Abstract
Studies of periventricular white matter injury (PWMI) in preterm infants suggest the involvement of the transient cortical subplate zone. We studied the cortical wall of non‐cystic and cystic PWMI cases and controls. Non‐cystic PWMI corresponded to diffuse white matter lesions, the predominant injury currently detected by imaging. Glial cell populations were analyzed in post‐mortem human frontal lobes from very preterm [24–29 postconceptional weeks (pcw)] and preterm infants (30–34 pcw) using immunohistochemistry for glial fibrillary acidic protein (GFAP), monocarboxylate transporter 1 (MCT1), ionized calcium‐binding adapter molecule 1 (Iba1), CD68 and oligodendrocyte lineage (Olig2). Glial activation extended into the subplate in non‐cystic PWMI but was restricted to the white matter in cystic PWMI. Two major age‐related and laminar differences were observed in non‐cystic PWMI: in very preterm cases, activated microglial cells were increased and extended into the subplate adjacent to the lesion, whereas in preterm cases, an astroglial reaction was seen not only in the subplate but throughout the cortical plate. There were no differences in Olig2‐positive pre‐oligodendrocytes in the subplate in PWMI cases compared with controls. The involvement of gliosis in the deep subplate supports the concept of the complex cellular vulnerability of the subplate zone during the preterm period and may explain widespread changes in magnetic resonance signal intensity in early PWMI.
Keywords: astrogliosis, development, glia, human, microglial activation, periventricular leukomalacia
Introduction
Despite critical advances in neonatology, the incidence of encephalopathy of prematurity has gradually increased over the past decade 44, 75. Infants born before 33 gestational weeks or 31 postconceptional weeks (pcw) 13 are at high risk of brain damage and subsequent cognitive, behavioral and/or motor deficits of varying intensity 3, 43, 47, 58, 74, 75. Over the last few decades, cystic periventricular white matter injury (PWMI) has been the major neuropathological correlate of focal white matter damage associated with cerebral palsy in premature infants. However, recent magnetic resonance imaging (MRI) and sonogram studies have shown that 95% of white matter abnormalities in preterm infants are more subtle, being diffuse and non‐cystic 8, 21, 43, 52, 75. In addition, volumetric MRI studies in infants with diffuse PWMI show a significant reduction in the grey matter, in particular in cerebral cortical volume, in premature infants studied at term‐equivalent age when compared with term infants 21, 22. These studies and others have introduced and emphasized the notion that neuronal injury/deficits underline the long‐term cognitive and/or behavioral impairments seen in these premature children 44, 51, 52. Pathological studies have revealed that this neuronal injury is manifested as a decrease in the density of pyramidal and GABAergic neurons in the different compartments of the cerebral wall 2, 20, 31, 60. In addition, various authors have suggested that the lesioning of the subplate (SP) zone, a transient layer located between the white matter and the cerebral cortex 39, could be the source of some cognitive deficits detected in the follow‐up of premature infants 35, 51, 75. This transient layer presents a huge expansion in primates especially in human and could play an important morphogenic role in human cortical organization 29, 35, 73. Interestingly, it displays a peak of thickness at 20–35 pcw when premature infants displayed the highest risk period for PWMI 39.
In a previous work, we studied the glial reaction at the axonal crossroads of the white matter in a large cohort of very preterm and preterm infants with PWMI 71. Within this cohort, most cases selected were recent non‐cystic cases (18/25) matching with discrete lesions corresponding to lesions described by imaging. In the present work, we have focused on the involvement of the SP and developing cerebral cortex in terms of glial reaction and neuronal injury in the same cohort of PWMI cases when compared with age‐matched controls. We used immunolabeling for different markers of astrocytes [glial fibrillary acidic protein (GFAP), monocarboxylate transporter 1 (MCT1)], microglia/macrophages [ionized calcium‐binding adapter molecule 1 (Iba1), CD68], vessel walls (CD34, MCT1), marker of oligodendrocyte lineage (Olig2) and neurofilaments (SMI31–SMI32) to analyze the glial reaction and neuronal injury in different layers of the cerebral wall, in particular the deep and superficial subplate (sSP) zones. We hypothesized that the astrocytic and microglial activation would vary between cystic and non‐cystic PWMI, and that layer‐specific responses related to age would be observed for these glial reactions. Indeed, differences in the density, size and morphology of glial cells were detected in the different layers of the cerebral wall of very preterm cases when compared with preterm cases with non‐cystic PWMI.
Material and methods
Based on the diagnosis of two experienced neuropathologists (CF‐B, HA‐B), 25 premature (24–34 pcw) brains with PWMI and 10 control brains were selected post‐mortem (Table 1). Most cases were obtained in France and half of the control cases in Croatia. All procedures were approved by the Institutional Review Board of the Paris North Hospitals, Paris 7 University, AP‐HP (N°09–062) and the Internal Review Board of the Ethics Committee, School of Medicine, University of Zagreb (N°108–1081870‐1876). The post‐mortem delay was not more than 48 h. The specimens were formalin fixed and embedded in paraffin. Ten micron‐thick sections were cut on a microtome and mounted on Superfrost slides (Fisher Scientific, Pittsburgh, PA, USA). All sections were processed for hemalum‐phloxine (HP) staining and histological examination. Cystic cases (seven cases) were considered to consist of focal lesions, as defined in Volpe 75; they all displayed macrocopic cysts associated or not with necrosis and/or calcifications surrounded by diffuse pallor (Table 2, Supporting Information Figure S1A–D). Non‐cystic cases without tissue loss displayed pallor and gliosis (18/18 cases) associated with microscopic necrotic foci in a few cases (4/18 cases) and were considered to consist of diffuse lesions 16, 30, 71, 75 (Table 3, Figure 1E and F). For pathological cases, the survival time is indicated in Table 1. The post‐mortem interval is longer than one week for two cases out of seven for cystic PWMI and two cases out of 18 for the non‐cystic cases. It could slightly influence the amount, morphology and intensity of both microglial and astrocytic response to injury.
Table 1.
Control cases (age at death: postconceptional weeks + days)
| Case | Age at birth (modifier) | Sex | Clinical context |
|---|---|---|---|
| 1 | 24 | Female | Twin |
| 2 | 25 + 2 d | Female | Medical abortion (cleft lip and palate) |
| 3 | 25 + 2 d | Male | Spontaneous abortion |
| 4 | 25 + 5 d | Male | Medical abortion (growth retardation) |
| 5 | 27 | Male | Spontaneous abortion |
| 6 | 29 + 2 d | Male | Medical abortion (posterior urethral valve) |
| 7 | 30 + 4 d | Female | Spontaneous abortion |
| 8 | 32 | Female | Uropathy |
| 9 | 33 | Male | Spontaneous abortion |
| 10 | 33 | Female | Spontaneous abortion |
Table 2.
Cases with cystic PWMI (age at death: postconceptional weeks + days). Abbreviation: PWMI = periventricular white matter injury
| Cases | Age at birth (pcw) | Survival time (week/day) | Sex | Macroscopic lesions | ||
|---|---|---|---|---|---|---|
| Pallor | Necrosis | Cyst | ||||
| 1 | 25 | 0 d | Female | + | − | ++Calcifications |
| 2 | 24 + 4 | 5 d | Male | + | ++ | ++ |
| 3 | 26 | 4 d | Male | − | + | ++ |
| 4 | 27 | 0 d | Male | + | + | ++Calcifications |
| 5 | 28 | 4 d | Male | + | + | + |
| 6 | 27 | 1 w + 3 d | Female | + | − | +Calcifications |
| 7 | 27 | 4 w + 1 d | Male | + | + | +Calcifications |
Pallor: −, absent; +, present.
Necrosis: −, absent; +, microscopic necrosis; ++, macroscopic necrosis visible by naked eyes on the histologic sections.
Macroscopic cyst: +, small cyst; ++, large cysts.
Table 3.
