differential diagnosis with metastastic melanoma and with other (partly) pigmented primary CNS tumors, remained a challenge for both clinicians and pathologists.
In the past decade, much has been learned about the genetic alterations in distinct subtypes of melanomas 31, 141. A milestone was the discovery of oncogenic mutations in the BRAF gene in about 50% of cutaneous melanomas 32. This finding led to successful targeted therapy with BRAF inhibitors for patients with metastatic BRAFV600‐mutated melanoma 24. These developments evoked increased interest in molecular subclassification of especially cutaneous and uveal melanoma. However, for some time, the genetic background of primary LMNs remained largely unexplored, probably because these tumors are rare and it is difficult to assemble larger series.
In this review, we focus on recently obtained insights in the molecular alterations in primary melanocytic neoplasms of the CNS, and we discuss implications for pathogenesis, differential diagnosis and potential targeted therapy. Prior, main clinical and pathologic characteristics of these tumors are briefly addressed.
Main Clinicopathologic Features of Primary LMNs
Classification
According to the present (ie, 2007) World Health Organization (WHO) classification of tumors of the CNS, proliferation of melanocytes in the leptomeninges may give rise to circumscribed or diffuse neoplasms that can either be benign or malignant. Primary leptomeningeal melanomas may arise de novo or by transformation from a non‐malignant precursor. Circumscribed LMNs include melanocytoma (benign) and melanoma (malignant), with in‐between lesions histologically showing features of intermediate‐grade malignancy. Diffuse melanocytic proliferations in the leptomeninges include melanocytosis and melanomatosis and are characterized by diffuse growth of cytologically benign and malignant melanocytic cells, respectively (Table 1) 17.
Table 1.
World Health Organization (WHO) 2007 classification of primary leptomeningeal melanocytic neoplasms. Abbreviation: NCM = neurocutaneous melanosis
| WHO 2007 Primary leptomeningeal melanocytic neoplasms | |||
|---|---|---|---|
| Circumscribed | |||
| Melanocytoma | Benign/low‐grade | Mostly in adults (de novo) | |
| Intermediate‐grade melanocytic tumor | Intermediate‐grade | ||
| Melanoma | Frankly malignant | ||
| Diffuse | |||
| Melanocytosis | Cytologically benign | Mostly in children (often in the context of NCM) | |
| Melanomatosis* | Malignant | ||
The distinction between adults and children is not always strict, as cases of circumscribed leptomeningeal melanoma has been described in children with NCM, and melanomatosis* may also become manifest in adults, mostly in the fourth decade 103, 105, 124. Moreover, adult patients can still have a congenital background of their disease, and it is not clear whether all leptomeningeal melanocytic neoplasms in children are congenital in origin.
Circumscribed primary LMNs mostly occur in adults. Children more often present with diffuse leptomeningeal proliferations, often in the context of the congenital syndrome NCM with large and/or multiple congenital melanocytic nevi in the skin. However, diffuse leptomeningeal melanocytosis and melanomatosis may also occur in adult patients 8, 89, 100, 105, 124 while circumscribed primary leptomeningeal melanoma has been described in children 70, 84, 103. Benign and malignant primary LMNs can be associated with other melanocytic skin lesions such as blue nevi and nevi of Ota (or oculo‐cutaneous melanosis if the eye is involved) 2, 10, 65, 95.
Circumscribed neoplasms
Melanocytoma
Melanocytoma is generally a slow‐growing neoplasm with a peak incidence in the fifth decade and a slight predilection for women. It has an estimated incidence of 1 per 10 million per year 17. Melanocytoma occurs especially in the cervical and thoracic spinal region, the posterior cranial fossa and Meckle's cave, which is probably related to the higher density of melanocytes in the normal leptomeninges at these sites (Table 2) 17.
Table 2.
Main clinicopathologic features of primary leptomeningeal melanocytic neoplasms. Abbreviations: CNS = central nervous system; LMNs = leptomeningeal melanocytic neoplasms; NCM = neurocutaneous melanosis
| Incidence | Sex, age | Localization | Behavior/prognosis | Treatment | ||
|---|---|---|---|---|---|---|
| Circumscribed LMNs | Melanocytoma | 1/107/year 17 | F > M (1.5:1) median; 45–50 years 17 | Spinal region (cervical, thoracic)**; posterior fossa; Meckel's cave 17 | Frequent local recurrence, esp. in case of incomplete resection*** 108; malignant transformation rare 72, 132 | Complete resection if possible. Radiation therapy in case of incomplete resection or recurrence 108 |
| Melanoma | 0.5–0.9/107/year 14, 17, 54 | M > F median; 4 years 17 | Along the neuroaxis, slight predilection for spinal cord and posterior fossa 17 | Distant metastases rare 72; better prognosis in case of complete resection compared with CNS melanoma metastasis 113, 123 | Complete resection if possible, postoperative radiotherapy is recommended 113; usefulness of chemotherapy is not established 124 | |
| Diffuse LMNs | Melanocytosis | Very rare* | Children, mostly in context of NCM, rarely without cutaneous melanocytic lesions 17, 18 | Supra‐ and/or infratentorial/spinal leptomeninges; most frequent in/near cerebellum, pons, medulla and temporal lobes 17 | Poor prognosis, once symptomatic 65 | Efficacy of chemotherapy and radiotherapy is not established. Chemotherapy might show some benefit 90. |
| Melanomatosis | Very rare* | Bimodal distribution: children, mostly in context of NCM or adults, fourth decade 3, 105 | Supra‐ and/or infratentorial/spinal leptomeninges, and/or superficial brain parenchyma 17 | Dismal prognosis 3, 105 | Not established. Chemotherapy and radiotherapy was reported to have benefit in a NCM patient with melanomatosis 127. | |
*Population‐based incidence is not available 17.
**Mostly in the extramedullary, intradural compartment of the spinal canal.
***The biologic behavior of intermediate‐grade melanocytomas is variable.
While most melanocytomas are intradural, extramedullary tumors, occasionally intramedullary or paraspinal cases have been reported 39, 97. Melanocytomas contain variable amounts of melanin pigment and may macroscopically appear black, red‐brown or blue, and even amelanotic 17. The unique paramagnetic properties of melanin result in a fairly characteristic isointensity to hyperintensity on T1‐weighted magnetic resonance imaging (MRI) and iso‐or hypointensity on T2‐weighted images 124. Symptoms are related to their location and include myelopathy, radiculopathy, cranial nerve deficiency, seizures and hydrocephalus 124. Especially in case of incomplete resection, melanocytomas frequently recur (up to 50% recurrence reported 1 year after surgery) 108. Also, aggressive behavior with leptomeningeal seeding or rarely malignant transformation into melanoma has been reported 20, 72, 108, 132. Whenever possible, melanocytomas should be completely resected. In case of incomplete resection or recurrence adjuvant radiation therapy is advised 108.
Histologically, melanocytomas consist of variably pigmented, well‐differentiated melanocytes showing little cytonuclear atypia and low proliferative activity [zero to one mitosis per 10 high‐power fields (HPFs) and MIB‐1 labeling index (LI) < 1%–2%] 16, 17. At present, histologic features that reliably predict more aggressive behavior are lacking.
Melanoma
Circumscribed primary melanoma of the leptomeninges occurs along the neuroaxis with a slight predilection for the spinal cord and posterior fossa. It most frequently occurs in adults, particularly men, with a peak incidence in the fifth decade (Table 2) 14, 17. Reported incidences vary between 0.5 and 0.9 per 10 million per year, but this is likely an underestimation because of under‐recognition 14, 54. Distant metastases from a primary leptomeningeal melanoma are rare and have been reported in the liver, bones and lungs 14, 72, 75, 138. Prognosis is variable and several studies have reported a better prognosis compared with metastatic melanoma to the CNS with long‐term survival up to 12 years in case of localized disease and complete resection 49, 110, 113. Whether cases with prolonged survival may have been in fact cases that would now meet the criteria of melanocytomas or intermediate‐grade lesions is unclear.
Melanocytoma of intermediate‐grade malignancy
Unequivocal classification of primary LMNs as either melanocytoma or melanoma can be difficult. In some cases, obvious cytologic atypia is lacking, while microscopic CNS invasion and/or increased mitotic activity may be present (one to three mitoses per 10 HPFs and MIB‐1 LI ranging from 1% to 4%) 16. According to the WHO 2007 classification, such neoplasms can be diagnosed as intermediate‐grade melanocytic tumors 17. The clinical behavior of these intermediate‐grade melanocytic neoplasms is uncertain and needs further study 16, 17.
Differential diagnosis of circumscribed primary LMNs from other pigmented nervous system tumors
The main differential diagnosis includes metastatic melanoma to the CNS. These tumors are much more frequent as cutaneous melanoma has a higher incidence combined with a high propensity to metastasize to the CNS (CNS involvement in approximately 10% of cutaneous melanoma patients in clinical studies) 27, 136. This distinction is important as the prognosis of a patient with a primary LMN is substantially better than that of a metastatic melanoma to the CNS, which has a life expectancy of generally less than a year 19, 49, 110, 113. Primary leptomeningeal melanoma and metastatic lesions show histologic overlap, and histopathologic discrimination between primary or metastatic melanoma in individual patients is often impossible 16, 17. In addition, it is important to realize that primary cutaneous melanoma may show “spontaneous regression,” a prior excision of a cutaneous melanoma may not have been histologically examined or may have been misdiagnosed as a benign lesion, and primary melanomas may originate from less visible locations such as acral surfaces, nail beds, mucosal surfaces (genitals, sinonasal cavity, gastrointestinal tract) and the uveal tract. Therefore, before concluding that a CNS melanoma is a primary tumor, a thorough clinical examination as well as histologic revision of previously removed melanocytic tumors should be performed.
Clinically and radiologically, circumscribed primary LMNs can resemble meningeoma as both are leptomeningeal‐based tumors. Even histologically, the differential diagnosis may be challenging as both may demonstrate a nested growth pattern. Immunohistochemistry for EMA is helpful in this respect as melanocytomas are non‐reactive for Epithelial Membrane Antigen (EMA) 16.
