Abstract
Increased tumor‐associated macrophages (TAMs) have been reported to be associated with poor prognosis in various tumors; however, the importance of TAMs in primary central nervous system lymphoma (PCNSL) has not been clarified. In 47 patients with PCNSL who were treated with high‐dose methotrexate (MTX) and radiotherapy, the relationships between the infiltration levels of TAMs and the clinicopathological parameters were analyzed. Univariate analysis of the Cox proportional hazards model using continuous scales revealed that increased CD68 positive (+) TAMs was significantly associated with inferior progression‐free survival (PFS) (P = 0.04), and trends were observed for the increased CD163+ TAMs and having shorter PFS (P = 0.05). However, increased TAMs were not associated with overall survival. Because TAMs are known to produce various cytokines, we examined the relationships between cerebrospinal fluid (CSF) cytokines and TAMs. CSF interleukin‐6 (IL‐6) and soluble IL‐2 receptor were not correlated with the infiltration rate of TAMs; however, CSF IL‐10 level was correlated with infiltration levels of CD68 and CD163+ TAMs. We also confirmed the expression of IL‐10 in CD68+ and CD163+ TAMs by double immunostaining analysis. Our results indicate that a high level of IL‐10 in CSF may be positively associated with the infiltration level of TAMs in PCNSLs.
Keywords: cerebrospinal fluid, IL‐10, primary central nervous system lymphoma, prognosis, tumor‐associated macrophage
Introduction
Primary central nervous system lymphoma (PCNSL) is an aggressive extra‐nodal non‐Hodgkin lymphoma (NHL) that involves the brain, spinal cord and eyes. PCNSL occurs in both immunocompetent and immunocompromised patients, as well as in approximately 4% of all brain tumors 21. High‐dose methotrexate (MTX)‐based treatment has elicited good responses in PCNSL patients, and combined modality therapy has produced response rates of up to 80–90% and a median overall survival (OS) of up to 4 years; however, PCNSL has a poor prognosis compared with other extranodal NHLs 21.
The tumor microenvironment consists of tumor cells and heterogeneous populations of stromal cells, such as fibroblasts, endothelial cells and infiltrating immune cells 7. Tumor–stromal interactions have been implicated in the regulation of tumor cell growth, metastasis and resistance to antitumor therapy 20. It is well known that numerous macrophages are present in tumor tissues, termed tumor‐associated macrophages (TAMs). However, the role of TAMs has been controversial, and many studies have shown the protumoral functions of TAMs and suggested that TAMs can contribute to tumor progression, angiogenesis, invasion and immunosuppression 20, 24, 32. Macrophages are differentiated cells of the mononuclear phagocytic lineage that are characterized by specific phenotypic characteristics and by the expression of specific markers 17. In humans, CD68, CD163, CD204, CD16, CD206, CD312 and CD115 are the major markers of macrophage lineage 17.
TAMs can be classified into two functionally distinct types: classically activated macrophages (M1), which promote inflammation, and alternatively activated macrophages (M2), which inhibit inflammation 11, 25. M1 macrophages produce high levels of inflammatory cytokines to limit tumor growth. In contrast, M2 macrophages release a considerable amount of immunosuppressive cytokines/chemokines and promote tumor growth. Differentiation into TAMs is induced by various factors produced by tumor cells 24, 32. Most TAMs are polarized to M2 macrophages, and these TAMs have enhanced expression of CD163 (hemoglobin scavenger receptor), CD204 (class A macrophage scavenger receptor), CD206 (mannose receptor C type 1), stabiline‐1 and arginase‐1 15, 20. However, CD68, which is a glycoprotein, is a pan‐monocyte/macrophage marker and is expressed in both M1 and M2 macrophages 15.
Numerous studies have shown that higher levels of CD68‐positive (CD68+) TAM infiltration in human malignant tumors are associated with lower survival rates 6, 28, 30, 31. In addition, recent studies have demonstrated that increased expression of M2 polarized TAMs is associated with worse clinical prognosis in various tumors, including glioma 4, 9, renal cell cancer 8 and T‐cell leukemia/lymphoma 12. With regard to malignant lymphoma, the importance of TAMs for poor prognosis has been reported for Hodgkin's lymphoma 28, 31, follicular lymphoma 2, 3 and systemic diffuse large B‐cell lymphoma 33, but the importance of TAMs in PCNSL has not been elucidated. In the present study, we examined the infiltration level of TAMs in PCNSL and analyzed the association of TAMs with clinical findings, cytokine levels and prognosis.
Materials and Methods
Patients
Patients with PCNSL were treated at the Department of Neurosurgery, University of Kobe, between August 2003 and July 2015. All patients were histologically newly diagnosed as PCNSL. To exclude systemic lymphoma, computed tomography scans of the chest and abdomen were performed before treatment in all patients, and patients with coexisting extracranial lymphoma and CNS lymphoma (secondary CNS lymphoma) were excluded. Therefore, all patients analyzed in the present study were stage I according to the Ann Arbor classification. Patients with T‐cell lymphoma, Burkitt lymphoma and human immunodeficiency virus (HIV)‐associated lymphoma were excluded from this study. In addition, patients who did not receive high‐dose MTX therapy and radiotherapy (RT) were excluded from this study. Furthermore, patients who were treated with steroids prior to surgery were excluded because steroids may change immunological microenvironment in the tumor. This study was approved by the ethical review board of our institutions (no. 1312).