Cases with non‐cystic PWMI (age at death: postconceptional weeks + days). Abbreviation: PWMI = periventricular white matter injury
| Cases | Age at birth (pcw) | Survival time (week/day) | Sex | Macroscopic lesions | Microscopic lesions | |||
|---|---|---|---|---|---|---|---|---|
| Pallor | Necrosis | Cyst | Pallor | Necrosis | ||||
| 1 | 24 + 2 d | 2 d | Female | − | − | − | + | − |
| 2 | 24 | 7 d | Female | + | − | − | + | − |
| 3 | 25 + 5 d | 2 d | Male | + | − | − | + | + |
| 4 | 25 + 6 d | 1 d | Male | + | − | − | + | − |
| 5 | 26 | 1 d | Female | + | − | − | + | − |
| 6 | 26 | 2 d | Male | + | − | − | + | − |
| 7 | 27 + 3 d | 3 d | Male | + | − | − | + | − |
| 8 | 27 + 6 d | 0 d | Female | + | − | − | + | − |
| 9 | 27 + 4 d | 3 d | Male | − | − | − | + | − |
| 10 | 28 + 5 d | 1 d | Male | + | − | − | + | + |
| 11 | 28 + 6 d | 0 d | Male | + | − | − | + | − |
| 12 | 25 + 6 d | 1 w + 4 d | Male | + | − | − | + | −Microcalcifications |
| 13 | 30 | 2 d | Male | + | − | − | + | + |
| 14 | 30 + 2 d | 5 d | Female | + | − | − | + | −Microcalcifications |
| 15 | 28 | 4 w | Female | + | − | − | + | + |
| 16 | 33 | 0 d | Male | + | − | − | + | − |
| 17 | 33 | 3 d | Male | − | − | − | + | − |
| 18 | 34 | 4 d | Female | + | − | − | + | − |
Diffuse component was macroscopically and/or microscopically identified as a pallor on histological examination.
Necrosis: −, absent; +, microscopic necrosis.
Figure 1.
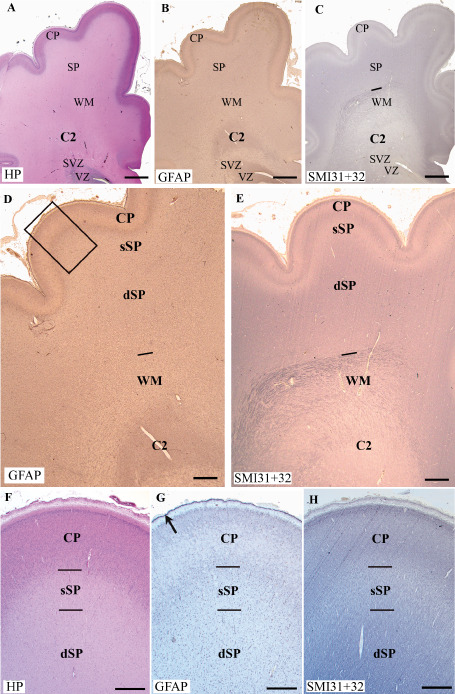
Coronal sections through the frontal lobe (premotor area, 29 pcw) of a control case: hemalum‐phloxine (HP) staining (A,F), immunolabeling for glial fibrillary acidic protein (GFAP) (B,D,G) and SMI31 + 32 (C,E,H). The crossroads area, C2, is indicated in A–E. The limit between the white matter (WM) and the subplate layer (SP) is clearly delineated by SMI31 + 32 labeling (lines in C and E). D and E show the cortical plate (CP) and the SP subdivided into the deep subplate (dSP) and superficial subplate (sSP). The frame in D indicates the level of the enlargements made on serial sections labeled with HP staining (F), GFAP labeling (G) and SMI31 + 32 (H). Note the low labeling for SMI311 in the sSP in E and H and the band of strongly GFAP‐positive cells in the marginal zone in G (arrow). Scale bars: A–C. 2 mm; D–E. 800 μm; F–H. 200 μm.
Immunohistochemistry
Serial paraffin sections were processed for immunohistochemistry. The primary antibodies used were directed against different antigens (Table 4): GFAP for astrocytes, Iba1 for microglia/macrophages, CD68 for macrophages and Olig2 as marker of oligodendrocyte lineage. CD34 antibodies label all the vascular endothelial cells 55, whereas MCT1 are detected in subpopulations of vessel walls and astrocytes at these time periods 15. The monoclonal mouse antibodies SMI32 are directed against non‐phosphorylated epitopes of neurofilament proteins (neuronal cell bodies), whereas SMI31 antibodies are directed against phosphorylated epitopes of neurofilament proteins (axonal field) 42, 68 (Sternberger Monoclonal Inc., Baltimore, MD, USA).
Table 4.
Primary antibodies used in the study. Abbreviations: GFAP = glial fibrillary acidic protein; Iba1 = ionized calcium‐binding adapter molecule 1; MCT1 = monocarboxylate transporter 1
| Antibodies | Cells/structures labeled | Source | Species | Dilution |
|---|---|---|---|---|
| GFAP | Astrocytes | Sigma (Saint Louis, USA) | Mouse | 1:500 |
| Iba1 | Ramified and activated microglia | Waco (Richmond, USA) | Rabbit | 1:200 |
| CD68 | Macrophages | Dako (Glostrup, Denmark) | Mouse | 1:300 |
| CD34 | Endothelial cells | Serotec (Oxford, UK) | Mouse | 1:200 |
| MCT1 | Vessel walls + astrocytes | Chemicon (Temecula, USA) | Chicken | 1:1000 |
| Olig2 | Oligodendrocytic lineage | IBL (Gunma, Japan) | Rabbit | 1:200 |
| SMI31‐32 | Neurofilaments | Affinity (Exeter, UK) | Mouse | 1:2000 |
Following deparaffinization, slides were incubated in 0.01 M citrate buffer (pH = 6) at 94°C for 45 minutes. The sections were then pretreated with hydrogen peroxide (H2O2, 0.25%) in phosphate buffered saline (PBS, 15 minutes) and rinsed in PBS (3 × 10 minutes). After a rinse in PBS/0.5% TritonX 100/20/00 gelatin (PBS‐TX‐gel, 2 × 10 minutes) 55, the sections were incubated with the appropriately diluted primary antibody (Table 4) in PBS‐TX‐gel with 8% human albumin and 0.02% sodium azide for 3–4 days at 4°C. The primary antibodies were visualized using the streptavidin‐biotin‐peroxidase (Amersham, Little Chafont, Buckinghamshire, UK) method as described in 69 using diaminobenzidine 0.02% (DAB) and 0.6% nickel ammonium sulfate as chromogens in the presence of 0.005% H2O2 in Tris (0.05 M, pH 7.4–7.6). Sections were counterstained with neutral red (1%), dehydrated and mounted. The labeled antigen appeared black under the light microscope.
Double immunolabeling
Double immunolabeling for GFAP‐Iba1 and MCT1‐CD34 was performed using primary antibodies raised in different species (Table 4). The first primary antibody of each combination was visualized as a black reaction product using the streptavidin‐biotin‐peroxidase method described above. Then, the sections were incubated with the second primary antibody, which was subsequently visualized by the peroxidase‐antiperoxidase (PAP) (Dako, Glostrup, Denmark) method as described in Monier et al 55. Briefly, peroxidase reacted with H2O2 (0.005%) in the presence of DAB (0.05%) in 0.1 M PBS (pH 7.5) to yield a brown reaction product. For both methods, controls without the primary antibody were carried out to verify the absence of cross reactivity.
Microscopy and quantification
Images were acquired with a CDD camera (Evolution MP, Media Cybernetics, Bethesda, MD, USA) adapted to a Zeiss Axioplan microscope (Carl Zeiss, Jena, Germany) and organized using adobe photoshop CS2 (Adobe Systems, San Jose, CA, USA).
Quantification of immunoreactive cells
The densities of cells labeled by GFAP, Iba1 and Olig2 in the five areas 1, 2, 3, 4, 5 described in the next section were assessed in each brain. For each brain, labeled cells were counted at 400× magnification in four fields of 0.065 mm2 each. The assessment of Olig2‐positive pre‐oligodendrocytes was performed on 15 non‐cystic PWMI cases when compared with nine control ones. The size (in μm2) of GFAP‐positive cell bodies was assessed in microscopic fields as mentioned for the measurement of the density. Images were processed and analyzed using the Fiji distribution (http://fiji.sc) of imagej U.S. National Institutes of Health, Bethesda, MD, USA (http://rsbweb.nih.gov/ij/). Results were compared using two‐way ANOVA with Bonferroni's multiple comparison test (graphpad prism, GraphPad Software Inc., La Jolla, CA, USA), **P < 0.01, ***P < 0.001.