Melanocytoma should also be differentiated from melanotic schwannoma, a rare variant of schwannoma composed of cells having the ultrastructure and immunophenotype of Schwann cells and demonstrating some melanocytic features in the form of intracytoplasmic melanosomes and being reactive for melanoma markers 6, 119. Melanotic schwannoma frequently occurs in association with cranial nerves and spinal nerve roots and the paraspinal sympathic chain, thereby showing overlap with primary LMNs. Discrimination is important because especially the psammomatous form of melanotic schwannoma is associated with the autosomal dominantly inherited Carney complex, a rare multiple neoplasia syndrome characterized by (cardiac) myxomas, multiple types of skin tumors and pigmented lesions, and endocrine tumors including pigmented nodular adrenocortical disease 21, 112, 115. Furthermore, a small percentage of melanotic schwannomas may show malignant behavior with distant metastases 119, 130. Immunohistochemistry is often of little help as both neoplasms are neural crest‐derived and generally positive for S100 and (other) melanocytic markers such as MelanA and HMB‐45. Reticulin stains and immunohistochemistry for basement membrane components can be helpful as there is generally pericellular staining in schwannomas compared with nested staining in melanocytoma, although the pericellular staining pattern is usually less pronounced in melanotic schwannomas 6, 60, 76.
Occasionally, other primary CNS tumors contain melanin pigment, for example melanotic medulloblastoma, melanotic neuro‐ectodermal tumor of infancy, pigmented glial/ependymal and choroid plexus tumors, and teratoma 124, 131. Further discussion of these extremely rare and non‐ or non‐pure melanocytic tumors is beyond the scope of this review.
Diffuse neoplasms
Melanocytosis
Melanocytosis is a diffuse proliferation of histologically benign appearing melanocytes in the leptomeninges, often with extension in the Virchow‐Robin spaces, but without frank invasion of the CNS parenchyma. Macroscopically, melanocytosis is visible as brown to black discoloration of the leptomeninges 17. Melanocytosis can remain asymptomatic, but once neurologic symptoms develop prognosis is generally poor, even in the absence of malignant transformation.
Melanocytosis most frequently occurs in children in the context of NCM. This rare, congenital neurocutaneous syndrome is characterized by the presence of large and/or multiple congenital melanocytic nevi (CMN) of the skin in association with primary melanotic lesions of the CNS. The latter include melanin depositions in the brain parenchyma (visible on T1‐weighted MRI) and/or a benign or malignant, primary LMN 65, 69. NCM shows no clear pattern of inheritance. Rarely, non‐melanocytic CNS neoplasms have been reported in association with CMN and NCM, including neurocristic hamartoma, choroid plexus papilloma and meningeoma 70.
Melanomatosis
Diffuse spread of malignant appearing primary melanocytic cells along the leptomeninges, often with superficial invasion of the brain parenchyma, is known as melanomatosis (Table 2) 17. This disease has a bimodal age distribution and may become manifest in children, with or without a context of NCM, as well as in adults, mostly in the fourth decade 3, 8, 105.
Melanomatosis is an extremely aggressive disease with high mortality. Especially in small biopsies, melanomatosis can histologically mimic metastasis of for example, cutaneous melanoma to the CNS. The absence of melanoma elsewhere in the body and its diffuse growth pattern on radiologic images (in contrast to localized lesions found in metastatic CNS melanoma) contribute to the diagnosis 124.
Molecular Characteristics of LMNs
Mutations in melanomas other than primary LMNs
Especially during the last decade molecular studies showed that melanoma is a heterogeneous disease with distinct oncogenic mutations in different subgroups of melanoma (Figure 2, Table 3) 31, 141. Activating mutations in the BRAF gene, encoding a member of the RAF family of serine/threonine kinases, are frequent in cutaneous melanoma, especially in those occurring in skin subject to intermittent sun exposure (present in approximately 50% of those tumors) 86. Activating BRAF mutations lead to constitutive activation of the mitogen‐activated protein kinase (MAPK) pathway, by transduction of signals from the cell surface to the nucleus through phosphorylation of a cascade of kinases that results in cell proliferation 41. Approximately, 75% of BRAF mutations involve the c.1799T>A [p.(Val600Glu)] alias BRAFV600E hotspot mutation 24, 48.
Figure 2.
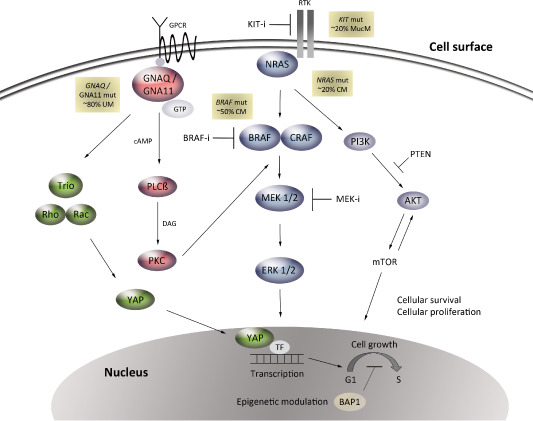
Main intracellular pathways implicated in different subtypes of melanoma. Mutations in KIT are relatively frequent in mucosal melanoma (mucm, ∼20%), result in activation of the MAPK and PI3/AKT/mTOR pathways, and can be treated with KIT inhibitors. Approximately 50% of CM harbor mutations in BRAFV600, which provide a therapeutic target for selective BRAF inhibitors. Mutations in NRAS (∼20% of CM) signal to MEK/ERK through CRAF, but also to the PI3/AKT/mTOR pathway. MEK inhibitors have shown activity in some patients with NRAS mutant melanoma. Signaling pathways downstream of GNAQ/GNA11 (∼80% of UM) include activation of PKC through the release of diacylglycerol (DAG) by β‐PLC, which can activate the MAPK pathway. Another downstream pathway is the Hippo‐YAP pathway resulting in activation and translocation of the transcriptional coactivator YAP into the nucleus through a mechanism of actin polymerization regulated by the GTPases Rho and Rac. Metastatic UMs frequently harbor inactivating mutations in BAP1, which is involved in multiple functions including regulation of the cell cycle and epigenetic modulation. Inhibition of downstream components such as MEK, PKC, BAP1 and YAP are now under (pre) clinical investigation for treatment of GNAQ/GNA11‐mutated UM. CM = cutaneous melanoma; MAPK = mitogen‐activated protein kinase; MucM = mucosal melanoma; PKC = protein kinase C; PLC = phospholipase C; UM = uveal melanoma; ERK = extracellular signal‐regulated kinase; MEK = mitogen‐activated ERK kinase; PI3K/AKT/mTOR = phosphatidylinositol 3‐kinase/protein kinase AKT/mammalian target of rapamycin.
Table 3.
Frequency of oncogenic hotspot mutations in circumscribed LMNs in adults compared with melanoma of other body locations. Abbreviations: LMNs = leptomeningeal melanocytic neoplasms
| GNAQ codon 209 | GNA11 codon 209 | BRAF codon 600 | NRAS codons 61, 12, and 13 | HRAS codons 61, 12, and 13 | KRAS codons 61, 12, and 13 | KIT ex 11, 17 | |
|---|---|---|---|---|---|---|---|
| LMNs | |||||||
| Melanocytoma | 6/12 (50%) 77 | 0/11 75 | 0/12 77 | 0/10 77 | 0/9 77 | 1/18 (6%)e 71 | 0/5 45 |
| 3/6 (50%) 45 | 0/6 45 | 0/5 45 | 0/6 45 | 0/6 45 | 0/18 71 | ||
| 0/5 96 | 1/5 (20% ) 96 | 1/18 (6%)a 71 | 0/18 71 | 0/8 139 | |||
| 8/18 (44%) 71 | 6/18 (33%) 71 | 0/11 139 | 0/8 139 | ||||
| 41% | 18% | 2%a | 0% | 0% | 6%e | 0% | |
| Intermediate‐grade melanocytic tumor | 3/7 45 | 1/7 (14%) 45 | 0/3 77 | 0/1 77 | 0/1 77 | Not reported yet | 0/7 45 |
| 0/3 77 | 1/5 (20%) 75 | 0/7 45 | 1/7 (14%) 45 | 0/7 45 | |||
| 30% | 17% | 0% | 13% | 0% | Unknown | 0% | |
| Melanoma | 1/3 (33%) 77 | 1/3 (33%) 45 | 0/3b 77 | 0/2c 77 | 0/3 77 | Not reported yet | 0/3 45 |
| 0/3 45 | 1/4 (25%) 75 | 0/3 45 | 0/3c 45 | 0/3 45 | |||
| 17% | 29% | 0% | 0% | 0% | Not reported yet | 0% | |
| Melanoma of other body locations | |||||||
| Uveal | 45%–49% 102, 134 | 32% 134 | <1% 29, 85, 148 | <1% 29, 148 | 0% 125, 148 | 0% 140 | 10% 137 |
| Skin | 1% 22, 134 | 1% 134 | ∼50% 86 | 15%–20% 66, 81 | 2% 43 | 1%–2% 140 | 10% 30 |
| Mucosal | <1%d 22, 68, 134 | 0% 134 | ∼10% 30, 31, 86 | ∼20% 13 | <2% 64, 101 | Rare 79 | 20% 30 |
This table includes all series reported up to now for LMNs in adults; case reports are not included in this table, but are discussed in the text.
Koelsche et al reported one BRAFV600E mutation in their series of 18 melanocytomas. By unsupervised hierarchical cluster analysis of tumor methylomes the DNA methylation profile of this case clustered with the metastatic melanoma group instead of with melanocytomas, raising the question whether this case was truly a melanocytoma or rather a (metastatic) melanoma.
The single BRAFV600E mutation we previously detected in a leptomeningeal melanoma is not included here as we cannot rule out in retrospect that this was a metastasis from a skin melanoma; the patient underwent a skin excision with histology that could fit a regressed melanoma.
In adult cases. Of note, NRAS mutations in LMNs in the pediatric setting are a frequent event; see text for further reading.
A single case of GNAQ mutation occuring in a mucosal melanoma has been reported 68.
Up to now, only one study looked for mutations in KRAS in primary LMNs. Koelsche et al found one KRAS mutation (c.179G>A) in 18 melanocytomas. The DNA methylation profile of this case, however, clustered with the melanotic schwannoma group instead of with melanocytomas.
Activating mutations in NRAS, one of the three major isoforms of the RAS family of GTPase proteins and an upstream signaling component in the MAPK pathway, are present in approximately 15%–20% of cutaneous melanomas and 20% of mucosal melanomas, but are very rare in uveal melanomas 13, 81, 148. NRAS mutations usually occur in codons 12, 13 or 61, and are mutually exclusive with BRAFV600 mutations 37. Activating NRAS mutations cause aberrant signaling in several downstream cascades including the MAPK and the phosphatidylinositol 3‐kinase/protein kinase AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) pathways that are implicated in cell proliferation and survival 106. Cutaneous melanomas also frequently harbor mutations in the promoter region of the telomerase reverse transcriptase TERT (70%) 59.