Treatment and follow‐up evaluation
Of all of the 66 patients with PCNSLs, high‐dose MTX + RT were performed in 47 patients. MTX (3–6 g/m2) was administered as an intravenous infusion over a 4‐h period on day 1. Leucovorin rescue began 8 h after the MTX infusion. High‐dose MTX were performed for 1–4 cycles in each patient. In whole‐brain irradiation, both eyes were included in the RT field, and the radiation dose was typically 36 Gy (1.8 Gy × 20 fractions). The dose of boost irradiation for the tumor was 10–20 Gy. After completion of the treatments, the patients underwent a magnetic resonance imaging to evaluate the treatment response. Additional follow‐up scans were performed every 3–6 months or if clinically necessary. Progression‐free survival (PFS) was determined from the onset of treatment until relapse, disease progression or the last follow‐up evaluation. OS was determined from the onset of treatment until the last follow‐up evaluation or death from any cause.
Cerebrospinal fluid (CSF) examination and measurement of CSF interleukin (IL)‐10, IL‐6 and sIL‐2R
For the preoperative diagnosis, CSF was withdrawn from the patients via a lumbar puncture after obtaining informed consent when not contraindicated. A lumber puncture was performed on 39 patients. The CSF samples were immediately centrifuged and stored at −70°C. The CSF concentration of IL‐10 was measured using the human IL‐10 enzyme‐linked immunosorbent assay (ELISA) kit (KHC0101) (Life Technologies, Carlsbad, CA, USA) and a plate reader (Emax; Molecular Devices, Sunnyvale, CA, USA) at the SRL company (SRL, Tokyo, Japan). The limit of the quantification of IL‐10 was 2 pg/mL. To measure the CSF IL‐6 level, chemiluminescent enzyme immuno assay (CLEIA), using a two‐step sandwich method with a cartridge for IL‐6 measurement (human IL‐6 CLEIA Fujirebio, Fujirebio, Tokyo), was developed specifically for the fully automated chemiluminescent enzyme immunoassay system (Lumipulse, Fujirebio, Tokyo, Japan) at the SRL company (SRL). The limit of the quantification of IL‐6 was 0.3 pg/mL. The CSF concentration of sIL‐2R was measured using a sandwich ELISA test kit (Cellfree N IL‐2R; Kyowa Medex Co., Ltd., Tokyo, Japan) in the fully automated EIA analytical instruments (AP‐X, Kyowa Medex Co., Ltd.) at the SRL company (SRL). The limit of the quantification was 50 U/mL.
Immunohistochemistry
Antibodies to CD68, CD163, CD204 and IL‐10 were purchased from Dako Cytomation (Glostrup, Denmark), Abcam (Cambridge, MA, USA), TransGenic, Inc. (Kumamoto, Japan) and Abcam, respectively. Archived paraffin blocks of PCNSL patient tissues from the Department of Pathology of our hospital were used. The serial sections were deparaffinized, immersed in methanol with 3% hydrogen peroxide and heated in 0.01 M citrate buffer (pH 6.0) for 15 minutes by autoclaving (121°C, 2 atm). The sections were then incubated with primary antibodies at 4°C overnight. The sections were allowed to react with either a peroxidase‐conjugated anti‐rabbit or anti‐mouse IgG polyclonal antibody (Histofine Simple Stain MAX‐PO; Nichirei, Tokyo, Japan) for 60 minutes, and the reaction products were visualized by immersing the sections in 0.03% diaminobenzidine solution containing 2 mM hydrogen peroxide for 5 minutes.
Assessment of staining
Immunohistochemical staining of CD68, CD163 and CD204 was independently evaluated and scored by the neuro‐oncologist, KT, and pathologist, TH, who were blind to the clinical data and patient outcomes. The mean percentage of the immunoreactive area (% area) was calculated from five randomly selected fields per section under ×200 middle‐power magnification. The final % area value was calculated by averaging the average values of each observer. Images were analyzed using Image J 1.48 software (NIH, Bethesda, MD, USA). Only areas containing the tumor were analyzed, and areas without tumor cells, including areas with necrosis, fibrosis and blood vessels, were excluded from the analysis. The number of positive pixels was divided by the total number of pixels (negative + positive) in the analyzed area and multiplied by 100 to determine the percentage of positive pixels.