Topographical criteria and neuropathological survey of cystic and non‐cystic PWMI in the cohort of premature infants
HP‐stained sections were examined blindly by two neuropathologists (CF‐B, HA‐B) to differentiate between control cases free of neuropathological abnormalities and cystic and non‐cystic PWMI cases (Table 1). Our observation focused on cortical regions located in the posterior part of the superior, middle and inferior frontal gyri and sulci, and the precentral gyrus and central sulcus according to levels 4–8 of the atlas 5. They correspond to the presumptive premotor and motor areas (areas 8‐6‐4) and contiguous prefrontal areas. The levels studied varied in each case but always included the basal ganglia and the anterior thalamic nuclei. The cortical wall was composed from the ventricle to the pial surface of different tangential layers: the ventricular (VZ) and subventricular zones (SVZ), the white matter (WM), the SP and the cortical plate (CP) 7, 34, 35, 36, 37, 38, 39 (Figure 1A–C). The limit between the deep subplate (dSP) and the white matter (intermediate zone) was ascertained by the presence of dense SMI31 axonal neurofilament labeling in the white matter (Figure 1C and E). The sSP was defined by faint SMI31 labeling (Figure 1C,E and H), and its upper limit by the densely cell packed cells of the CP (Figure 1A and F). Within the presumptive white matter, two segments were defined: the axonal crossroads (C2) and the semioval center (WM; Figure 1A and E) 25, 71. HP staining, GFAP immunolabeling and, in particular, SMI31 neurofilament labeling (Figure 1) allowed the improved delineation of the upper layers of the cerebral wall, in particular the outer limit of the white matter, the dSP, the sSP and the CP (Figure 1D–H).
Results
Glial characteristics in the different layers of the cerebral wall in very preterm and preterm control cases
In premotor and motor areas (areas 8‐6‐4) and contiguous prefrontal areas, no obvious phenotypic differences were detected in the expression of GFAP or Iba1 in the gyri vs. the sulci (data not shown) of control cases.
Very preterm control cases (24–29 pcw)
GFAP‐positive astrocytes were dense and showed small round cell bodies and a few processes in the C2 crossroads area and deep white matter (Figures 1D and 2A), whereas they were more fibrous in the superficial white matter (Figure 2D, Supporting Information Figure S2A) and dSP (Figure 2G, Supporting Information Figure S2B). They were sparse with thin processes and small cell bodies in the sSP (Figure 2J) and CP (Figure 2M). Quantitative analysis showed that there was a decrease in the density of GFAP‐positive cell bodies from deep to superficial layers (Figure 3A).
Figure 2.
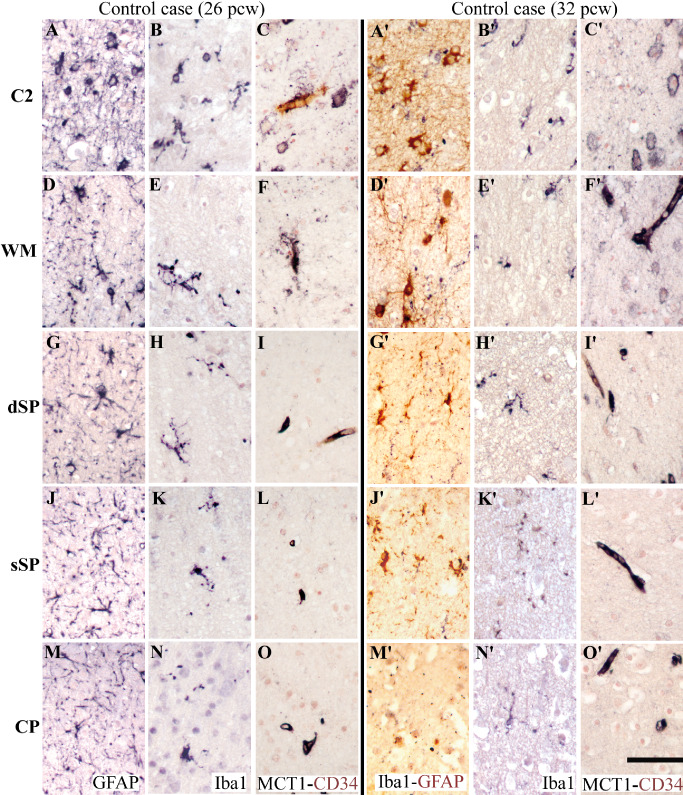
Immunolabeling for glial markers from the deep to the superficial cerebral wall, as indicated in Figure 1: area C2 (A–C, A′–C′); superficial white matter (WM) (D–F, D′–F′); deep subplate (dSP) (G–I,G′–I′); superficial subplate (sSP) (J–L,J′–L′); cortical plate (CP) (M–O, M′–O′). First column: a very preterm control case (26 pcw); second column: a preterm control case (32 pcw). Glial fibrillary acidic protein (GFAP)‐positive astrocytes (A,D,G,J,M), ionized calcium‐binding adapter molecule 1 (Iba1)‐positive microglia/macrophages (B,B′,E,E′,H,H′,K,K′,N,N′), double‐labeling for Iba1 (black) and GFAP (brown) (A′,D′,G′,J′,M′), and double‐labeling for MCT1 (black) and CD34 (brown) vessel walls and astrocytic cell bodies (C,C′,F,F′,I,I′,L,L′,O,O′). Scale bar: 50 μm
Figure 3.
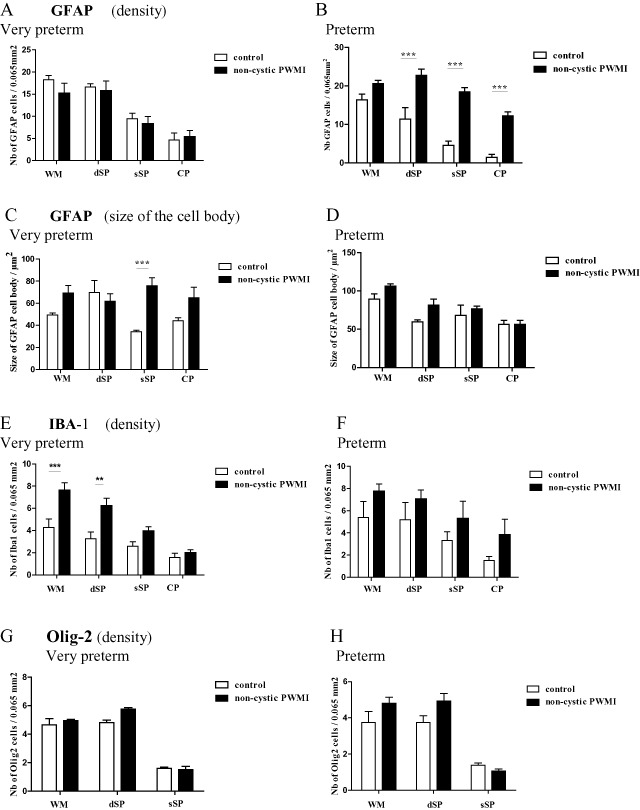
Density (number per unit area) of glial fibrillary acidic protein (GFAP)‐positive astrocytes (A,B), size (in μm2) of GFAP‐positive cell bodies (C,D), density (number per unit area) of ionized calcium‐binding adapter molecule 1 (Iba1)‐positive microglia/macrophages (E,F) and Olig2‐positive pre‐oligodendrocytes (G,H) in the superficial white matter (WM), the deep subplate (dSP), the superficial subplate (sSP) and the cortical plate (CP) in non‐cystic periventricular white matter injury (PWMI) in very preterm and preterm cases, compared with control cases. **P < 0.01, ***P < 0.001.
GFAP‐positive cell bodies were not quantified in the marginal zone, but they formed a dense band in this transiently organized fetal zone (Figure 1G). Thus, the distribution of GFAP‐positive cell bodies in the cortical layers shows a bilaminar distribution, with a lower density region in the sSP and CP sandwiched between higher density areas in the dSP.