In comparison with cutaneous melanoma, BRAF mutations are infrequent in mucosal melanomas (∼10%), in melanomas of the “non‐hair‐bearing skin” (acral melanomas, approximately 15%) and virtually absent in uveal melanomas 29, 86. Similarly, TERT promoter mutations are rare in these melanomas subtypes (eg, found in 0.5% of uveal melanomas) 35.
Instead, mucosal melanomas and acral melanomas relatively frequently harbor activating mutations and/or increased copy numbers of the gene encoding the receptor tyrosine kinase KIT (approximately 20%–40%) 30. Uveal melanomas and blue nevi frequently harbor activating, mutually exclusive GNAQ or GNA11 mutations. GNAQ mutations are found in about 45% of uveal melanomas and 83% of blue nevi, GNA11 mutations in about 32% of uveal melanomas and 6.5% of blue nevi. In both genes, the mutations most often involve codon 209 with only rarely mutations in codon 183 (1%–3%, in either gene) 102, 133, 134. Although so far a clear association between GNA11 or GNAQ mutational status and survival in uveal melanoma patients is lacking, the study of Van Raamsdonk et al suggested that GNA11Q209 mutations occur especially in aggressive, metastatic tumors 12, 74, 134. Of note, oncogenic mutations in the GNAQ and GNA11 genes are extremely rare in cutaneous, mucosal and acral melanomas, as well as in common melanocytic, congenital and spitz nevi (Table 3) 22, 104, 107, 134.
Furthermore, inactivating mutations in the tumor suppressor BAP1 are frequent in metastatic uveal melanoma as well (approximately 80%) and are strongly associated with monosomy 3, which is a significant predictor for liver metastasis 52. Recently, hotspot mutations of the SF3B1 gene, encoding the splicing factor 3B subunit 1 and affecting RNA splicing, was found in 30% of uveal melanomas (especially in those tumors without monosomy 3) compared with only 1% of cutaneous melanomas 73, 88.
Adult patients with circumscribed primary LMNs
High frequency of GNAQ and GNA11 mutations
Only in 2009 resp. 2012, GNAQ and GNA11 mutations were reported for the first time to occur in LMNs 77, 96. Oncogenic mutations in these genes are found in benign as well as malignant LMNs and have up to now only been reported in adult patients with circumscribed tumors, except for a 15‐year‐old boy with a GNA11‐mutated melanocytoma in the series of Koelsche et al 28, 44, 45, 71, 75, 77, 96, 126. Like in uveal melanomas, activating mutations in GNAQ and GNA11 in LMNs mainly affect codon 209 and consist of substitution of glutamine to leucine [c.626 A>T (p.(Gln209Leu))] or, less frequently, by substitution to proline [c.626 A>C (p.(Gln209Pro))] 45, 71, 75, 77. Only a single case of spinal melanocytoma with a codon 183 GNAQ mutation {c.548 G>A [p.(Arg183Gln)]} has been described 96. A summary of mutation frequencies in LMNs is presented in Table 3. Based on published series so far, GNAQ mutations are present in about 39% of melanocytomas and 17% of primary leptomeningeal melanomas, while GNA11 mutations are present in approximately 17% of melanocytomas (including intermediate‐grade melanocytic neoplasms) and 29% of primary leptomeningeal melanomas. Up till now, the prognostic significance of GNAQ and GNA11 mutations in primary LMNs is unclear, nor is there a clear association with other clinicopathologic characteristics.
Other molecular findings
In the few larger series on primary LMNs reported up to now, BRAFV600E mutations are very rare. (Table 3) Of note, the number of esp. primary leptomeningeal melanoma cases analyzed is limited 45, 71, 77, 139. A BRAFV600E mutation has recently been reported in an unusual case of leptomeningeal melanocytoma in assocation with a congenital nevus of Ota in a 15‐year‐old boy. This melanocytoma did not harbor any GNAQQ209 mutations, however, codon 183 and GNA11 mutation status was not investigated 95. Koelsche et al found a single case with a BRAFV600E mutation in their series of 18 melanocytomas. By unsupervised hierarchical cluster analysis of tumor methylomes, however, the DNA methylation profile of this case clustered with the metastatic melanoma group instead of with the melanocytoma group 71. This latter finding may be explained as proof of an unusual BRAF‐mutational profile in a primary leptomeningeal tumor as well as a reason to consider this lesion rather as metastatic melanoma. Based on current literature, the BRAFV600E mutation seems to be very rare in primary LMNs, but more cases need to be analyzed.
Most studies have reported absence of NRAS mutations in primary LMNs in adult. A single NRASQ61K‐mutated intermediate‐grade melanocytoma was found in a series of 15 primary LMN cases in adult patients. This concerned a tumor in the spinal region in a 40‐year‐old woman. Whether this patient had a congenital background of her disease is unknown 45. Importantly, in primary LMNs in children NRAS mutations occur more frequently (see later). In a few studies, HRAS, KRAS and KIT mutations were examined in primary LMNs and reported to be absent (Table 3). Only the study of Koelsche et al reported a KRAS mutation in a melanocytoma (c.179G>A, 1/18) although the DNA methylation profile of this case clustered with the melanotic schwannoma group rather than with the melanocytomas 71.
Similar to uveal melanoma, TERT promoter mutations are reported to be absent in primary LMNs 46. It remains to be determined whether inactivating mutations in BAP1 frequently present in uveal melanoma also characterize primary LMNs.
In a recent study, we demonstrated monosomy 3 in a GNAQ‐mutated leptomeningeal melanoma. This patient showed distant metastases in bone and lungs, but no liver metastases. A gain of chromosome 6p was found in a GNA11‐mutated leptomeningeal melanoma without distant metastases, while gain of whole chromosome 6 was present in an intermediate‐grade melanocytic tumor showing local recurrence 75. Koelsche et al also demonstrated that gain of chromomosome 6 or 6p is frequently present in melanocytomas (8 out of 18 cases) in addition to loss of chromosome 3 or 3q (4/18) 71. These results suggest that primary LMNs share not only mutations, but also copy number variations (CNVs) with uveal melanoma. It is presently unclear whether these CNVs have prognostic value in LMNs as well.
GNAQ and GNA11 mutations: diagnostic potential
Acknowledging the relatively high incidence of cutaneous melanoma and its frequent spread to the CNS vs. the low incidence of primary LMNs, it is much more likely that a melanoma in the CNS represents a metastatic lesion than a primary LMN. Still, discriminating these entities is relevant because of different patient work‐up and the substantially better prognosis for patients with a primary LMN 49, 113, 123.
GNAQ and GNA11 mutational analysis can be useful in this differential diagnosis as presence of a GNAQ or GNA11 mutation in a CNS melanocytic tumor strongly favors a primary leptomeningeal origin over metastasis of cutaneous melanoma. (Figure 3) Theoretically, in case of a GNAQ or GNA11 mutation, metastasis of uveal melanoma or malignant blue nevus to the CNS should be considered in the differential diagnosis. An example illustrating the diagnostic potential of this analysis is given in Figure 4. Conversely, knowing that about 50% of cutaneous melanomas carry a BRAFV600 mutation and that this mutation is very rare in primary LMNs, demonstration of this BRAF mutation in a CNS melanocytic tumor strongly favors metastatic cutaneous melanoma over primary LMN 45, 71, 75, 139.
Figure 3.
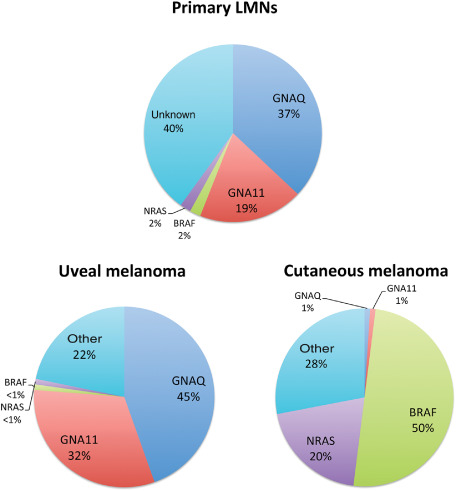
Relative frequency of oncogenic mutations in primary LMNs in adults vs. uveal and cutaneous melanomas. Primary leptomeningeal melanocytic neoplasms (LMNs) in adults frequently harbor oncogenic mutations in GNAQ and to a lesser extent in GNA11. The mutation profile is comparable with that of uveal melanoma, but distinct from cutaneous melanoma. GNAQ/GNA11/BRAF mutation analysis can be used as a diagnostic tool in the differential diagnosis of primary LMNs vs. metastasis of cutaneous melanoma to the central nervous system (CNS). The mutation frequencies depicted are based on the studies summarized in Table 3.
Figure 4.