Double immunostaining analysis
The sections were deparaffinized and heated in 0.01 M citrate buffer (pH 6.0) for 15 minutes by autoclaving (121°C, 2 atm) and were treated with 0.5% Triton X‐100 in PBS. After incubation for 60 minutes with 5% bovine serum albumin in PBS, the sections were incubated with primary antibodies overnight at 4°C and then for 60 minutes at room temperature with Alexa‐488 conjugated goat antibodies to rabbit IgG (Abcam) and rhodamine‐conjugated goat antibodies to mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The specimens were examined using a confocal microscope LSM 700 (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
A Mann–Whitney U‐test was used to analyze the differences between the two groups. When we decided on each threshold of the CSF cytokines, we considered their median values. The correlation between two groups was assessed using the Spearman rank correlation coefficient test. Survival was estimated using Cox's proportional hazards model. PFS was defined as the time from the onset of treatment to relapse, disease progression or the last follow‐up evaluation. OS was defined as the time from the onset of treatment to the last follow‐up evaluation or death from any cause. The cut‐off values for % area of CD68+, CD163+ and CD204+ TAMs in the survival analysis were set at the mean values of the counts. A P‐value < 0.05 was considered to be statistically significant. Statistical analysis was performed using the JMP 11 software (JMP Institute, Cary, NC, USA).
Results
Clinicopathological findings
The clinicopathological findings in patients with PCNSL are summarized in Table 1. Forty‐seven patients with PCNSL were enrolled in this study. The mean age was 64 (range, 36–79), with 33 men and 14 women. Twenty‐five patients (53%) had a single tumor lesion, and 22 patients (47%) had multiple tumor lesions. The main tumor locations were the frontal (14 cases), parietal (two cases), temporal (nine cases) and occipital lobes (two cases); basal ganglia (seven cases); carpus callosum (four cases); brainstem (one case); cerebellum (four cases); and other brain regions (four cases). Thirty patients had a performance status (PS) of 0–2, and 17 patients had a PS of 3–4. The surgeries are shown in Table 1. The median follow‐up period was 25.2 months (range 7–138). At the end of the follow‐up period, 34 patients had experienced relapse, 20 patients were alive and 27 patients were dead. In the pretreatment CSF examination, the concentrations of IL‐10, IL‐6, sIL‐2R and lactate dehydrogenase (LDH) were tested. Median value of CSF IL‐10, IL‐6, IL‐10/IL‐6 ratio, sIL‐2R and LDH were 51 pg/mL, 6.7 pg/mL, 8.6‐fold, 200 U/mL and 34 mg/L, respectively.
Table 1.
Clinicopathological findings of patients with primary central nervous system lymphoma
| Clinical factors | Number of case (rate) | |
|---|---|---|
| Age (mean 64) | <60 | 13 (28%) |
| ≥60 | 34 (72%) | |
| Sex | Male | 33 (70%) |
| Female | 14 (30%) | |
| Tumor number | Single | 25 (53%) |
| Multiple | 22 (47%) | |
| Main tumor location | Frontal | 14 (29%) |
| Parietal | 2 (4%) | |
| Temporal | 9 (18%) | |
| Occipital | 2 (4%) | |
| Basal ganglia | 7 (18%) | |
| Corpus callosum | 4 (8%) | |
| Brainstem | 1 (2%) | |
| Cerebellum | 4 (10%) | |
| Others | 4 (8%) | |
| Performance status | 0–2 | 30 (63%) |
| 3–4 | 17 (37%) | |
| Surgery | Biopsy | 37 (79%) |
| Resection | 10 (21%) | |
| Relapse | Relapse (+) | 34 (73%) |
| Relapse (−) | 13 (27%) | |
| Status | Alive | 20 (42%) |
| Dead | 27 (58%) |
Infiltration of TAMs in PCNSL
Positive (CD68+) TAMs were diffusely infiltrated in PCNSL specimens (Figure 1A), and the rate of the CD68‐immunopositive area (CD68+ %area) ranged from 0.8 to 20.3% (mean 8.4%) (Figure 1B). In addition to CD68+ TAMs, CD163+ and CD204+ TAMs were diffusely infiltrated and present in PCNSL tissues (Figure 1A), and the mean CD163+ and CD204+ %areas were 7.7% (0.3–21.0%) and 3.3% (0.1–11.5%), respectively (Figure 1B). The rates of CD68+ and CD163+ TAMs were statistically higher than that of CD204+ TAMs (P < 0.001, P < 0.001, respectively). However, there was no difference between the rate of CD163+ TAMs and the rate of CD163+ TAMs. The level of CD68+ TAM infiltration was statistically correlated with CD163+ TAMs infiltration [correlation coefficient (r) = 0.885, P < 0.001] and CD204+ TAMs infiltration (r = 0.712, P < 0.001), and the level of CD163+ TAMs infiltration was statistically correlated with CD204+ TAMs infiltration (r = 0.635, P < 0.001). However, the infiltration levels of CD68+, CD163+ and CD204+ TAMs were not always identical (Figure 1C).
Figure 1.