As described in Verney et al 71, intermediate Iba1‐positive microglia were densely distributed in the C2 crossroads area (Figure 2B). In the white matter above and the dSP, they were less dense, with a ramified phenotype characterized by dots on their processes (Figures 2E,H and 3C). A gradual decrease in density was detected from the white matter to the CP during the very preterm period (Figure 3C), and the cells were less ramified in the sSP (Figure 2K) and the CP (Figure 2N). At this stage, all vessel walls show CD34 labeling 15. MCT1/CD34 double labeling showed that not all vessel walls expressed MCT1 in the C2 crossroads area (Figure 2C), white matter (Figure 2F), SP (Figure 2I and L) or CP (Figure 2O). However, most astrocytes expressed low levels of MCT1 in the C2 crossroads, whereas sparse expression was detected in the overlying white matter (Figure 2C and F). In contrast, almost no expression of MCT1 was detected in astrocytes in the SP (Figure 2I and L) and CP (Figure 2O).
Preterm control cases (30–34 pcw)
GFAP‐positive astrocytes in control preterm cases showed bigger cell bodies throughout the various layers (Figure 2A′,D′,G′ and J′) than those detected in very preterm infants (Figure 2A,D,G and J) (compare Supporting Information Figure S2A–D with Figure S2M–P). A decrease in density from deep to superficial layers was observed, similar to that detected in very preterm cases (Figure 3B).
The density of GFAP‐positive astrocytes in preterm control cases decreased (non‐significantly) when compared with very preterm cases (Figure 3A and B, Supporting Information Figure S3A), but their cell bodies increased in size (Figure 3C and D, Supporting Information Figure S3C). Interestingly, this increase was significant in the white matter and the sSP (Supporting Information Figure S3C, P < 0.01) but was not present in the dSP.
Microglial cells visualized by Iba1 immunoreactivity were ramified, with dots of varying densities on their processes (Figure 2B′,E′,H′,K′ and N′). Their density decreased from the white matter to the CP (Figure 3D). Almost all vessel walls were MCT1‐positive at these later stages (Figure 2C′,F′,I′,L′ and O′). Numerous astrocytes with MCT1‐positive cell bodies were present in the C2 crossroads area and white matter (Figure 2C′ and F′), whereas these were fewer in the dSP (Figure 2I) and almost absent in the sSP and CP (Figure 2L′ and O′).
Glial features in the cerebral wall of cystic PWMI cases
Most of the cystic PWMI cases were very preterm (six out of seven). In all cystic cases, the cysts were confined to the white matter without extending into superficial layers of the cerebral wall such as the SP and CP 71 (Figure 4). In a single case, the lesion was so large that it invaded all the layers of the cortical wall. GFAP‐positive astrocytes displayed few processes (Figure 5A,E,I and M, Supporting Information Figure S2I–L). In the white matter surrounding the cyst, they were significantly denser when compared with control cases (Supporting Information Figure S2A compared with Figure S2I, Figure 6B, P < 0.001), whereas no increase in density was detected in the SP and CP.
Figure 4.
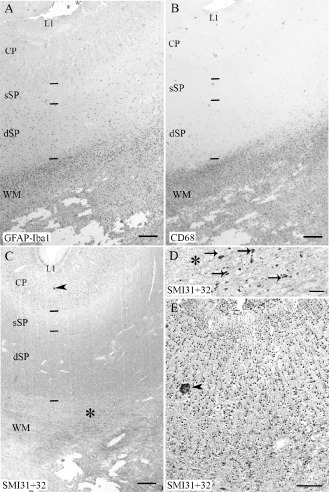
Serial sections of the cerebral wall of a cystic periventricular white matter injury (PWMI) case (26 pcw) immunolabeled with glial fibrillary acidic protein (GFAP)‐ionized calcium‐binding adapter molecule 1 (Iba1) (A), CD68 (B) and SMI31 + 32 (C–E). Note the glial reaction restricted to the white matter (WM) in A and B, as well as the SMI‐positive spheroids (arrows in D) near the area of tissue loss (asterisks in C and D). The cortical plate (CP) in C—at the level of an artifact (arrowhead), enlarged in E (arrowhead)—does not display any obvious abnormalities in SMI‐positive neuronal cell bodies. L1, layer 1; dSP, deep subplate; sSP, superficial subplate. Scale bars: A,B,C. 500 μm; D. 200 μm; E. 100 μm.
Figure 5.
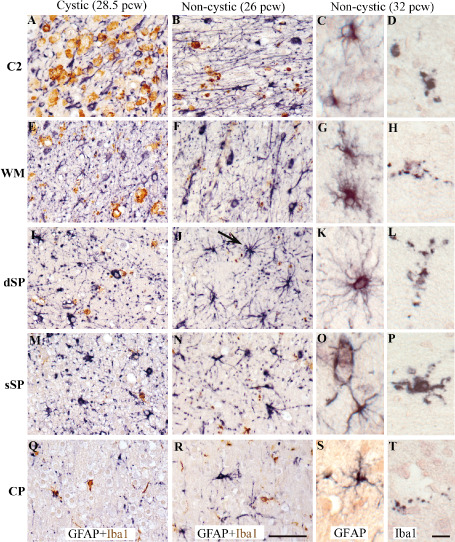
In vertical columns: different cases of periventricular white matter injury (PWMI): a cystic case at 28.5 pcw (A,E,I,M,Q), a non‐cystic case at 26 pcw (B,F,J,N,R) and a non‐cystic case at 32 pcw (C,D,G,H,K,L,O,P,S,T). Immunolabeling for glial markers from deep to superficial cerebral wall layers: area C2 (A–D), superficial white matter (WM) (E–H), deep subplate (dSP) (I–L), superficial subplate (sSP) (M–P), cortical plate (CP) (Q–T). Double‐labeling of glial fibrillary acidic protein (GFAP)‐positive astrocytes (black) + ionized calcium‐binding adapter molecule 1 (Iba1)‐positive microglia/macrophages (brown) (A,D,G,J,M and B,E,H,K,N). GFAP‐positive astrocytes (C,G,K,Q,S) and Iba1‐positive microglia/macrophages at higher magnifications (C,F,I,L,O and D,H,L,P,T). The arrow in J shows a big protoplasmic astrocyte with long processes located in the dSP in a non‐cystic lesion. Scale bars: A,B,E,F,I,J,M,N,Q,R. 50 μm; C,D,G,H,K,L,O,P,S,T. 15 μm.
Figure 6.
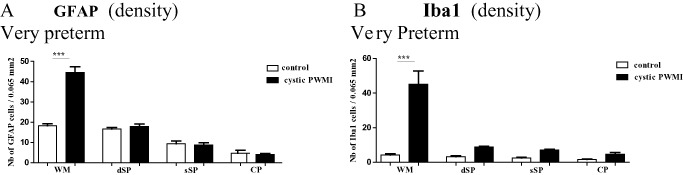
Density (number per unit area) of glial fibrillary acidic protein (GFAP)‐positive astrocytes (A) and of ionized calcium‐binding adapter molecule 1 (Iba1)‐positive microglia/macrophages (B) in the superficial white matter (WM), the deep subplate (dSP), the superficial subplate (sSP) and the cortical plate (CP) in cystic periventricular white matter injury (PWMI) in very preterm compared with control cases. ***P < 0.001.
Around the cyst, numerous Iba1‐positive and CD68‐positive macrophages were present, more or less restricted to the white matter (Figures 4A,B and 5A). The density of Iba1 positive cells was significantly increased in the white matter (Figure 6B, P < 0.001), whereas only a few Iba1‐positive intermediate/ramified microglial cells were detected in the upper layers of the cerebral wall (Figure 5I,M and Q). No change in MCT1 labeling was seen in the SP or CP when compared with age‐matched control cases (data not shown). SMI32 and 31 immunolabeling showed axonal spheroids around areas of white matter tissue loss (Figure 4C and D), without obvious neuronal lesions in the SP or CP (Figure 4E).