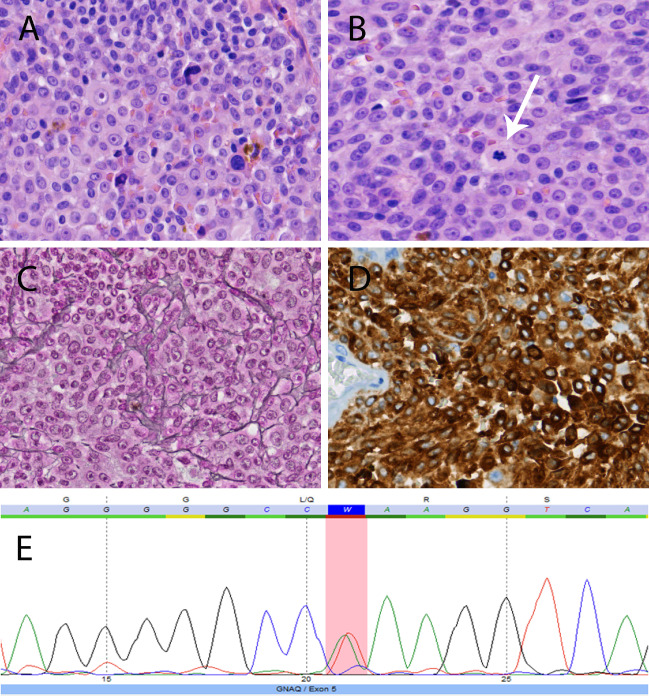
Example of the diagnostic potential of molecular analysis in a patient with a pigmented tumor around the spinal cord. A 62‐year‐old man presented with neurologic symptoms consistent with myelopathy at the thoracic level. Magnetic resonance imaging showed an intra‐ and extramedullary spinal tumor at thoracic levels 7–9 and the lesion was resected. Histology revealed a cellular tumor with a mixture of spindle and epithelioid cells, arranged in nests and sheets, and with oval nuclei with prominent nucleolus and moderate to strong nuclear atypia. Focally, melanin pigment was present. (A) Proliferation activity was limited (three mitoses per 10 high‐power fields, arrow in B; MIB‐1 labeling index around 5%) and necrosis was absent. The tumor showed invasion in CNS tissue. In the reticulin stain (Laquesse), nests of tumor cells rather than individual tumor cells were encircled by reticulin fibers, indicating melanocytic rather than schwannian differentiation (C). Tumor cells were strongly reactive with Melan‐A (D) and HMB‐45. Based on histology and knowing that the patient did not have a (history of) cutaneous melanoma, a diagnosis of primary leptomeningeal melanoma was made. Indeed, mutation analysis of the hotspot regions of GNAQ, GNA11, BRAF, NRAS and KIT revealed a GNAQQ209L mutation {c.626A > T[p.(Gln209Leu)]}, fully supporting the diagnosis of a primary melanocytic tumor of the CNS over a metastatic cutaneous melanoma or melanotic schwannoma (E, forward sequence, mutation area is marked as a red column). Of note, metastatic uveal melanoma and malignant blue nevus still needs to be in the differential diagnosis in case of a GNAQ‐mutated melanocytic tumor in/around the CNS, but these options were ruled out in our patient as well. CNS = central nervous system
GNAQ and GNA11 mutational analysis can have some value in the differential diagnosis of primary LMN vs. melanotic schwannoma 76. Correct diagnosis of (psammomatous) melanotic schwannoma is important to select patients who need further clinical work‐up for Carney complex, a potentially life‐threatening disease especially because of the frequent occurrence of cardiac myxomas 115, 120. Inactivating mutations in the regulatory subunit type 1 alpha gene of protein kinase A (PKA) (PRKAR1A, at chromosome region 17q22–24) are present in up to 70% of patients with Carney complex 58, 115. Mutations in PRKAR1A mostly result in PRKAR1A haploinsufficienty with loss of PRKAR1A expression and inactivation of the regulatory subunit type 1 alpha resulting in increased PKA activity 118 Instead of hotspot mutations, PRKAR1A mutations are spread over ten exons with approximately 120 pathogenic sequence variants described 15, 58. PRKAR1A mutational analysis is therefore not practical for routine testing when a melanotic schwannoma is considered. We previously showed that GNAQQ209 mutations were absent in a series of nine melanotic schwannomas 76 and subsequently found that GNAQR183 and GNA11Q209/R183 mutations were absent in these tumors as well (unpub. data). Koelsche et al identified in their series of 14 melanotic schwannomas one case with a GNAQQ209L mutation and another one with a GNA11Q209P mutation. The DNA methylation profiles of both cases, however, clustered with the melanocytomas and not with the melanotic schwannomas, suggesting that these cases might have been melanocytomas 71. The data at present thus suggest that GNAQ and GNA11 mutation analysis are helpful in the differential diagnosis of primary LMN vs. melanotic schwannoma although more cases of especially these latter tumors need to be tested.
Investigation of the mutational status of the BRAF, NRAS, GNAQ and GNA11 genes can easily be performed by Sanger sequencing or next generation sequencing (NGS) technologies 38. The relevant mutations in these genes are hotspot mutations, allowing targeted sequencing of the predefined regions of the respective genes. The major advantage of NGS is the lower amount of DNA that is needed for the analysis and the higher sensitivity compared with Sanger sequencing (detection of 2%–10% vs. 15%–25% allele frequency). For sequencing technologies, the DNA specimens are extracted via standard procedures, although the quality of the sequencing result from highly pigmented lesions may improve by an additional purification step.
GNAQ and GNA11 mutations: pathobiologic aspects
The preferential occurrence of GNAQ and GNA11 mutations in primary LMNs, uveal melanomas and blue nevi suggests a common pathogenetic mechanism. These tumors originate from melanocytes that are not epithelium related (blue nevi are thought to be derived from a small pool of melanocytes located in the dermis that have not reached the epidermis during embryogenesis 82 ). In contrast, common melanocytic nevi and cutaneous melanomas are derived from intra‐epidermally located melanocytes 92. Apparently, epidermal and non‐epidermal melanocytic cells are vulnerable to different oncogenic mutations. Interestingly, in mice indeed differences in epidermal vs. non‐epidermal melanocytes were found regarding their requirement of growth and differentiation signals 7.
GNAQ and its paralogue GNA11 map on chromosomes 9q21.2 and 19p13.3, respectively 61. They encode the α subunit of G‐proteins, which couple G‐protein coupled receptors (GPCRs) to various intracellular pathways 62. G‐proteins are heterotrimeric GTP‐binding proteins and consist of α and βγ subunits 62. The α subunit genes are grouped into four classes; Gαs, Gαi/o, Gαq/11 and Gα12/13; GNAQ and GNA11 belong to the Gq/11 class of α subunit genes 61, 99, 142. G‐proteins function as a molecular switch: activation of the GPCR catalyzes the exchange of guanosine diphosphate (GDP), bound to the inactive α subunit, for guanosine triphosphate (GTP), resulting in dissociation of the complex 62. The Gα subunit then activates diverse effector proteins, depending on the Gα subunit type, by the release of second messengers. GTP hydrolysis and re‐association of the complex into its inactive, GDP‐bound state is largely regulated by the intrinsic GTPase activity of the α subunit 62.
The glutamine at codon 209 or arginine at codon 183 of GNAQ and GNA11 is essential for GTP hydrolysis and mutations at these codons cause impaired GTPase activity. This leads to constitutive activation of downstream intracellular pathways including the MAPK pathway via activation of protein kinase C (PKC) by β‐phospholipase C 53, 61, 87, 114, 133. Another pathway regulated by GPCRs is the Hippo‐YAP signaling pathway 147. Recently, it was demonstrated that this pathway is implicated in GNAQ/GNA11‐mutated uveal melanoma, that the transcription co‐activator YAP can be specifically activated by GNAQ/GNA11 mutations (Q209), and that YAP is important for GNAQ‐induced neoplastic transformation (Figure 2) 42, 146.
GNAQ/GNA11 mutations: therapeutic potential
Patients with metastatic cutaneous melanoma carrying the BRAFV600 mutation can nowadays be treated with selective BRAF inhibitors (eg, vemurafenib and dabrafenib), sometimes with impressive (albeit temporary) clinical and radiologic response 24, 36, 48. Treatment with the MEK inhibitor trametinib is approved by the Food and Drugs Administration as monotherapy for unresectable or metastatic BRAFV600‐mutated melanoma in the USA while the MEK inhibitor MEK162 has shown efficacy in NRAS‐mutated advanced melanoma in a phase 2 study 9, 143. Similarly, treatment with KIT inhibitors such as imatinib and sorafenib has shown objective responses in advanced KIT‐mutated cutaneous melanomas 50, 56.
At present, there are neither GNAQ‐ nor GNA11‐mutated cell lines from primary human LMNs available and preclinical and clinical evidence for therapeutic efficacy of targeted treatment in patients with primary LMNs is lacking. Still, inhibition of GNAQ/GNA11‐dependent signaling pathways may as well be of interest for targeting LMNs carrying these mutations.
To date, there are no direct inhibitors of mutant‐GNAQ (or GNA11) and current focus is on inhibition of components of downstream‐activated pathways. One of these downstream pathways is the MAPK pathway that is activated by PKC through the release of diacylglycerol by β‐phospholipase C (Figure 2) 53, 61, 114, 133. The PKC inhibitor enzastaurin was shown to have antitumor activity against GNAQ‐mutated uveal melanoma cell lines via inhibition of the PKC/ERK 1/2 pathway 144, 145 Very recently, a phase 1 trial with the PKC inhibitor AEB071 completed dose escalation in GNAQ/GNA11 mutant uveal melanoma patients 122. Combination of PKC inhibition and MEK inhibition was demonstrated to be synergistic in suppressing MAPK signaling in GNAQ‐mutated uveal melanoma cell lines and combination trials are ongoing 25, 122. Also, GNAQ‐mutated uveal melanoma cell lines were shown to be sensitive to inhibition of MEK, resulting in decreased levels of pERK and decreased proliferation 5, 40, 91, 133. Recently, a phase II trial showed that the MEK inhibitor selumetinib (AZD6244) resulted in a modestly improved progression‐free survival compared with chemotherapy in metastatic uveal melanoma patients with GNAQ or GNA11 mutation 23.
Using a siRNA strategy, knockdown of GNAQ was demonstrated to inhibit the PI3K/AKT/mTOR pathway as well 4. Dual pathway inhibition with a combination of MEK inhibitor and PI3K inhibitor or mTOR kinase inhibitor was described to be synergistic in inducing apoptosis and reducing cell viability in GNAQ mutant uveal melanoma cell lines, respectively 4, 55, 67. A combination phase 2 trial of the MEK inhibitor trametinib or trametinib plus the AKT inhibitor GSK2141795 is currently randomizing patients 122. Also, as the YAP inhibitor verteporfin was shown to selectively inhibit growth of GNAQ/GNA11 mutant uveal melanoma cell lines, this might be a promising therapeutic strategy for patients with primary LMNs as well 42, 146.
Children with primary LMNs
NRAS mutations in pediatric LMNs and NCM
Post‐zygotic, somatic mutations in NRAS were recently shown to underlie the pathogenesis of NCM in most of the cases. These mutations mainly affect codon 61 of NRAS and have been detected in diffuse as well as circumscribed LMNs in children 45, 70, 78, 103, 117. In a very recent series, 12 of 16 patients with NCM harbored a somatic NRASQ61 mutation in their CMN (75%) 117. Moreover, several studies on NCM have demonstrated an identical, somatic NRASQ61 mutation in multiple of the CMN as well as in the LMN in the same patients 70, 78, 103. Table 4. This phenomenon can be explained by assuming that early during embryogenesis a melanocyte precursor cell acquires a somatic NRAS mutation, which gives rise to a mosaic pattern of NRAS‐mutated cells that further migrate and colonize the skin and/or leptomeninges. This pathogenetic mechanism fits the spectrum of mosaic RASopathies that is characterized by post‐zygotic mutations resulting in the presence of two genetically distinct cell populations in the same organism 51. The exact timing of acquisition of the NRAS mutation during embryogenesis (and thus the position of the migrating precursor cell at that time) might influence the eventual phenotype (Figure 5).