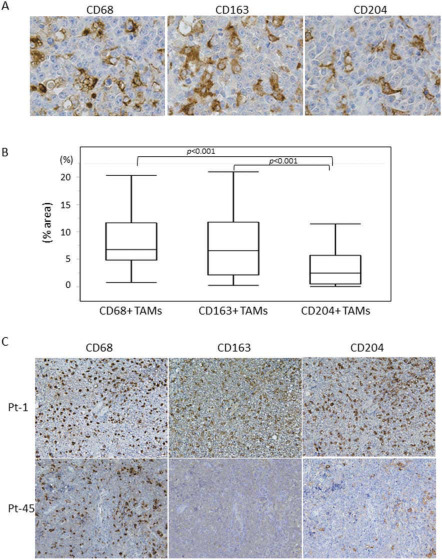
A. Immunohistochemical staining for CD68, CD163 and CD204 in primary central nervous system lymphoma (PCNSL) specimens (original magnification: ×400). B. Infiltration level (% area) of CD68+, CD163+ and CD204+ tumor‐associated macrophages (TAMs) in all PCNSLs. The rates of CD68+ and CD163+ TAMs were statistically higher than that of CD204+ TAMs. C. Immunohistochemical staining for CD68, CD163 and CD204 in PCNSL specimens obtained from two patients. Upper panels: patient—1, lower panels; patient—45. High infiltration of CD68+ TAMs is observed in both patients; however, the infiltration levels of CD163+ and CD204+ TAMs were different between these patients (original magnification: ×200).
Relationship between TAM infiltration and clinicopathological factors or prognosis
Relationship between the infiltration of CD68+, CD163+ and CD204+ TAMs and clinicopathological parameters are shown in Figure 2. Age (<60 or ≥60 years), number of tumor (single or multiple) and PS (0–2 or 3–4) were not associated with the infiltration levels of CD68+, CD163+ and CD204+ TAMs in PCNSLs. Of all 47 patients, the median OS was 36.4 months, and the median PFS was 30.8 months. Using a continuous scale for the scores in age, PS, CD68, CD163 and CD204 %area, univariate analysis revealed that number of tumor and CD68+ %area were significantly associated with inferior PFS (P = 0.02, P = 0.04, respectively); however, age, sex, PS, CD163+ %area and CD204+ %area were not associated with PFS (Table 2). But, trends were observed for the increased CD163+ TAMs and having shorter PFS (P = 0.05). And the univariate analysis revealed that only PS was significantly associated with inferior OS (P = 0.001). The multivariate analysis revealed that the number of tumor was independently associated with PFS (P = 0.03), and PS was independently associated with OS (P = 0.001) (Table 2); however, CD68, CD163 and CD204 %area were not associated with neither PFS nor OS.
Figure 2.
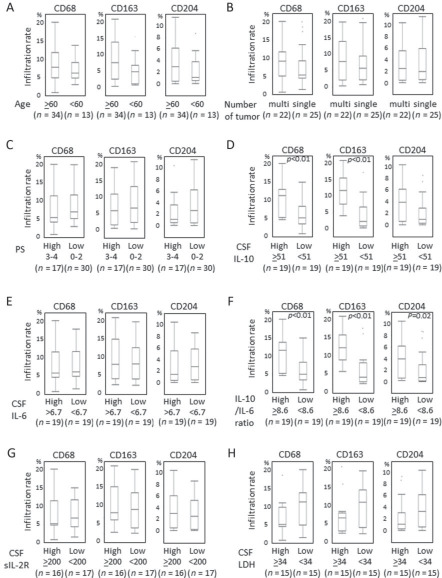
Relationship between clinicopathological or CSF factors and tumor‐associated macrophage (TAM) infiltration rate: (A) age, (B) number of tumor, (C) performance status (PS), (D) cerebrospinal fluid (CSF) interleukin‐10 (IL‐10), (E) CSF IL‐6, (F) CSF IL‐10/IL‐6 ratio, (G) CSF sIL‐2R, (H) CSF LDH. Higher CSF IL‐10 levels (≥51 pg/mL) were statistically correlated with a higher infiltration of CD68+ and CD163+ TAMs. Higher CSF IL‐10/IL‐6 ratio (≥8.6) was statistically correlated with a higher infiltration of CD68+, CD163+ and CD204+ TAMs.
Table 2.
Univariate and multivariate analyses. Abbreviations: CI = confidence interval; HR = hazard ratio; m = multiple; OS = overall survival; PFS = progression free survival; PS = performance status; s = single
| Parameters | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| PFS | OS | PFS | OS | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| CD68+ %area* | 1.08 (1.00–1.15) | 0.04 | 1.04 (0.96–1.12) | 0.34 | 1.14 (0.96–1.38) | 0.14 | 1.09 (0.87–1.35) | 0.39 |
| CD163+ %area* | 1.06 (0.998–1.12) | 0.05 | 1.03 (0.96–1.09) | 0.37 | 0.95 (0.81–1.12) | 0.56 | 0.93 (0.78–1.11) | 0.41 |
| CD204+ %area* | 1.06 (0.95–1.18) | 0.28 | 1.04 (0.91–1.16) | 0.52 | 1.01 (0.81–1.24) | 0.89 | 1.09 (0.87–1.35) | 0.45 |
| Age (years)* | 1.01 (0.96–1.05) | 0.63 | 1.01 (0.96–1.06) | 0.53 | 0.97 (0.92–1.03) | 0.42 | 0.98 (0.91–1.04) | 0.43 |
| Sex (male : female) | 1.53 (0.68–3.25) | 0.28 | 1.62 (0.64–3.81) | 0.29 | 1.08 (0.91–1.30) | 0.09 | 1.70 (0.59–4.68) | 0.31 |
| Number of tumor (m : s) | 2.36 (1.13–5.12) | 0.02 | 1.74 (0.80–3.93) | 0.15 | 1.00 (0.86–1.18) | 0.03 | 1.79 (0.68–4.87) | 0.23 |
| PS* | 1.51 (0.98–2.30) | 0.06 | 2.07 (1.33–3.21) | 0.001 | 1.57 (0.94–2.63) | 0.08 | 2.39 (1.39–4.22) | 0.001 |
*Continuous variables.