Glial features in the cerebral wall of non‐cystic PWMI cases
In very preterm non‐cystic PWMI cases, GFAP‐positive astrocytes displayed more processes from dSP to the CP compared with control cases (Figure 6J,N and R, compared with 2G,J and M; Supporting Information Figure S2F–H compared with B–D). Their density in each layer was similar to that of control cases (Figure 3A), but they were hypertrophic as shown by the increased size of their cell bodies (Figure 3C). Interestingly, this increase was significant (P < 0.001) in the sSP when compared with control cases. Iba1‐positive macrophagic cells were detected in the crossroads area (Figure 5B) but displayed an intermediate/ramified phenotype with Iba1‐positive dots in the layers of the cerebral wall (Figure 5F,J,N and R). The density of Iba1‐positive cells increased in all layers in non‐cystic PWMI cases when compared with control cases (Figure 3E). However, this increase was significant only in the white matter (P < 0.001) and dSP (P < 0.01).
In preterm non‐cystic PWMI cases, the GFAP‐positive astrocytes were larger, with various hypertrophic phenotypes in the different layers. Some big astrocytes displayed numerous hairy processes in the white matter (Figure 5G) and dSP (Figure 5K), unlike the protoplasmic astrocytes detected in the dSP in very preterm cases (Figure 5J). In the sSP and CP, they displayed more end‐feet extending toward vessel walls (Figure 5O and S). Interestingly, the density of GFAP‐positive astrocytes was significantly increased in the SP as a whole and in the CP when compared with control cases (Figure 3B, P > 0.001). In parallel, the density GFAP positive astrocytes of non‐cystic PWMI increased in preterm period compared with very preterm and significantly in the sSP (Supporting Information Figure S3B). Also, an increase in size of the astrocytic GFAP positive cell body was detected in preterm period compared with very preterm period but was significant only in the white matter (Supporting Information Figure S3D, P < 0.01).
In four out of 18 preterm non‐cystic PWMI cases, small necrotic foci with no tissue loss invaded the SP (Figure 7A–D). GFAP‐positive astrocytes were particularly numerous in the dSP, similar to the white matter, whereas their density decreased in the sSP and CP (Figure 7C). In parallel, Iba1‐positive microglial cells showed a rather macrophagic phenotype around necrotic foci with no tissue loss in the dSP (Figure 7D and E), whereas they exhibited a more intermediate phenotype in the sSP (Figure 7F) and CP. No significant increase in density was detected in the different cortical layers for Iba1‐positive cells when compared with controls (Figure 3D). Disrupted SMI31 + 32‐positive axons were observed around the foci located in the dSP (Figure 6H).
Figure 7.
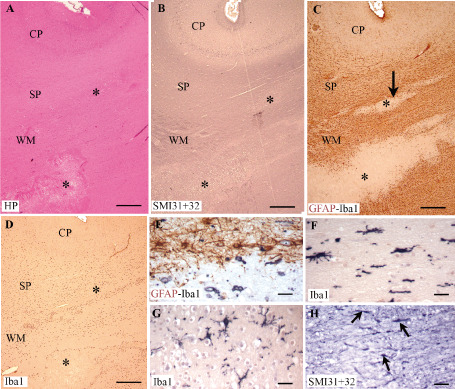
Serial sections of a non‐cystic lesion in the cerebral wall of a preterm infant (34.5 pcw) showing necrotic foci in the white matter (WM) and subplate (SP) (asterisks). A. Hemalum‐phloxine (HP) staining. B,H. SMI31 + 32 immunolabeling. C,E. Double‐labeling for GFAP (brown) + ionized calcium‐binding adapter molecule 1 (Iba1) (black). D,F,G. Iba1‐positive microglia/macrophages. The arrow in C indicates the lesion in the SP, enlarged in E and H. Note the Iba1‐positive macrophage in the deep SP in E, whereas Iba1‐positive microglia have an intermediate phenotype in the superficial SP (G) and cortical plate (CP) (F). In H, note the SMI‐positive axonal spheroids (small arrows) present in the deep SP. Scale bars: A,B,C,D. 500 μm; E,F,G,H. 50 μm.
In very preterm and preterm cases, Olig2‐positive nucleus were detected essentially in the white matter and dSP (Supporting Information Figure S4); very few were present in the sSP and none in the CP (Figure 3G and H). No difference in density was detected non‐cystic PWMI cases compared with control cases during very preterm and preterm period.
In one case, neuronal disorganization was detected in the CP upon HP staining (Figure 8A). In contiguous sections, patches of hypertrophic astrocytes were detected in the CP (Figure 8B), intermingled with Iba1‐positive patches (Figure 8C and D). Interestingly, these latter patches corresponded to a disruption of SMI31 + 32 expression in the developing pyramidal neurons of the CP (Figure 8E). The association of MCT1 with CD34 immunolabeling displayed similar features to those observed in controls with one exception: in the CP, some CD34‐positive vessels did express MCT1, possibly corresponding to increased energy supply (data not shown).
Figure 8.
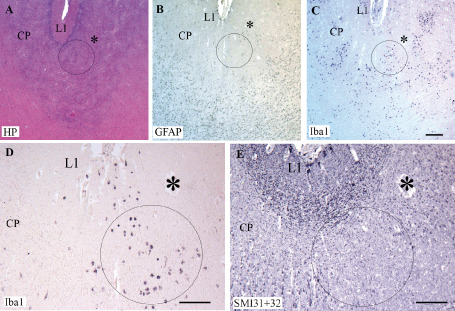
Serial sections of a non‐cystic lesion in the cortical plate (CP) of a preterm infant (33.5 pcw). Hemalum‐phloxine (HP) staining of a lesion located in the CP (A). Patches of cells positive for glial fibrillary acidic protein (GFAP) (B) and ionized calcium‐binding adapter molecule 1 (Iba1) (C,D) in the same area delineated by a circle. The asterisk show the same vessel visualized on the different serial sections in A–E. The circle in C is enlarged in D. In E, note the spot with no SMI31 + 32‐positive neuronal labeling within the circle at the level where numerous Iba1 macrophages are present in D. L1, layer 1. Scale bars: A–C. 200 μm; D,E. 100 μm.
Discussion
We previously analyzed the microglial cells and astrocytes in the white matter and crossroads in very preterm and preterm infants with PWMI 71. The selection of the pathological cases displayed two groups: a large cohort of subtle non‐cystic PWMI (diffuse lesions) and a few cystic PWMI all displaying macroscopic cysts. The present study focuses on astrocytic activation and microglial cells activation in the SP of the cerebral wall of the same PWMI cases.
During very preterm and preterm period, the transient SP layer reaches its maximum thickness with a maximum extend of approximately four times thicker than the CP when premature infants display the highest risk of PWMI 39. Our first results compare the laminar characteristics of astrocytes and microglial cells in the SP of the non‐cystic PWMI cases compared with cystic PWMI during very preterm period. In non‐cystic PWMI, the density of astrocytes and microglial cells increased in the white matter and the SP, whereas the increase of density of these cells was restricted to the white matter in cystic PWMI during very preterm period. Second, in non‐cystic PWMI detected in the very preterm period, the dSP is a target for microglial activation, whereas in preterm cases, the SP displayed an increase in the density of reactive astroglia when compared with age‐matched control cases.
In the following paragraphs, we will discuss the laminar distribution of astrocytes and microglial cells in the normal and lesioned cerebral wall, and the possible and specific vulnerability of the SP.
Laminar distribution of glia in the normal and lesioned cerebral wall
As described in a former article 71, astrocytes and microglial cells are readily found in the white matter of very preterm infants, in both control and PWMI cases. The presence of astrocytes and microglial cells is to be expected in the cerebral wall of normal cases at this time period, when resident microglia invade the different layers of the developing cerebral wall 55, 70. The SP displays Olig2, a marker of the oligodendrocyte lineage at this time period 23. In our results, the highest density (number per unit area) of GFAP‐positive astrocytes was seen in the white matter, with intermediate densities in the SP and the lowest density in the CP. This is consistent with the original observations of Schmechel and Rakic 62 describing the transformation of radial glial fibers in the deep moiety of the cerebral wall and recent concepts of radial glial lineage 41, 61. Our results showed that the size of GFAP‐positive cell bodies is two times bigger in preterm period when compared with the very preterm period in the sSP. Also, we observed a bilaminar distribution of GFAP‐positive cells, which were predominant in the SP and marginal zone, concurring with the description of DeAzevedo et al 9. Interestingly, this bilaminar distribution of GFAP‐positive cell bodies corresponds to the distribution of the first synapses detected in the fetal human brain 53 above and below the CP in the sSP.