Table 4.
Overview of NRAS mutations detected up to now in both CNS lesions as well as in CMN of patients with NCM. Abbreviations: CMN = congenital melanocytic nevus; CNS = central nervous system; NCM = neurocutaneous melanosis
| Age (years) | CNS melanocytic/melanotic lesion | Location | NRAS mutation in the CNS tumor | Identical NRAS mutation in the patient's CMN | Reference |
|---|---|---|---|---|---|
| 7 | Circumscribed melanoma | Right frontotemporal | Q61K | Yes | Pedersen et al 103 |
| 8 | Melanocytosis | Not otherwise specified | Q61R | Yes, in multiple CMN | Kinsler et al 70 |
| 10 | Primary melanomaa | Cerebellum | Q61R | Yes, in multiple CMN | Kinsler et al 70 |
| 13 | Melanocytosis | Spinal | Q61K | Yes | Küsters‐Vandevelde et al 78 |
| 2 months | Melanin deposition on T1‐weighted MRI | Right cerebellopontine cisternb | G13Rc | Yes | Shih et al 121 |
In a background of diffuse leptomeningeal melanocytosis.
At the age of 21 months, this patient also presented with a myxoid, non‐melanocytic mesenchymal tumor in the left occipital region; this tumor harbored an NRASG13R mutation.
Which was also present in the patient's CMN.
Figure 5.
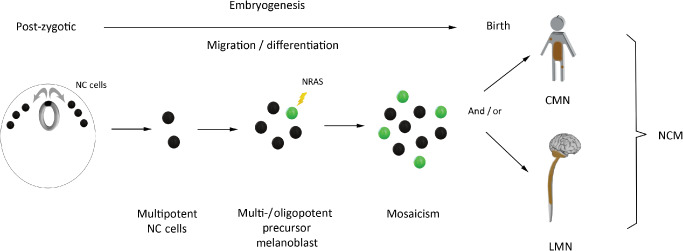
Acquisition of somatic NRAS mutations early during embryogenesis underlies the pathogenesis of NCM. Neural crest cells are a multipotent population of cells that arise early during embryogenesis; they give rise to diverse cell lineages including melanocytes (depicted at the left: neural crest cells are represented by black dots in cross‐section of human embryo). Early acquisition of a post‐zygotic, somatic NRAS mutation is thought to underlie the pathogenesis of NCM: one of the neural crest cell‐derived precursor cells acquires an NRAS mutation and gives rise to a mosaic pattern of NRAS‐mutated cells that further migrate and colonize the skin and/or leptomeninges. The timing of acquisition of the mutation in the precursor cell is then a co‐determinant for the resulting phenotype. NC = neural crest cells; NCM = neurocutaneous melanosis; CMN = congenital melanocytic nevus; LMN = leptomeningeal melanocytic neoplasm.
There is some evidence suggesting that post‐zygotic NRAS mutations in NCM (and CMN) might occur before specification of neural crest cells to the melanocytic lineage, and thus possibly leading to neuroectodermal‐derived tumors other than melanocytic tumors in these patients. Kinsler et al for instance detected a somatic NRASQ61R mutation in a non‐melanocytic CNS tumor, that is, a spinal neurocristic hamartoma, occuring in an 8‐year‐old child with NCM and leptomeningeal melanocytosis 70. In addition, they detected a somatic NRASQ61R mutation in a meningeoma and choroid plexus papilloma in two patients with large CMN. Very recently, Shih et al demonstrated an NRASG13R mutation in a CNS mesenchymal (but non‐melanocytic) tumor occurring in a young child with NCM 121. These NRAS mutations were also present in the CMN of these patients suggesting that they occurred very early during embryogenesis in multi‐ or oligopotent precursor cells. Of note, the NRASG13R mutation in the NCM patient in the study of Shih et al is the first report of an NRAS mutation other than NRASQ61 in NCM, although it is uncertain whether this NRASG13R mutation was truly a post‐zygotic mutation as it was also present in normal tissue albeit in lower allelic frequency. This patient in addition had a germline, single‐nucleotide polymorphism in the MET gene that encodes the receptor for hepatocyte growth factor/scatter factor (HGF/SF). In a transgenic mouse model, inappropriate expression of HGF/SF has been shown to result in a phenotype similar to NCM 128.
We recently presented an NRAS‐driven mouse model of NCM and primary leptomeningeal melanoma using the Cre‐LoxP technology 103. In this model, the embryonic expression of oncogenic NRASG12D in melanocytes of developing mice embryos induced congenital melanocytic lesions of the skin, congenital melanocytosis of the leptomeninges and early onset primary leptomeningeal melanoma. However, NRAS mutations are in itself insufficient for melanoma development, as is illustrated by their occurrence in benign CMN 11. Identifying the events that synergize with oncogenic NRAS is needed in order to better understand the pathogenesis of NRAS‐mutated leptomeningeal melanoma. A possible contributing event could be deletion of CDKN2A (encoding p16INK4A), as deletion of CDKN2A has been shown to cooperate with NRASQ61K to induce melanoma in mouse models earlier 1, 26 and was detected in a primary NRAS‐mutated melanoma arising in the cerebellum of a 10‐year‐old child 70.
Other molecular findings
A very recent study of Saldago et al reported a BRAFV600E mutation in the CMN of two NCM patients (2/16, 12.5%) 117. Although the LMNs of these two patients were not investigated, these results suggest that BRAF mutations might play a role in some NCM patients. Also, a minority of patients with large or giant CMN outside the context of NCM harbors BRAFV600E instead of NRASQ61 mutations 33. Additional studies including testing of CNS lesions will have to confirm a role for BRAF mutations in some NCM patients, as this could have consequences for targeted therapy.
Currently, knowledge on CNVs in LMNs in children is largely lacking. In the single study that has been published on this subject, Kinsler et al reported absence of large gains or losses in one case of NRAS‐mutated melanocytosis in an 8‐year‐old boy with NCM (using array CGH) 70. In contrast, they detected multiple gains and losses in a primary NRAS‐mutated melanoma in the cerebellum of a 10‐year‐old child with NCM that developed liver metastases. The aberrations in this melanoma included, among others, loss of whole chromosome 3, gain of chromosome 8 and loss of 9p24.3–p21.1, the latter including the CDKN2A locus 70. However, given the small number of cases studied, the diagnostic and prognostic significance of these CNVs is unclear.
NRAS mutations: therapeutic potential
In contrast to mutant BRAF, small molecules that selectively inhibit mutant NRAS are presently not available 106. One of the strategies for NRAS‐mutated neoplasms is the use of inhibitors that target downstream‐activated cascades such as the MAPK pathway and the PI3K/AKT/mTOR pathway (Figure 3) 66. Melanoma cell lines harboring NRAS mutations were shown to be sensitive to inhibition of MEK 40. A recent phase 2 trial with MEK162, a potent MEK inhibitor, has revealed efficacy in some patients with NRAS mutant melanoma, including two patients with NRAS‐mutated brain melanoma metastases 9. As NRAS activates multiple downstream pathways, there is evidence that combination of inhibitors of proteins downstream of NRAS such as MEK, ERK and PI3K/mTOR effectors will be more effective, and clinical trials with such combinatorial approaches are currently underway 66, 109, 111.
We previously demonstrated that melanoma cells derived from primary NRASG12D‐mutated brain melanomas of mice are sensitive to MEK inhibitors in vitro, and that the MEK inhibitor PD184352 delayed tumor growth in in vivo allograft experiments using NRASG12D‐mutant cells from a primary mouse brain melanoma 103. The YP‐MEL cell line is one of the few human NCM cell lines available for study. This cell line harbors an NRASQ61K mutation and was recently shown to be sensitive to several MEK inhibitors and inhibitors of mTOR and PI3K in vitro 98, 116. Whether MEK inhibitors can effectively target NRAS‐mutated LMNs is to be further investigated, but some effect on pERK protein level and MIB‐1 LI was demonstrated in a patient with NRAS‐mutated leptomeningeal melanocytosis experimentally treated with MEK162 78.
Conclusions
Primary LMNs represent a spectrum of rare neoplasms occurring in adults as well as in children. Distinct clinicopathologic and genetic features characterize these tumors. Oncogenic mutations in hotspots of the GNAQ and GNA11 genes are frequent in circumscribed primary LMNs in adults, while in children, oncogenic mutations in NRAS are frequent both in circumscribed and diffused LMNs. The presence of mutations in GNAQ and GNA11 in LMNs suggests a pathogenetic mechanism similar to uveal melanoma and blue nevi and supports the idea of distinct subtypes of melanocytes that are preferentially targeted by distinct oncogenic mutations. In the congenital setting, acquisition of somatic mutations in NRAS early during embryonic development underlies the pathogenesis of NCM. Of note, the distinction between adult and pediatric setting is somewhat arbitrary, as adult patients may have a congenital background of their disease, but were only diagnosed later in life while not all LMNs that occur in children are necessarily truly congenital in origin.
The frequent presence of GNAQ/GNA11 mutations in primary LMNs is helpful in the differential diagnosis with metastasis of especially cutaneous melanoma to the CNS. Adequate diagnosis of primary vs. metastatic melanoma in/around the CNS has prognostic relevance, the patients with melanoma metastatic to the CNS having a grimmer prognosis. As for other CNS tumors, smart integration of histologic and molecular information can be expected to result in a more robust and clinically relevant classification of primary LMNs 83. Also, further elucidation of the molecular aberrations underlying primary LMNs may well provide novel therapeutic options for these patients.
We have no disclosures or conflicts of interest to declare.