P‐value < 0.05 was considered to be statistically significant in bold.
Relationship between TAM infiltration and CSF cytokines
As TAMs are known to produce various cytokines, including IL‐10 and IL‐6, thus, we examined the relationship between the infiltration levels of TAMs and CSF IL‐10, IL‐6 and sIL‐2R. Higher CSF IL‐10 level (≥51 pg/mL) was statistically correlated with a higher infiltration of CD68+ TAMs (P = 0.005) and CD163+ TAMs (P < 0.001) (Figure 2D). However, higher CSF IL‐6 (≥6.7 pg/mL), CSF sIL‐2R (≥200 U/mL) and CSF LDH (≥34 mg/dL) were not correlated with higher infiltration levels of CD68+, CD163+ and CD204+ TAMs (Figure 2E, G, H). However, higher CSF IL‐10/IL‐6 ratio was statistically associated with a higher infiltration of CD68+ TAMs (P = 0.005), CD163+ TAMs (P < 0.001) and CD204+ TAMs (P = 0.02) (Figure 2F). Spearman rank correlation coefficient test using continuous scales revealed that CSF IL‐10 level was statistically correlated with the infiltration levels of CD163+ TAMs (P < 0.001, r = 0.70) and CD68+ TAMs (P = 0.005, r = 0.44), but not with the infiltration level of CD204+ TAMs (P = 0.07, r = 0.29) (Figure 3A–C). The levels of CSF IL‐6, sIL‐2R and LDH were not statistically correlated with any type of TAM (Figure 3D–F and data not shown). On the other hand, the IL‐10/IL‐6 ratio was statistically correlated with all type of TAM (Figure 3G–I). Next, we confirmed that both CD68+ and CD163+ TAMs expressed IL‐10 protein using double immunofluorescence staining (Figure 4 and Supporting Information Figure S1). Taken together, these results suggested that IL‐10 in CSF may be secreted from infiltrated CD68+ and CD163+ TAMs in PCNSLs, and CSF IL‐10 levels may be a marker of the infiltration levels of TAMs in PCNSLs.
Figure 3.
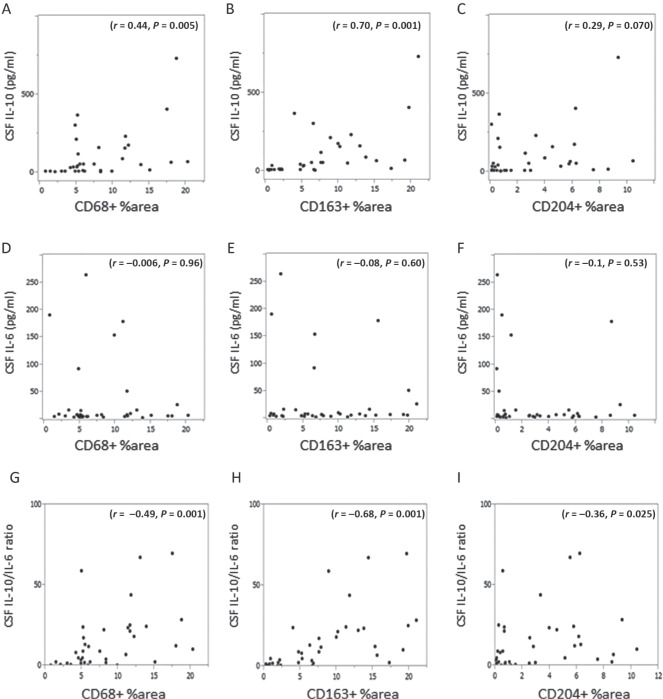
(A–I) Correlation between the infiltration levels of tumor‐associated macrophage (CD68+, CD163+, CD204+) and cerebrospinal fluid (CSF) interleukin (IL)‐10, IL‐6 and IL‐10/IL‐6 ratio (Spearman rank correlation coefficient test).
Figure 4.
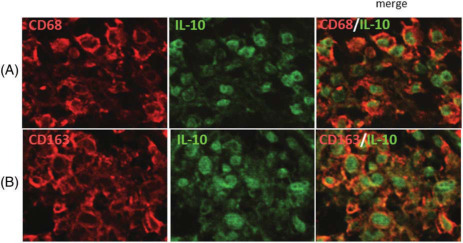
Double‐staining immunofluorescence analysis of CD68 and interleukin (IL)‐10 (A), and CD163 and IL‐10 (B) in primary central nervous system lymphoma (PCNSL) specimens. Sections are stained with the indicated antibodies. A merged image is shown in the right panels. Both CD68‐positive and CD163‐positive cells express IL‐10 protein. CD68 and CD163: rhodamine (red); IL‐10: Alexa‐488 (green) (original magnification: ×400).