In PWMI cases, we saw significant differences in astrocytic and microglial features depending on whether the damage was cystic or non‐cystic. In cystic PWMI during very preterm period, increase in density of astrocytes and of microglial cells was restricted to the white matter, comparable with the well‐described focal lesions seen in periventricular leukomalacia (PVL) 3, 18, 59, 75. In the opposite, the non‐cystic PWMI cases showed an increase in density of astrocytes highly significant in the different layers of the cerebral wall including the SP during the preterm period. Also the density of microglia cells significantly increased in the white matter and the dSP during during the very preterm period. We have previously described a similar pattern of glial activation (microglia in very preterm infants but astrogliosis in preterm infants) in the deepest segment of the white matter in preterm infants, within the so‐called periventricular axonal crossroads 71. The significance of diffuse non‐cystic white matter lesions as a possible substrate for later cognitive deficits in preterm children has been thoroughly discussed 51, 75 and described in numerous MRI studies 8, 47, 52.
Vulnerability of sublamina regions of the cortical SP
Injuries of the cerebral cortical wall, including the SP, are associated with prematurity in humans 73 and in several animal models 10, 24, 49, 50, 63, 64, 72. For instance, we have shown in a non‐human primate (baboon) model of prematurity that specific ventilatory strategies can have a significant impact on cortical neuronal subpopulations 72. In a preterm rat model, following neonatal hypoxic‐ischemic injury on postnatal day 3 (P3), neuronal lesions have been noted in parietal deep cortical layers, including the SP 64. Furthermore, the specific vulnerability of the SP has been described following hypoxic‐ischemic injury in neonatal rats at P1‐P2 49, 50 and in premature rabbits in which excitotoxic lesions of the white matter invade the large SP and cortical layers 63. Interest in the SP as a substrate for injury in premature infants has increased after the finding that this layer can be readily defined in magnetic resonance images in humans 34, 47 and in animal models 50, 51, 52.
In humans, the SP, a transient compartment, is the most prominent zone of the preterm cerebral wall and is composed of well‐differentiated postmigratory neurons, glia, growing axonal connections and the earliest cortical synaptic contacts associated with extensive amounts of extracellular matrix 32, 33, 34, 37, 38. The SP has been suggested to play a significant role in the development of cortical connectivity and plasticity 1, 17, 28, 29, 35, 36, 37, 39, 48. Dynamic changes in the SP in fetal, preterm and neonatal brains involve developmental changes in all its major components: cells, fibers and extracellular matrix 40. However, the real cytological and histopathological substrate of prospective SP lesions has never been convincingly documented 31, 49, 57, and the SP has been considered a uniform and static layer despite the fact that it shows reorganization, especially during the stage of resolution 34, 40. The glial characteristics presented in this paper support the idea of a sublaminar organization of the SP into deep and superficial compartments. In both control and PWMI cases, GFAP‐positive astrocytes increased in density, from the white matter‐dSP to the sSP‐CP 46. In the preterm period in non‐cystic PWMI cases, GFAP‐positive astrocytes with numerous processes were present in the white matter and dSP, an observation that fits well with the probable involvement and special vulnerability of the dSP compartment in pathology. The development of a transient vascular network 66 in the SP could also contribute to the selective vulnerability of this layer. In our results, the low expression levels of MCT1 in astrocytes in the white matter and SP in very preterm non‐cystic PWMI cases could indicate astrocytic immaturity. Conversely, the higher levels of MCT1 expression in astrocytes in an inside‐out gradient from the white matter to the SP during the preterm period could reflect their functional maturation and involvement in energy supply.
The vulnerability of axonal fields, including the axonal crossroads region of the white matter, is well documented in PWMI 25, 71. In parallel, the SP displays axonal pathways such as those of catecholaminergic axons 69 and the growth of corticocortical afferents through the SP from deep to superficial layers 32, 39. In diffuse non‐cystic lesions, the pattern of abnormal glial activation is likely to reflect the laminar distribution of vulnerable target elements such as growing fibers and synaptic contacts. Further investigation is needed to understand the possible role of glial cells in the vulnerability of the different components of the SP.
Age‐related shifts in microglial and astroglial activation in the SP in PWMI
Activated microglia in our study invaded the entire extent of the SP, whereas the CP displayed only a few cells, as described for resident microglia during normal development 54. The early presence of activated microglia/macrophages has been shown in various animal models of PWMI involving excitotoxicity, inflammatory, hypoxic or hypoxic‐ischemic insults 4, 45, 56, 65. This protection of the CP even when the white matter undergoes pathological changes is consistent with the general notion that in hypoxic‐ischemic lesions of the early developing cerebrum, the white matter is the main target, whereas cortical cells are more or less spared 18, 50, 75. However, the presence of activated microglia throughout the SP may perturb the morphogenic role of this layer in human cortical organization 35. The preterm period is characterized by the intensive growth of subcorticocortical and callosal fibers through the periventricular axonal crossroads, sagittal strata and SP. During this period, some axons fail to reach their postsynaptic targets, and microglia are activated in order to remove the debris of axons that are either retracted or that change course during growth. This phenomenon has been observed in the periventricular axonal crossroads during both normal 25, 55 and pathological development 70, 71. However, the further activation of microglia, particularly under pathological conditions, is probably related to the axonal damage caused by hypoxic‐ischemic/inflammatory lesions. Moreover, one could ask whether this microglial activation in the very preterm period is deleterious or neuroprotective 11, 12, 65. The role of these microglia is probably different from that of the Iba1‐positive, CD68‐positive macrophages described around small necrotic foci or in clear cystic lesions 6, 18, 19, 27, 75. In addition, the expression of cytokines such as IL‐1β, IL‐2, IL‐6 and TNF‐α has been shown in the microglia/macrophages of newborns with PWMI 26, 27. Studying the expression of various cytokines during the spatiotemporal onset of PWMI could provide new insights into the pathologic events that occur in the cerebral wall following PWMI 14.
Our results also showed the preferential involvement of astroglial activation within the SP in non‐cystic PWMI during the preterm period (30–34 pcw). In preterm cases, this astroglial reaction, which extends to the SP and CP (as previously discussed), could correspond to a new wave of growth of long corticocortical pathways 33, 67. Even though inflammatory mechanisms have been suggested to play a role in astrogliosis in PWMI 59, more investigations are needed to understand the possible role of these cells in the vulnerability of the SP.
Our results emphasize the involvement of activated microglia and astrocytes in the SP, which parallels that described in the white matter for non‐cystic PWMI 65. No change in density of oligodendrocyte lineage marker Olig2 was observed in the SP and white matter that is in accord with the results published by Billiard et al 6, 14. The next step is to pinpoint the precise correlation between variations in the density of glial cells measured in the different compartments of the cerebral wall—in particular the deep and sSP—with imaging data obtained from these compartments in the same brains. Signal abnormalities and disturbances in diffusion parameters may vary according to the spatiotemporal sequence of glial proliferation and activation described in the cerebral wall in this study. These milestones could provide significant insights into the evaluation of the outcome of premature infants.
Supporting information
Figure S1. Hemalum‐phloxine staining of cystic (A–D) and non‐cystic (E,F) periventricular white matter injury (PWMI) cases. A,B. Large cysts surrounded of necrotic foci (arrows) (necrosis ++) and pallor. C. High magnification of a necrotic focus (arrow). D,F. High magnification of pallor (diffuse lesion). E. spots of necrosis +. Scale bars: A. 1 mm, B. 500 μm, C. 100 μm, D. 50 μm, E,F. 200 μm.
Figure S2. Microphotographs of glial fibrillary acidic protein (GFAP)+ astrocytes of the layers of the cerebral wall showed in columns: white matter (WM) (A,E,I,M,Q), the deep subplate (dSP) (B,F,J,N,R), the superficial subplate (sSP) (C,G,K,O,S) and the cortical plate (CP) (D,H,J,P,T) in very preterm cases (VPT) (A–L) and preterm (PT) cases (M–T) in control cases (A–D,M–P) and in non‐cystic periventricular white matter injury (PWMI) (E–H,Q–T) and cystic PWMI (I–L). Scale bar: 50 μm.