References
- 1. Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F (2005) Metastasizing melanoma formation caused by expression of activated N‐RasQ61K on an INK4a‐deficient background. Cancer Res 65:4005–4011. [DOI] [PubMed] [Google Scholar]
- 2. Alla P, Carsuzaa F, Fesselet J, Muyard B, Carloz E, Piersecki MD et al (1998) Blue nevus of the scalp associated with a meningeal melanocytoma. Ann Dermatol Venereol 125:129–131. [PubMed] [Google Scholar]
- 3. Allcutt D, Michowiz S, Weitzman S, Becker L, Blaser S, Hoffman HJ et al (1993) Primary leptomeningeal melanoma: an unusually aggressive tumor in childhood. Neurosurgery 32:721–729. [DOI] [PubMed] [Google Scholar]
- 4. Ambrosini G, Musi E, Ho AL, de Stanchina E, Schwartz GK (2013) Inhibition of mutant GNAQ signaling in uveal melanoma induces AMPK‐dependent autophagic cell death. Mol Cancer Ther 12:768–776. [DOI] [PubMed] [Google Scholar]
- 5. Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, Schwartz GK (2012) Identification of unique MEK‐dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res 18:3552–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antonescu CR, Scheithauer BW, Woodruff JM (2013) Tumors of the peripheral nervous system. In: AFIP Atlas of Tumor Pathology. American Registry of Pathology Press (ed.), American Registry of Pathology: Silver Spring, Maryland. [Google Scholar]
- 7. Aoki H, Yamada Y, Hara A, Kunisada T (2009) Two distinct types of mouse melanocyte: differential signaling requirement for the maintenance of non‐cutaneous and dermal versus epidermal melanocytes. Development 136:2511–2521. [DOI] [PubMed] [Google Scholar]
- 8. Arias M, Alberte‐Woodward M, Arias S, Dapena D, Prieto A, Suarez‐Penaranda JM (2011) Primary malignant meningeal melanomatosis: a clinical, radiological and pathologic case study. Acta Neurol Belg 111:228–231. [PubMed] [Google Scholar]
- 9. Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P et al (2013) MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non‐randomised, open‐label phase 2 study. Lancet Oncol 14:249–256. [DOI] [PubMed] [Google Scholar]
- 10. Balmaceda CM, Fetell MR, O'Brien JL, Housepian EH (1993) Nevus of Ota and leptomeningeal melanocytic lesions. Neurology 43:381–386. [DOI] [PubMed] [Google Scholar]
- 11. Bauer J, Curtin JA, Pinkel D, Bastian BC (2007) Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol 127:179–182. [DOI] [PubMed] [Google Scholar]
- 12. Bauer J, Kilic E, Vaarwater J, Bastian BC, Garbe C, de Klein A (2009) Oncogenic GNAQ mutations are not correlated with disease‐free survival in uveal melanoma. Br J Cancer 101:813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beadling C, Jacobson‐Dunlop E, Hodi FS, Le C, Warrick A, Patterson J et al (2008) KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res 14:6821–6828. [DOI] [PubMed] [Google Scholar]
- 14. Bergdahl L, Boquist L, Liliequist B, Thulin CA, Tovi D (1972) Primary malignant melanoma of the central nervous system. A report of 10 cases. Acta Neurochir 26:139–149. [DOI] [PubMed] [Google Scholar]
- 15. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L et al (2009) Mutations in regulatory subunit type 1A of cyclic adenosine 5′‐monophosphate‐dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brat DJ, Giannini C, Scheithauer BW, Burger PC (1999) Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol 23:745–754. [DOI] [PubMed] [Google Scholar]
- 17. Brat DJ, Perry A (2007) Melanocytic lesions. Chapter 10. In: WHO Classification of Tumours of the Central Nervous System. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), pp. 181–183. International Agency for Research on Cancer: Lyon. [Google Scholar]
- 18. Brunsvig KL, Zenobi M, Rilliet B, El Hassani Y, de Haller R, Ansari M et al (2011) Primary leptomeningeal melanocytosis in a 10‐year‐old girl: a challenging diagnosis with a poor prognosis. J Child Neurol 26:1444–1448. [DOI] [PubMed] [Google Scholar]
- 19. Bullard DE, Cox EB, Seigler HF (1981) Central nervous system metastases in malignant melanoma. Neurosurgery 8:26–30. [DOI] [PubMed] [Google Scholar]
- 20. Bydon A, Gutierrez JA, Mahmood A (2003) Meningeal melanocytoma: an aggressive course for a benign tumor. J Neurooncol 64:259–263. [DOI] [PubMed] [Google Scholar]
- 21. Carney JA (1990) Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol 14:206–222. [PubMed] [Google Scholar]
- 22. Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J et al (2011) KIT as a therapeutic target in metastatic melanoma. JAMA 305:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR et al (2014) Effect of selumetinib vs chemotherapy on progression‐free survival in uveal melanoma: a randomized clinical trial. JAMA 311:2397–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Wu Q, Tan L, Porter D, Jager MJ, Emery C, Bastian BC (2013) Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene 33:4724–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon‐Cardo C et al (1997) Cooperative effects of INK4a and ras in melanoma susceptibility in vivo . Genes Dev 11:2822–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohn‐Cedermark G, Mansson‐Brahme E, Rutqvist LE, Larsson O, Johansson H, Ringborg U (1998) Central nervous system metastases of cutaneous malignant melanoma—a population‐based study. Acta Oncol 37:463–470. [DOI] [PubMed] [Google Scholar]
- 28. Cornejo KM, Hutchinson L, Cosar EF, Smith T, Tomaszewicz K, Dresser K, Deng A (2013) Is it a primary or metastatic melanocytic neoplasm of the central nervous system?: a molecular based approach. Pathol Int 63:559–564. [DOI] [PubMed] [Google Scholar]
- 29. Cruz F 3rd, Rubin BP, Wilson D, Town A, Schroeder A, Haley A et al (2003) Absence of BRAF and NRAS mutations in uveal melanoma. Cancer Res 63:5761–5766. [PubMed] [Google Scholar]
- 30. Curtin JA, Busam K, Pinkel D, Bastian BC (2006) Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 24:4340–4346. [DOI] [PubMed] [Google Scholar]
- 31. Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H et al (2005) Distinct sets of genetic alterations in melanoma. N Engl J Med 353:2135–2147. [DOI] [PubMed] [Google Scholar]
- 32. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954. [DOI] [PubMed] [Google Scholar]
- 33. Dessars B, De Raeve LE, Morandini R, Lefort A, El Housni H, Ghanem GE et al (2009) Genotypic and gene expression studies in congenital melanocytic nevi: insight into initial steps of melanotumorigenesis. J Invest Dermatol 129:139–147. [DOI] [PubMed] [Google Scholar]
- 34. Domingues MJ, Larue L, Bonaventure J (2013) Migration of melanocytic lineage‐derived cells. Med Sci 29:287–292. [DOI] [PubMed] [Google Scholar]
- 35. Dono M, Angelini G, Cecconi M, Amaro A, Esposito AI, Mirisola V et al (2014) Mutation frequencies of GNAQ, GNA11, BAP1, SF3B1, EIF1AX and TERT in uveal melanoma: detection of an activating mutation in the TERT gene promoter in a single case of uveal melanoma. Br J Cancer 110:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dubbink HJ, Deans ZC, Tops BB, van Kemenade FJ, Koljenovic S, van Krieken HJ et al (2014) Next generation diagnostic molecular pathology: critical appraisal of quality assurance in Europe. Mol Oncol 8:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edlundh‐Rose E, Egyhazi S, Omholt K, Mansson‐Brahme E, Platz A, Hansson J, Lundeberg J (2006) NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 16:471–478. [DOI] [PubMed] [Google Scholar]
- 38. van Engen‐van Grunsven IA, Kusters‐Vandevelde HVN, Groenen PJTA, Blokx WAM (2014) Update on molecular pathology of cutaneous melanocytic lesions: what is new in diagnosis and molecular testing for treatment? Front Med 1:39. doi: 10.3389/fmed.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eun SS, Kim HS, Lee SH, Liu WC, Lee JH (2011) Spinal meningeal melanocytoma in the S‐1 nerve root sheath with paraspinal extension mimicking schwannoma. World Neurosurg 75:303–306. [DOI] [PubMed] [Google Scholar]
- 40. von Euw E, Atefi M, Attar N, Chu C, Zachariah S, Burgess BL et al (2012) Antitumor effects of the investigational selective MEK inhibitor TAK733 against cutaneous and uveal melanoma cell lines. Mol Cancer 11:22. doi: 10.1186/1476-4598-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fecher LA, Amaravadi RK, Flaherty KT (2008) The MAPK pathway in melanoma. Curr Opin Oncol 20:183–189. [DOI] [PubMed] [Google Scholar]
- 42. Feng X, Degese MS, Iglesias‐Bartolome R, Vaque JP, Molinolo AA, Rodrigues M et al (2014) Hippo‐independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio‐regulated rho GTPase signaling circuitry. Cancer Cell 25:831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fernandez‐Medarde A, Santos E (2011) Ras in cancer and developmental diseases. Genes Cancer 2:344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fuld AD, Speck ME, Harris BT, Simmons NE, Corless CL, Tsongalis GJ et al (2011) Primary melanoma of the spinal cord: a case report, molecular footprint, and review of the literature. J Clin Oncol 29:499–502. [DOI] [PubMed] [Google Scholar]
- 45. Gessi M, Hammes J, Lauriola L, Dorner E, Kirfel J, Kristiansen G et al (2012) GNA11 and N‐RAS mutations: alternatives for MAPK pathway activating GNAQ mutations in primary melanocytic tumours of the central nervous system. Neuropathol Appl Neurobiol 39:417–425. [DOI] [PubMed] [Google Scholar]
- 46. Gessi M, van de Nes J, Griewank K, Barresi V, Buckland ME, Kirfel J et al (2014) Absence of TERT promoter mutations in primary melanocytic tumours of the central nervous system. Neuropathol Appl Neurobiol 40:794–797. [DOI] [PubMed] [Google Scholar]
- 47. Goldgeier MH, Klein LE, Klein‐Angerer S, Moellmann G, Nordlund JJ (1984) The distribution of melanocytes in the leptomeninges of the human brain. J Invest Dermatol 82:235–238. [DOI] [PubMed] [Google Scholar]
- 48. Greaves WO, Verma S, Patel KP, Davies MA, Barkoh BA, Galbincea JM et al (2013) Frequency and spectrum of BRAF mutations in a retrospective, single‐institution study of 1112 cases of melanoma. J Mol Diagn 15:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greco Crasto S, Soffietti R, Bradac GB, Boldorini R (2001) Primitive cerebral melanoma: case report and review of the literature. Surg Neurol 55:163–168. [DOI] [PubMed] [Google Scholar]