Discussion
In the present study, we demonstrated that the increased infiltration of CD68+ TAMs was associated with poorer PFS in patients with PCNSL, and trends were observed for the increased CD163+ TAMs and poorer PFS; however, there was no association between increased TAMs and OS. The cut‐off value was set at the mean, and increased CD68+ TAMs and increased CD163+ TAMs were significantly associated with inferior PFS (Figure 3B, D), and increased CD68+ TAMs were significantly associated with inferior OS. In addition, TAM infiltration levels are correlated with CSF IL‐10 levels. Also, we confirmed that IL‐10 was expressed in CD68+ and CD163+ TAMs in PCNSL specimens using double‐immunostaining analysis. Taken together, these findings suggested that increased TAMs may be one of the useful prognostic factors in PCNSLs, and CSF IL‐10 levels may be a marker of infiltration levels of TAMs in PCNSLs.
Relationship between TAMs and clinical prognosis in tumors
Numerous studies found that a higher level of TAM infiltration is associated with a poorer clinical outcome in various tumors, such as thyroid, lung and hepatocellular cancers, and hematopoietic malignancies 6, 8, 9, 12, 13, 18, 22, 28, 29, 30, 31, 37. Based on one meta‐analysis, 67% of studies showed a correlation between macrophage density and poor patient prognosis 1, and recent studies have further supported this conclusion. Steidl et al reported that an increased number of CD68+ TAMs was highly associated with shortened OS and PFS and with an increased likelihood of relapse after autologous hematopoietic stem cell transplantation in patients with classic Hodgkin's lymphoma 28. Tan et al described similar results in which increased CD68 or CD163 expression in classic Hodgkin's lymphoma was a significant independent predictor of decreased failure‐free survival and OS in the multivariate analysis 31. However, other reports of cancers, such as gastric, colon and prostate cancer, showed that greater TAM infiltration results in a better outcome 11, 24.
With regard to PCNSL, there has only been one report about TAMs and prognosis. Komohara et al analyzed the relationship between M2 TAMs and prognosis in 43 patients with PCNSL and reported that no significant correlation was found between the number of CD163+ or CD204+ TAMs and OS 10. However, detailed clinical data and the treatment regimen of the patients were not shown. In addition, they have not analyzed the relationship between CD68+ TAMs and prognosis. In their report, they mainly focused on the association between the infiltration level of TAMs and STAT3 expression in PCNSLs.
Because CD68 is a pan‐macrophage marker, the rate of immunoreactive area of CD68 was usually higher compared with CD163 and CD204. Although both CD163 and CD204 are considered to be M2 macrophage markers, the rate of the immunoreactive area of CD163 was higher compared with CD204 in the present study. In addition, CD163+ TAMs was relatively associated with poorer PFS; however, CD204+ TAMs was not associated with PFS at all. The reason for these results is not clear; however, Komohara et al reported similar results in patients with renal cell carcinoma, in which the number of CD163+ TAMs, but not CD204+ TAMs, was significantly associated with poor clinical prognosis 8. Taken together, these results indicated that CD68 is a better prognostic marker than CD163 or CD204 for the patients with PCNSL. The scores made by the two observers were highly similar and statistically correlated. However, the scoring of large resection samples had relatively low reproducibility compared with the scoring of small biopsy samples because infiltration of TAMs was not homogenous in large resection samples.
M2 macrophages
Although the previous literature has equated TAMs with a M2‐like phenotype, it has become increasingly clear that TAMs consist of multiple distinct populations with overlapping features that are dependent on a variety of factors, including location, microenvironment, tumor type and tumor stage 11, 14, 15, 23, 24, 25, 26. However, it is still largely unknown whether TAM diversity results from the maturation of unique monocytic precursors or from differences in microanatomical factors. However, in general, TAMs have “trophic” immunomodulatory M2‐like phenotypes similar to those involved in the development processes. Although both CD163 and CD204 are considered to be markers for M2 macrophages, some differences do exist between CD163+ and CD204+ TAMs 10. CD163 is the acute phase‐regulated macrophage protein and was identified as a receptor that scavenges hemoglobin by mediating the endocytosis of haptoglobin–hemoglobin complexes 16. CD204 is the class A macrophage scavenger receptor, which has been implicated in many macrophage‐associated physiological and pathological processes 19. Recently, Qian et al classified TAMs into six types: activated macrophages, immunosuppressive macrophages, angiogenic macrophages, invasive macrophages, perivascular macrophages and metastasis‐associated macrophages 24. However, not all of these macrophage types of TAMs show the phenotype of M2 macrophages.