Figure S3. Density (number per unit area) (A,B) and size of cell bodies (in μm2) (C,D) of glial fibrillary acidic protein (GFAP)‐positive astrocytes in the superficial white matter (WM), the deep subplate (dSP), the superficial subplate (sSP) and the cortical plate (CP) in very preterm compared with preterm in control cases (A,C) and in non‐cystic periventricular white matter injury (PWMI) (B,D). **P < 0.01.
Figure S4. Olig2‐immunolabeling of the nucleus of cells of the oligodendrocytic lineage in the deep subplate of a very preterm control case (A) and a non‐cystic periventricular white matter injury (PWMI) case (B). Scale bar: 25 μm.
Acknowledgments
This study was supported by grants from the Inserm, Université Paris Diderot, PremUP, Seventh Framework Program of the European Union (grant agreement #HEALTH‐F2–2009‐241778/NEUROBID), Fondation Leducq, Fondation Grace de Monaco, Fondation Roger de Spoelberch, ELA Foundation, Fondation Planiol, Assistance Publique Hôpitaux de Paris (APHP‐Contrat Hospitalier de Recherche Translationnelle to PG), the Ministry of Science, Education and Sport, Croatia (grant 108–1081870‐1876 to IK and IP) and a scholarship from the French Government to IP.
References
- 1. Allendoerfer KL, Shatz CJ (1994) The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci 17:185–218. [DOI] [PubMed] [Google Scholar]
- 2. Andiman SE, Haynes RL, Trachtenberg FL, Billiards SS, Folkerth RD, Volpe JJ, Kinney HC (2010) The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol 20:803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banker BQ, Larroche JC (1962) Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol 7:386–410. [DOI] [PubMed] [Google Scholar]
- 4. Baud O, Daire JL, Dalmaz Y, Fontaine RH, Krueger RC, Sebag G et al (2004) Gestational hypoxia induces white matter damage in neonatal rats: a new model of periventricular leukomalacia. Brain Pathol 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayer SA, Altman J (2004) The Human Brain During the Third Trimester. CRC Press: London. [Google Scholar]
- 6. Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH et al (2008) Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol 18:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: boulder committee revisited. Nat Rev Neurosci 9:110–122. [DOI] [PubMed] [Google Scholar]
- 8. Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O et al (2003) Diffusion‐weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 112(Pt 1):1–7. [DOI] [PubMed] [Google Scholar]
- 9. deAzevedo LC, Fallet C, Moura‐Neto V, Daumas‐Duport C, Hedin‐Pereira C, Lent R (2003) Cortical radial glial cells in human fetuses: depth‐correlated transformation into astrocytes. J Neurobiol 55:288–298. [DOI] [PubMed] [Google Scholar]
- 10. Delcour M, Olivier P, Chambon C, Pansiot J, Russier M, Liberge M et al (2012) Neuroanatomical, sensorimotor and cognitive deficits in adult rats with white matter injury following prenatal ischemia. Brain Pathol 22:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng W, Pleasure J, Pleasure D (2008) Progress in periventricular leukomalacia. Arch Neurol 65:1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dommergues MA, Plaisant F, Verney C, Gressens P (2003) Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience 121:619–628. [DOI] [PubMed] [Google Scholar]
- 13. Engle WA (2004) Age terminology during the perinatal period. Pediatrics 114:1362–1364. [DOI] [PubMed] [Google Scholar]
- 14. Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg‐Didinger G et al (2011) Systemic inflammation disrupts the developmental program of white matter. Ann Neurol 70:550–565. [DOI] [PubMed] [Google Scholar]
- 15. Fayol L, Baud O, Monier A, Pellerin L, Magistretti P, Evrard P, Verney C (2004) Immunocytochemical expression of monocarboxylate transporters in the human visual cortex at midgestation. Brain Res Dev Brain Res 148:69–76. [DOI] [PubMed] [Google Scholar]
- 16. Folkerth RD (2005) Neuropathologic substrate of cerebral palsy. J Child Neurol 20:940–949. [DOI] [PubMed] [Google Scholar]
- 17. Ghosh A, Antonini A, McConnell SK, Shatz CJ (1990) Requirement for subplate neurons in the formation of thalamocortical connections. Nature 347(6289):179–181. [DOI] [PubMed] [Google Scholar]
- 18. Haynes RL, Baud O, Li J, Kinney HC, Volpe JJ, Folkerth DR (2005) Oxidative and nitrative injury in periventricular leukomalacia: a review. Brain Pathol 15:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haynes RL, Folkerth RD, Trachtenberg FL, Volpe JJ, Kinney HC (2009) Nitrosative stress and inducible nitric oxide synthase expression in periventricular leukomalacia. Acta Neuropathol 118:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haynes RL, Xu G, Folkerth RD, Trachtenberg FL, Volpe JJ, Kinney HC (2011) Potential neuronal repair in cerebral white matter injury in the human neonate. Pediatr Res 69:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294. [DOI] [PubMed] [Google Scholar]
- 22. Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ (2003) Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 143:171–179. [DOI] [PubMed] [Google Scholar]
- 23. Jakovcevski I, Zecevic N (2005) Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 49:480–491. [DOI] [PubMed] [Google Scholar]
- 24. Johnston MV, Trescher WH, Ishida A, Nakajima W (2001) Neurobiology of hypoxic‐ischemic injury in the developing brain. Pediatr Res 49:735–741. [DOI] [PubMed] [Google Scholar]
- 25. Judas M, Rados M, Jovanov‐Milosevic N, Hrabac P, Stern‐Padovan R, Kostovic I (2005) Structural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol 26:2671–2684. [PMC free article] [PubMed] [Google Scholar]
- 26. Kadhim H, Tabarki B, De Prez C, Rona A‐M, Sébire G (2002) Interleukin‐2 in the pathogenesis of perinatal white matter damage. Neurology 58:1125–1128. [DOI] [PubMed] [Google Scholar]
- 27. Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sébire G (2001) Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology 56:1278–1284. [DOI] [PubMed] [Google Scholar]
- 28. Kanold PO, Kara P, Reid RC, Shatz CJ (2003) Role of subplate neurons in functional maturation of visual cortical columns. Science 301(5632):521–525. [DOI] [PubMed] [Google Scholar]
- 29. Kanold PO, Luhmann HJ (2010) The subplate and early cortical circuits. Annu Rev Neurosci 33:23–48. [DOI] [PubMed] [Google Scholar]
- 30. Kinney HC, Haynes RL, Folkerth RD (2004) White matter lesions in the perinatal period. In: Developmental Neuropathology Pathology and Genetics , Neuropath Press. Golden JA, Harding BN (eds), pp. 156–170. Basel, Switzerland. [Google Scholar]
- 31. Kinney HC, Haynes RL, Xu G, Andiman SE, Folkerth RD, Sleeper LA, Volpe JJ (2012) Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann Neurol 71:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kostovic I, Goldman‐Rakic PS (1983) Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol 219:431–447. [DOI] [PubMed] [Google Scholar]
- 33. Kostovic I, Judas M (2002) Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec 267:1–6. [DOI] [PubMed] [Google Scholar]
- 34. Kostovic I, Judas M, Rados M, Hrabac P (2002) Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 12:536–544. [DOI] [PubMed] [Google Scholar]
- 35. Kostovic I, Lukinovic N, Judas M, Bogdanovic N, Mrzljak L, Zecevic N, Kubat M (1989) Structural basis of the developmental plasticity in the human cerebral cortex: the role of the transient subplate zone. Metab Brain Dis 4:17–23. [DOI] [PubMed] [Google Scholar]
- 36. Kostovic I, Molliver ME (1974) A new interpretation of the laminar development of cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. Anat Rec 178:395. [Google Scholar]
- 37. Kostovic I, Rakic P (1980) Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol 9:219–242. [DOI] [PubMed] [Google Scholar]
- 38. Kostovic I, Rakic P (1984) Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci 4:25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kostovic I, Rakic P (1990) Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297:441–470. [DOI] [PubMed] [Google Scholar]