- 50. Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y et al (2011) Phase II, open‐label, single‐arm trial of imatinib mesylate in patients with metastatic melanoma harboring c‐Kit mutation or amplification. J Clin Oncol 29:2904–2909. [DOI] [PubMed] [Google Scholar]
- 51. Hafner C, Groesser L (2013) Mosaic RASopathies. Cell Cycle 12:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA et al (2010) Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330:1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hawes BE, van Biesen T, Koch WJ, Luttrell LM, Lefkowitz RJ (1995) Distinct pathways of Gi‐ and Gq‐mediated mitogen‐activated protein kinase activation. J Biol Chem 270:17148–17153. [DOI] [PubMed] [Google Scholar]
- 54. Helseth A, Helseth E, Unsgaard G (1989) Primary meningeal melanoma. Acta Oncol 28:103–104. [DOI] [PubMed] [Google Scholar]
- 55. Ho AL, Musi E, Ambrosini G, Nair JS, Deraje Vasudeva S, de Stanchina E, Schwartz GK (2012) Impact of combined mTOR and MEK inhibition in uveal melanoma is driven by tumor genotype. PLoS ONE 7:e40439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hodi FS, Corless CL, Giobbie‐Hurder A, Fletcher JA, Zhu M, Marino‐Enriquez A et al (2013) Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun‐damaged skin. J Clin Oncol 31:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Holbrook KA, Underwood RA, Vogel AM, Gown AM, Kimball H (1989) The appearance, density and distribution of melanocytes in human embryonic and fetal skin revealed by the anti‐melanoma monoclonal antibody, HMB‐45. Anat Embryol (Berl) 180:443–455. [DOI] [PubMed] [Google Scholar]
- 58. Horvath A, Bertherat J, Groussin L, Guillaud‐Bataille M, Tsang K, Cazabat L et al (2010) Mutations and polymorphisms in the gene encoding regulatory subunit type 1‐alpha of protein kinase A (PRKAR1A): an update. Hum Mutat 31:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA (2013) Highly recurrent TERT promoter mutations in human melanoma. Science 339:957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang HY, Park N, Erlandson RA, Antonescu CR (2004) Immunohistochemical and ultrastructural comparative study of external lamina structure in 31 cases of cellular, classical, and melanotic schwannomas. Appl Immunohistochem Mol Morphol 12:50–58. [DOI] [PubMed] [Google Scholar]
- 61. Hubbard KB, Hepler JR (2006) Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal 18:135–150. [DOI] [PubMed] [Google Scholar]
- 62. Hurowitz EH, Melnyk JM, Chen YJ, Kouros‐Mehr H, Simon MI, Shizuya H (2000) Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res 7:111–120. [DOI] [PubMed] [Google Scholar]
- 63. Hwang HW, Pavan WJ (2010) Melanocytes don't always take the high road. Pigment Cell Melanoma Res 23:11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jiveskog S, Ragnarsson‐Olding B, Platz A, Ringborg U (1998) N‐ras mutations are common in melanomas from sun‐exposed skin of humans but rare in mucosal membranes or unexposed skin. J Invest Dermatol 111:757–761. [DOI] [PubMed] [Google Scholar]
- 65. Kadonaga JN, Frieden IJ (1991) Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol 24:747–755. [DOI] [PubMed] [Google Scholar]
- 66. Kelleher FC, McArthur GA (2012) Targeting NRAS in melanoma. Cancer J 18:132–136. [DOI] [PubMed] [Google Scholar]
- 67. Khalili JS, Yu X, Wang J, Hayes BC, Davies MA, Lizee G et al (2012) Combination small molecule MEK and PI3K inhibition enhances uveal melanoma cell death in a mutant GNAQ‐ and GNA11‐dependent manner. Clin Cancer Res 18:4345–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim CY, Kim DW, Kim K, Curry J, Torres‐Cabala C, Patel S (2014) GNAQ mutation in a patient with metastatic mucosal melanoma. BMC Cancer 14:516. doi: 10.1186/1471-2407-14-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kinsler VA, Paine SM, Anderson GW, Wijesekara DS, Sebire NJ, Chong WK et al (2012) Neuropathology of neurocutaneous melanosis: histological foci of melanotic neurones and glia may be undetectable on MRI. Acta Neuropathol 123:453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kinsler VA, Thomas AC, Ishida M, Bulstrode NW, Loughlin S, Hing S et al (2013) Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. J Invest Dermatol 133:2229–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koelsche C, Hovestadt V, Jones D, Capper D, Sturm D, Sahm F et al (2014) Melanotic tumors of the nervous system are characterized by distinct mutational, chromosomal and epigenomic profiles. Brain Pathol doi: 10.1111/bpa.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koenigsmann M, Jautzke G, Unger M, Theallier‐Janko A, Wiegel T, Stoltenburg‐Didinger G (2002) June 2002: 57‐year‐old male with leptomeningeal and liver tumors. Brain Pathol 12:519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kong Y, Krauthammer M, Halaban R (2014) Rare SF3B1 R625 mutations in cutaneous melanoma. Melanoma Res 24:332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Koopmans AE, Vaarwater J, Paridaens D, Naus NC, Kilic E, de Klein A (2013) Patient survival in uveal melanoma is not affected by oncogenic mutations in GNAQ and GNA11. Br J Cancer 109:493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kusters‐Vandevelde HV, van Engen‐van Grunsven IA, Coupland SE, Lake S, Rijntjes J, Pfundt R et al (2014) Mutations in G protein encoding genes and chromosomal alterations in primary leptomeningeal melanocytic neoplasms. Pathol Oncol Res doi: 10.1007/s12253-014-9841-3. [DOI] [PubMed] [Google Scholar]
- 76. Kusters‐Vandevelde HV, van Engen‐van Grunsven IA, Kusters B, van Dijk MR, Groenen PJ, Wesseling P, Blokx WA (2010) Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol 120:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kusters‐Vandevelde HV, Klaasen A, Kusters B, Groenen PJ, van Engen‐van Grunsven IA, van Dijk MR et al (2009) Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol 119:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kusters‐Vandevelde HV, Willemsen AE, Groenen PJ, Kusters B, Lammens M, Wesseling P et al (2014) Experimental treatment of NRAS‐mutated neurocutaneous melanocytosis with MEK162, a MEK‐inhibitor. Acta Neuropathol Commun 2:41. doi: 10.1186/2051-5960-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Langer R, Becker K, Feith M, Friess H, Hofler H, Keller G (2011) Genetic aberrations in primary esophageal melanomas: molecular analysis of c‐KIT, PDGFR, KRAS, NRAS and BRAF in a series of 10 cases. Mod Pathol 24:495–501. [DOI] [PubMed] [Google Scholar]
- 80. Le Douarin NMKC (1999) The Neural Crest. Cambridge University Press: Cambridge. [Google Scholar]
- 81. Lee JH, Choi JW, Kim YS (2011) Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta‐analysis. Br J Dermatol 164:776–784. [DOI] [PubMed] [Google Scholar]
- 82. Leopold JG, Richards DB (1968) The interrelationship of blue and common naevi. J Pathol Bacteriol 95:37–46. [DOI] [PubMed] [Google Scholar]
- 83. Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A et al (2014) International Society of Neuropathology––Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol 24:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Makin GW, Eden OB, Lashford LS, Moppett J, Gerrard MP, Davies HA et al (1999) Leptomeningeal melanoma in childhood. Cancer 86:878–886. [PubMed] [Google Scholar]
- 85. Malaponte G, Libra M, Gangemi P, Bevelacqua V, Mangano K, D'Amico F et al (2006) Detection of BRAF gene mutation in primary choroidal melanoma tissue. Cancer Biol Ther 5:225–227. [DOI] [PubMed] [Google Scholar]
- 86. Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T et al (2003) Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst 95:1878–1890. [DOI] [PubMed] [Google Scholar]
- 87. Markby DW, Onrust R, Bourne HR (1993) Separate GTP binding and GTPase activating domains of a G alpha subunit. Science 262:1895–1901. [DOI] [PubMed] [Google Scholar]
- 88. Martin M, Masshofer L, Temming P, Rahmann S, Metz C, Bornfeld N et al (2013) Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet 45:933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Michael BD, Syndikus I, Clark A, Baborie A (2010) Diffuse primary leptomeningeal melanocytosis in a patient receiving a novel cancer cell vaccine for prostate cancer. BMJ Case Rep doi: 10.1136/bcr.11.2009.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Miro J, Velasco R, Majos C, Gil M, Boluda S, Bruna J (2011) Meningeal melanocytosis: a possibly useful treatment for a rare primary brain neoplasm. J Neurol 258:1169–1171. [DOI] [PubMed] [Google Scholar]
- 91. Mitsiades N, Chew SA, He B, Riechardt AI, Karadedou T, Kotoula V, Poulaki V (2011) Genotype‐dependent sensitivity of uveal melanoma cell lines to inhibition of B‐Raf, MEK, and Akt kinases: rationale for personalized therapy. Invest Ophthalmol Vis Sci 52:7248–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mooi WJKT (2007a) Cutaneous nevi and other pigmented skin lesions. In: Pathology of Melanocytic Disorders. Shaw P (ed.), p. 52. Hodder Arnold: London. [Google Scholar]
- 93. Mooi WJKT (2007b) Melanin, melanocytes, skin. In: Pathology of Melanocytic Disorders. Shaw P (ed.), p. 4. Hodder Arnold: London. [Google Scholar]
- 94. Mooi WJKT (2007c) Melanin, melanocytes, skin. In: Pathology of Melanocytic Disorders. Shaw P (ed.), p. 6. Hodder Arnold: London. [Google Scholar]
- 95. Munoz‐Hidalgo L, Lopez‐Gines C, Navarro L, Callaghan RC, San Miguel T, Gil‐Benso R et al (2014) BRAF V600E mutation in two distinct meningeal melanocytomas associated with a nevus of Ota. J Clin Oncol 32:72–75. [DOI] [PubMed] [Google Scholar]
- 96. Murali R, Wiesner T, Rosenblum MK, Bastian BC (2012) GNAQ and GNA11 mutations in melanocytomas of the central nervous system. Acta Neuropathol 123:457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Muthappan M, Muthu T, Hussain Z, Lamont D, Balakrishnan V (2012) Cervical intramedullary melanocytoma: a case report and review of literature. J Clin Neurosci 19:1450–1453. [DOI] [PubMed] [Google Scholar]
- 98. Nagashima Y, Miyagi Y, Aoki I, Funabiki T, Ikuta K, Umeda M et al (1994) Establishment and characterization of a malignant melanoma cell line (YP‐MEL) derived from a patient with neurocutaneous melanosis. Pathol Res Pract 190:178–185. [DOI] [PubMed] [Google Scholar]