TAMs and IL‐10
It is well known that TAMs produce various angiogenic and invasive factors, such as vascular endothelial growth factor, IL‐8, basic fibroblast growth factor and matrix metalloprotease, which promote the formation of intratumoral blood vessels and invasion into peripheral tissues 15, 24, 25, 26. Furthermore, TAMs produce immunosuppressive factors, including IL‐10, prostaglandin E2 and indoleamine 2,3‐dioxygenase, which contribute to the formation of an immunosuppressive cancer‐specific environment 11, 25, 35. IL‐10 protein secreted from TAMs activates the JAK/STAT3 pathway in tumor cells, and recent studies have identified STAT3 as an important molecule that mediates tumor‐induced immunosuppression on many levels 35. STAT3 is not only a potent negative regulator of T helper 1 cell‐mediated inflammation but is also an important activator of many genes that are crucial for immunosuppression 34, 35. In addition to the immunosuppressive role of STAT3, STAT3 is crucial for tumor cell proliferation, survival angiogenesis and invasion 5, indicating that the IL‐10–STAT3 pathway may constitute a direct link between tumor progression and immunosuppression 35, 36. We previously reported that CSF IL‐10 levels are increased in patients with PCNSL compared with other brain tumors 27. In the present study, we revealed that the infiltration levels of TAMs are associated with CSF IL‐10 levels and TAMs highly express IL‐10 protein. Thus, we speculate that infiltrating TAMs in PCNSL secretes IL‐10 protein into the CSF, which activates the STAT3 pathway in tumor cells in a paracrine‐dependent manner.
Limitations of the study
A limitation of this study is the small number of patients with PCNSL. However, PCNSL is a rare disease and some patients were excluded in this study due to T‐cell lymphoma, other treatment regimens, preoperative steroid administration and HIV infection. Another limitation was that this study was retrospective and was performed at a single institute. In addition, the serum concentrations of IL‐10 and IL‐6 were not examined in this study. The CSF IL‐10 and IL‐6 index {(CSF/serum IL‐10 or IL‐6 quotient)/(CSF/serum albumin quotient)} is important for evaluating intrathecal synthesis. Although we showed that CD163+ and CD68+ TAMs expressed IL‐10 based on the immunohistochemical examination, we propose that the CSF IL‐10 index is better than the CSF IL‐10 value to evaluate intrathecal synthesis. However, the present study is the first report to describe the association of TAMs with clinical prognosis in patients with PCNSL.
In conclusion, our results indicate that increased infiltration of TAMs in PCNSLs may be associated with poorer prognosis. Moreover, our results indicate that CSF IL‐10 level may be a potential marker of infiltration of TAMs in PCNSLs. However, this study cohort was relatively small, and the correlations and associations identified were not very strong. Therefore, further evaluation of TAMs should be considered in a large prospective study. Patients with increased TAMs may benefit from more intensive chemotherapy or novel agents that are designed to disrupt TAM–tumor cell interactions.
Conflict of Interest
None declared.
Authors' Contributions
TS designed and performed the experiments, analyzed the data and wrote the manuscript. KT performed the assessment of immunohistochemical (IHC) experiments and statistical analysis. TM performed the experiments. KM and MN provided the clinical samples. SN, HT and HN performed the experiments. TH analyzed the IHC experiments. TI performed the pathological diagnosis. EK provided the clinical samples and interpreted the data.
Supporting information
Figure S1. Double‐staining immunofluorescence analysis of CD68 and IL‐10 (A) and CD163 and IL‐10 (B) in other two PCNSL specimens. Sections are stained with the indicated antibodies. A merged image is shown in the right panels. Both CD68‐positive and CD163‐positive cells express IL‐10 protein. CD68 and CD163: rhodamine (red); IL‐10: Alexa‐488 (green) (original magnification: ×400).