- 40. Kostović I, Jovanov‐Milošević N, Radoš M, Sedmak G, Benjak V, Kostović‐Srzentić M et al (2012) Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct 219:231–253. [DOI] [PubMed] [Google Scholar]
- 41. Kriegstein AR, Gotz M (2003) Radial glia diversity: a matter of cell fate. Glia 43:37–43. [DOI] [PubMed] [Google Scholar]
- 42. Kultas‐Ilinsky K, Ilinsky IA, Verney C (2011) Glutamic acid decarboxylase isoform 65 immunoreactivity in the motor thalamus of humans and monkeys: gamma‐aminobutyric acidergic connections and nuclear delineations. J Comp Neurol 519:2811–2837. [DOI] [PubMed] [Google Scholar]
- 43. Larroque B, Marret S, Ancel PY, Arnaud C, Marpeau L, Supernant K et al (2003) White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. J Pediatr 143:477–483. [DOI] [PubMed] [Google Scholar]
- 44. Leviton A, Gressens P (2007) Neuronal damage accompanies perinatal white‐matter damage. Trends Neurosci 30:473–478. [DOI] [PubMed] [Google Scholar]
- 45. Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I (2003) White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res 28:215–223. [DOI] [PubMed] [Google Scholar]
- 46. Marin‐Padilla M (1995) Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a Golgi study. J Comp Neurol 357:554–572. [DOI] [PubMed] [Google Scholar]
- 47. Mathur A, Inder T (2009) Magnetic resonance imaging—insights into brain injury and outcomes in premature infants. J Commun Disord 42:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McConnell SK, Ghosh A, Shatz CJ (1989) Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245(4921):978–982. [DOI] [PubMed] [Google Scholar]
- 49. McQuillen PS, Ferriero DM (2005) Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol 15:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM (2003) Selective vulnerability of subplate neurons after early neonatal hypoxia‐ischemia. J Neurosci 23:3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller SP, Ferriero DM (2009) From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci 32:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC et al (2005) Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 147:609–616. [DOI] [PubMed] [Google Scholar]
- 53. Molliver ME, Kostovic I, van der Loos H (1973) The development of synapses in cerebral cortex of the human fetus. Brain Res 50:403–407. [DOI] [PubMed] [Google Scholar]
- 54. Monier A, Adle‐Biassette H, Delezoide AL, Evrard P, Gressens P, Verney C (2007) Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol 66:372–382. [DOI] [PubMed] [Google Scholar]
- 55. Monier A, Evrard P, Gressens P, Verney C (2006) Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol 499:565–582. [DOI] [PubMed] [Google Scholar]
- 56. Olivier P, Baud O, Evrard P, Gressens P, Verney C (2005) Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol 64:998–1006. [DOI] [PubMed] [Google Scholar]
- 57. Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC (2007) Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol 114:619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Platt MJ (2008) Long‐term outcome for very preterm infants. Lancet 371(9615):787–788. [DOI] [PubMed] [Google Scholar]
- 59. Rezaie P, Dean A (2002) Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology 22:106–132. [DOI] [PubMed] [Google Scholar]
- 60. Robinson S, Li Q, Dechant A, Cohen ML (2006) Neonatal loss of gamma‐aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg 104(Suppl.):396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rowitch DH, Kriegstein AR (2010) Developmental genetics of vertebrate glial‐cell specification. Nature 468(7321):214–222. [DOI] [PubMed] [Google Scholar]
- 62. Schmechel DE, Rakic P (1979) A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 156:115–152. [DOI] [PubMed] [Google Scholar]
- 63. Sfaello I, Daire JL, Husson I, Kosofsky B, Sebag G, Gressens P (2005) Patterns of excitotoxin‐induced brain lesions in the newborn rabbit: a neuropathological and MRI correlation. Dev Neurosci 27(2–4):160–168. [DOI] [PubMed] [Google Scholar]
- 64. Sizonenko SV, Kiss JZ, Inder T, Gluckman PD, Williams CE (2005) Distinctive neuropathologic alterations in the deep layers of the parietal cortex after moderate ischemic‐hypoxic injury in the P3 immature rat brain. Pediatr Res 57:865–872. [DOI] [PubMed] [Google Scholar]
- 65. Tahraoui SL, Marret S, Bodenant C, Leroux P, Dommergues MA, Evrard P, Gressens P (2001) Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol 11:56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Takashima S, Itoh M, Oka A (2009) A history of our understanding of cerebral vascular development and pathogenesis of perinatal brain damage over the past 30 years. Semin Pediatr Neurol 16:226–236. [DOI] [PubMed] [Google Scholar]
- 67. Vasung L, Huang H, Jovanov‐Milosevic N, Pletikos M, Mori S, Kostovic I (2010) Development of axonal pathways in the human fetal fronto‐limbic brain: histochemical characterization and diffusion tensor imaging. J Anat 217:400–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Verney C, Derer P (1995) Cajal‐Retzius neurons in human cerebral cortex at midgestation show immunoreactivity for neurofilament and calcium‐binding proteins. J Comp Neurol 359:144–153. [DOI] [PubMed] [Google Scholar]
- 69. Verney C, Milosevic A, Alvarez C, Berger B (1993) Immunocytochemical evidence of well‐developed dopaminergic and noradrenergic innervations in the frontal cerebral cortex of human fetuses at midgestation. J Comp Neurol 336:331–344. [DOI] [PubMed] [Google Scholar]
- 70. Verney C, Monier A, Fallet‐Bianco C, Gressens P (2010) Early microglial colonization of the human forebrain and possible involvement in periventricular white‐matter injury of preterm infants. J Anat 217:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Verney C, Pogledic I, Biran V, Adle‐Biassette H, Fallet‐Bianco C, Gressens P (2012) Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J Neuropathol Exp Neurol 71:251–264. [DOI] [PubMed] [Google Scholar]
- 72. Verney C, Rees S, Biran V, Thompson M, Inder T, Gressens P (2010) Neuronal damage in the preterm baboon: impact of the mode of ventilatory support. J Neuropathol Exp Neurol 69:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Volpe JJ (1996) Subplate neurons—missing link in brain injury of the premature infant? Pediatrics 97:112–113. [PubMed] [Google Scholar]
- 74. Volpe JJ (2001) Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50:553–562. [DOI] [PubMed] [Google Scholar]
- 75. Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hemalum‐phloxine staining of cystic (A–D) and non‐cystic (E,F) periventricular white matter injury (PWMI) cases. A,B. Large cysts surrounded of necrotic foci (arrows) (necrosis ++) and pallor. C. High magnification of a necrotic focus (arrow). D,F. High magnification of pallor (diffuse lesion). E. spots of necrosis +. Scale bars: A. 1 mm, B. 500 μm, C. 100 μm, D. 50 μm, E,F. 200 μm.
Figure S2. Microphotographs of glial fibrillary acidic protein (GFAP)+ astrocytes of the layers of the cerebral wall showed in columns: white matter (WM) (A,E,I,M,Q), the deep subplate (dSP) (B,F,J,N,R), the superficial subplate (sSP) (C,G,K,O,S) and the cortical plate (CP) (D,H,J,P,T) in very preterm cases (VPT) (A–L) and preterm (PT) cases (M–T) in control cases (A–D,M–P) and in non‐cystic periventricular white matter injury (PWMI) (E–H,Q–T) and cystic PWMI (I–L). Scale bar: 50 μm.
Figure S3. Density (number per unit area) (A,B) and size of cell bodies (in μm2) (C,D) of glial fibrillary acidic protein (GFAP)‐positive astrocytes in the superficial white matter (WM), the deep subplate (dSP), the superficial subplate (sSP) and the cortical plate (CP) in very preterm compared with preterm in control cases (A,C) and in non‐cystic periventricular white matter injury (PWMI) (B,D). **P < 0.01.
Figure S4. Olig2‐immunolabeling of the nucleus of cells of the oligodendrocytic lineage in the deep subplate of a very preterm control case (A) and a non‐cystic periventricular white matter injury (PWMI) case (B). Scale bar: 25 μm.