- 99. Neer EJ (1995) Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80:249–257. [DOI] [PubMed] [Google Scholar]
- 100. Nikola Z, Dragan M, Mijovic Z, Milentijevic MJ (2012) Primary leptomeningeal melanocytosis––a case report with an autopsy diagnosis. Vojnosanit Pregl 69:631–634. [PubMed] [Google Scholar]
- 101. Niu HT, Zhou QM, Wang F, Shao Q, Guan YX, Wen XZ et al (2013) Identification of anaplastic lymphoma kinase break points and oncogenic mutation profiles in acral/mucosal melanomas. Pigment Cell Melanoma Res 26:646–653. [DOI] [PubMed] [Google Scholar]
- 102. Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, Harbour JW (2008) Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci 49:5230–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pedersen M, Kusters‐Vandevelde HV, Viros A, Groenen PJ, Sanchez‐Laorden B, Gilhuis JH et al (2013) Primary melanoma of the CNS in children is driven by congenital expression of oncogenic NRAS in melanocytes. Cancer Discov 3:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Phadke PA, Rakheja D, Le LP, Selim MA, Kapur P, Davis A et al (2011) Proliferative nodules arising within congenital melanocytic nevi: a histologic, immunohistochemical, and molecular analyses of 43 cases. Am J Surg Pathol 35:656–669. [DOI] [PubMed] [Google Scholar]
- 105. Pirini MG, Mascalchi M, Salvi F, Tassinari CA, Zanella L, Bacchini P et al (2003) Primary diffuse meningeal melanomatosis: radiologic–pathologic correlation. AJNR Am J Neuroradiol 24:115–118. [PMC free article] [PubMed] [Google Scholar]
- 106. Posch C, Ortiz‐Urda S (2013) NRAS mutant melanoma—undrugable? Oncotarget 4:494–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Puntervoll HE, Molven A, Akslen LA (2014) Frequencies of KIT and GNAQ mutations in acral melanoma. J Cutan Pathol 41:893–894. [DOI] [PubMed] [Google Scholar]
- 108. Rades D, Heidenreich F, Tatagiba M, Brandis A, Karstens JH (2001) Therapeutic options for meningeal melanocytoma. Case report. J Neurosurg Spine 95:225–231. [DOI] [PubMed] [Google Scholar]
- 109. Rebecca VW, Alicea GM, Paraiso KH, Lawrence H, Gibney GT, Smalley KS (2014) Vertical inhibition of the MAPK pathway enhances therapeutic responses in NRAS‐mutant melanoma. Pigment Cell Melanoma Res 27:1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rivers JK, Bhayana S, Martinka M (2001) Dural melanoma associated with ocular melanosis and multiple blue nevi. J Cutan Med Surg 5:381–385. [DOI] [PubMed] [Google Scholar]
- 111. Roberts PJ, Usary JE, Darr DB, Dillon PM, Pfefferle AD, Whittle MC et al (2012) Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res 18:5290–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rodriguez FJ, Stratakis CA, Evans DG (2012) Genetic predisposition to peripheral nerve neoplasia: diagnostic criteria and pathogenesis of neurofibromatoses, Carney complex, and related syndromes. Acta Neuropathol 123:349–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rodriguez y Baena R, Gaetani P, Danova M, Bosi F, Zappoli F (1992) Primary solitary intracranial melanoma: case report and review of the literature. Surg Neurol 38:26–37. [DOI] [PubMed] [Google Scholar]
- 114. Ross EM, Wilkie TM (2000) GTPase‐activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS‐like proteins. Annu Rev Biochem 69:795–827. [DOI] [PubMed] [Google Scholar]
- 115. Rothenbuhler A, Stratakis CA (2010) Clinical and molecular genetics of Carney complex. Best Pract Res Clin Endocrinol Metab 24:389–399. [DOI] [PubMed] [Google Scholar]
- 116. Ruan Y, Kovalchuk A, Jayanthan A, Lun X, Nagashima Y, Kovalchuk O et al (2014) Druggable targets in pediatric neurocutaneous melanocytosis: molecular and drug sensitivity studies in xenograft and ex vivo tumor cell culture to identify agents for therapy. Neuro‐Oncol doi: 10.1093/neuonc/nou310. [Epub 12 Nov 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Salgado CM, Basu D, Nikiforova M, Bauer BS, Johnson D, Rundell V et al (2014) BRAF mutations are also associated with neurocutaneous melanocytosis and large/giant congenital melanocytic nevi. Pediatr Dev Pathol [Epub 9 Dec 2014]. [DOI] [PubMed] [Google Scholar]
- 118. Salpea P, Stratakis CA (2014) Carney complex and McCune Albright syndrome: an overview of clinical manifestations and human molecular genetics. Mol Cell Endocrinol 386:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Scheithauer BW, Louis DN, Hunter S, Woodruff JM, Antonescu CR (2007) Schwannoma. Chapter 9. In: WHO Classification of Tumours of the Central Nervous System. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), pp. 152–155. International Agency for Research on Cancer: Lyon. [Google Scholar]
- 120. Seidl M, Zolnhofer G, Gunser S, Ennker J, Schafer W, Tietze L (2013) Psammomatous melanotic schwannoma as indicator of a Carney complex. Pathologe 34:343–346. [DOI] [PubMed] [Google Scholar]
- 121. Shih F, Yip S, McDonald PJ, Chudley AE, Del Bigio MR (2014) Oncogenic codon 13 NRAS mutation in a primary mesenchymal brain neoplasm and nevus of a child with neurocutaneous melanosis. Acta Neuropathol Commun 2:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shoushtari AN, Carvajal RD (2014) GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res 24:525–534. [DOI] [PubMed] [Google Scholar]
- 123. Skarli SO, Wolf AL, Kristt DA, Numaguchi Y (1994) Melanoma arising in a cervical spinal nerve root: report of a case with a benign course and malignant features. Neurosurgery 34:533–537. [DOI] [PubMed] [Google Scholar]
- 124. Smith AB, Rushing EJ, Smirniotopoulos JG (2009) Pigmented lesions of the central nervous system: radiologic–pathologic correlation. Radiographics 29:1503–1524. [DOI] [PubMed] [Google Scholar]
- 125. Soparker CN, O'Brien JM, Albert DM (1993) Investigation of the role of the ras protooncogene point mutation in human uveal melanomas. Invest Ophthalmol Vis Sci 34:2203–2209. [PubMed] [Google Scholar]
- 126. Strom RG, Shvartsbeyn M, Rosenblum MK, Hameed MR, Nafa K, Mikolaenko I, Babu RP (2012) Melanocytic tumor with GNA11 p.Q209L mutation mimicking a foramen magnum meningioma. Clin Neurol Neurosurg 114:1197–1200. [DOI] [PubMed] [Google Scholar]
- 127. Subbiah V, Wolff JE (2010) Rapid response to therapy of neurocutaneous melanosis with leptomeningeal melanoma. Pediatr Blood Cancer 54:180–181. [DOI] [PubMed] [Google Scholar]
- 128. Takayama H, La Rochelle WJ, Anver M, Bockman DE, Merlino G (1996) Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci U S A 93:5866–5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tolleson WH (2005) Human melanocyte biology, toxicology, and pathology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 23:105–161. [DOI] [PubMed] [Google Scholar]
- 130. Torres‐Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL (2014) Malignant melanotic schwannian tumor: a clinicopathologic, immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of “melanotic schwannoma”. Am J Surg Pathol 38:94–105. [DOI] [PubMed] [Google Scholar]
- 131. Tripathy K, Misra A, Misra D, Pujari S, Nayak M, Rath J (2011) Melanotic choroid plexus carcinoma of the posterior fossa. J Clin Neurol 7:105–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Uozumi Y, Kawano T, Kawaguchi T, Kaneko Y, Ooasa T, Ogasawara S et al (2003) Malignant transformation of meningeal melanocytoma: a case report. Brain Tumor Pathol 20:21–25. [DOI] [PubMed] [Google Scholar]
- 133. Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM et al (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T et al (2010) Mutations in GNA11 in uveal melanoma. N Engl J Med 363:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Virchow R (1859) Pigment und diffuse Melanose der Arachnoides. Virchows Arch 16:180–182. [Google Scholar]
- 136. de Vries E, Coebergh JW (2005) Melanoma incidence has risen in Europe. BMJ 331:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wallander ML, Layfield LJ, Emerson LL, Mamalis N, Davis D, Tripp SR, Holden JA (2011) KIT mutations in ocular melanoma: frequency and anatomic distribution. Mod Pathol 24:1031–1035. [DOI] [PubMed] [Google Scholar]
- 138. Wang F, Li X, Chen L, Pu X (2007) Malignant transformation of spinal meningeal melanocytoma. Case report and review of the literature. J Neurosurg Spine 6:451–454. [DOI] [PubMed] [Google Scholar]
- 139. Wang H, Zhang S, Wu C, Zhang Z, Qin T (2013) Melanocytomas of the central nervous system: a clinicopathological and molecular study. Eur J Clin Invest 43:809–815. [DOI] [PubMed] [Google Scholar]
- 140. Weber A, Hengge UR, Urbanik D, Markwart A, Mirmohammadsaegh A, Reichel MB et al (2003) Absence of mutations of the BRAF gene and constitutive activation of extracellular‐regulated kinase in malignant melanomas of the uvea. Lab Invest 83:1771–1776. [DOI] [PubMed] [Google Scholar]
- 141. Whiteman DC, Pavan WJ, Bastian BC (2011) The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res 24:879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wilkie TM, Gilbert DJ, Olsen AS, Chen XN, Amatruda TT, Korenberg JR et al (1992) Evolution of the mammalian G protein alpha subunit multigene family. Nat Genet 1:85–91. [DOI] [PubMed] [Google Scholar]
- 143. Wright CJ, McCormack PL (2013) Trametinib: first global approval. Drugs 73:1245–1254. [DOI] [PubMed] [Google Scholar]
- 144. Wu X, Li J, Zhu M, Fletcher JA, Hodi FS (2012) Protein kinase C inhibitor AEB071 targets ocular melanoma harboring GNAQ mutations via effects on the PKC/Erk1/2 and PKC/NF‐kappaB pathways. Mol Cancer Ther 11:1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wu X, Zhu M, Fletcher JA, Giobbie‐Hurder A, Hodi FS (2012) The protein kinase C inhibitor enzastaurin exhibits antitumor activity against uveal melanoma. PLoS ONE 7:e29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z et al (2014) Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 25:822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH et al (2012) Regulation of the Hippo‐YAP pathway by G‐protein‐coupled receptor signaling. Cell 150:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zuidervaart W, van Nieuwpoort F, Stark M, Dijkman R, Packer L, Borgstein AM et al (2005) Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer 92:2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]