Acknowledgments
We thank Kumi Takata and Naoko Satoh for performing the immunohistochemical experiments. In addition, we thank all of the doctors, nurses and medical staffs who took care of the patients with PCNSL at Kobe University Hospital. This study was supported in part by a Grant‐in‐Aid for Scientific Research to EK (25293309), TS (25462258) and KM (26462158), and by the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- 1. Bingle L, Brown NJ, Lewis CE (2002) The role of tumour‐associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196:254–265. [DOI] [PubMed] [Google Scholar]
- 2. Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F et al (2008) High numbers of tumor‐associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA‐GOELAMS FL‐2000 trial. J Clin Oncol 26:440–446. [DOI] [PubMed] [Google Scholar]
- 3. Clear AJ, Lee AM, Calaminici M, Ramsay AG, Morris KJ, Hallam S et al (2010) Increased angiogenic sprouting in poor prognosis FL is associated with elevated numbers of CD163+ macrophages within the immediate sprouting microenvironment. Blood 115:5053–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding P, Wang W, Wang J, Yang Z, Xue L (2014) Expression of tumor‐associated macrophage in progression of human glioma. Cell Biochem Biophys 70:1625–1631. [DOI] [PubMed] [Google Scholar]
- 5. Frank DA (2007) STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett 251:199–210. [DOI] [PubMed] [Google Scholar]
- 6. Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y et al (2011) Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol 179:1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, Takeya M (2011) Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci 102:1424–1431. [DOI] [PubMed] [Google Scholar]
- 9. Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, Nakagawa T et al (2012) Importance of direct macrophage‐tumor cell interaction on progression of human glioma. Cancer Sci 103:2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komohara Y, Horlad H, Ohnishi K, Ohta K, Makino K, Hondo H et al (2011) M2 macrophage/microglial cells induce activation of Stat3 in primary central nervous system lymphoma. J Clin Exp Hematop 51:93–99. [DOI] [PubMed] [Google Scholar]
- 11. Komohara Y, Jinushi M, Takeya M (2014) Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 105:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Komohara Y, Niino D, Saito Y, Ohnishi K, Horlad H, Ohshima K, Takeya M (2013) Clinical significance of CD163(+) tumor‐associated macrophages in patients with adult T‐cell leukemia/lymphoma. Cancer Sci 104:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T et al (2013) Expression of M2‐polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat 12:259–267. [DOI] [PubMed] [Google Scholar]
- 14. Lawrence T, Natoli G (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11:750–761. [DOI] [PubMed] [Google Scholar]
- 15. Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22:231–237. [DOI] [PubMed] [Google Scholar]
- 16. Moestrup SK, Moller HJ (2004) CD163: a regulated hemoglobin scavenger receptor with a role in the anti‐inflammatory response. Ann Med 36:347–354. [DOI] [PubMed] [Google Scholar]
- 17. Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niino D, Komohara Y, Murayama T, Aoki R, Kimura Y, Hashikawa K et al (2010) Ratio of M2 macrophage expression is closely associated with poor prognosis for Angioimmunoblastic T‐cell lymphoma (AITL). Pathol Int 60:278–283. [DOI] [PubMed] [Google Scholar]
- 19. Ohnishi K, Komohara Y, Fujiwara Y, Takemura K, Lei X, Nakagawa T et al (2011) Suppression of TLR4‐mediated inflammatory response by macrophage class A scavenger receptor (CD204). Biochem Biophys Res Commun 411:516–522. [DOI] [PubMed] [Google Scholar]
- 20. Pollard JW (2004) Tumour‐educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4:71–78. [DOI] [PubMed] [Google Scholar]
- 21. Prodduturi P, Bierman PJ (2012) Current and emerging pharmacotherapies for primary CNS lymphoma. Clin Med Insights Oncol 6:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV et al (2013) Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res 19:3776–3786. [DOI] [PubMed] [Google Scholar]
- 23. Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR et al (2011) CCL2 recruits inflammatory monocytes to facilitate breast‐tumour metastasis. Nature 475:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quatromoni JG, Eruslanov E (2012) Tumor‐associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res 4:376–389. [PMC free article] [PubMed] [Google Scholar]
- 26. Ramanathan S, Jagannathan N (2014) Tumor associated macrophage: a review on the phenotypes, traits and functions. Iran J Cancer Prev 7:1–8. [PMC free article] [PubMed] [Google Scholar]
- 27. Sasayama T, Nakamizo S, Nishihara M, Kawamura A, Tanaka H, Mizukawa K et al (2012) Cerebrospinal fluid interleukin‐10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro‐Oncol 14:368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T et al (2010) Tumor‐associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med 362:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suyani E, Sucak GT, Akyurek N, Sahin S, Baysal NA, Yagci M, Haznedar R (2013) Tumor‐associated macrophages as a prognostic parameter in multiple myeloma. Ann Hematol 92:669–677. [DOI] [PubMed] [Google Scholar]
- 30. Takayama H, Nishimura K, Tsujimura A, Nakai Y, Nakayama M, Aozasa K et al (2009) Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette‐Guerin instillation. J Urol 181:1894–1900. [DOI] [PubMed] [Google Scholar]
- 31. Tan KL, Scott DW, Hong F, Kahl BS, Fisher RI, Bartlett NL et al (2012) Tumor‐associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood 120:3280–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA, Sarukhan A (2014) Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Front Immunol 5:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wada N, Zaki MA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y et al (2012) Tumour‐associated macrophages in diffuse large B‐cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology 60:313–319. [DOI] [PubMed] [Google Scholar]
- 34. Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM (2004) Interleukin‐10 suppression of myeloid cell activation—a continuing puzzle. Immunology 113:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7:41–51. [DOI] [PubMed] [Google Scholar]
- 36. Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Cheng S, Zhang M, Zhen L, Pang D, Zhang Q, Li Z (2013) High‐infiltration of tumor‐associated macrophages predicts unfavorable clinical outcome for node‐negative breast cancer. PLoS ONE 8:e76147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Double‐staining immunofluorescence analysis of CD68 and IL‐10 (A) and CD163 and IL‐10 (B) in other two PCNSL specimens. Sections are stained with the indicated antibodies. A merged image is shown in the right panels. Both CD68‐positive and CD163‐positive cells express IL‐10 protein. CD68 and CD163: rhodamine (red); IL‐10: Alexa‐488 (green) (original magnification: ×400).


