Abstract
Epithelioid glioblastoma is among the rarest variants of glioblastoma and is not formally recognized in the World Health Organization classification; it is composed of monotonous, discohesive sheets of small, round cells with eccentric nuclei and eosinophilic cytoplasm devoid of cytoplasmic stellate processes, showing the retention of nuclear staining of INI‐1 protein. Here, we report a case involving a 22‐year‐old man with a right occipital lobe tumor, which comprised mainly epithelioid tumor cells with a small area of diffusely infiltrating less atypical astrocytoma cells showing a lower cell density. Array comparative genomic hybridization separately performed for each histologically distinct component demonstrated eight shared copy number alterations (CNAs) and three CNAs observed only in epithelioid cells; one of the latter was a homozygous deletion of a tumor suppressor gene, LSAMP, at 3q13.31. BRAF V600E mutation was observed both in epithelioid tumor cells and in diffusely infiltrating less atypical astrocytoma cells. Our findings suggest that the regional loss of LSAMP led to the aggressive nature of epithelioid cells in the present case of epithelioid glioblastoma.
Keywords: array CGH, BRAF, epithelioid glioblastoma, LSAMP
Introduction
Glioblastoma, the highest‐grade tumor in the spectrum of diffusely infiltrating astrocytic neoplasms, is morphologically heterogeneous and harbors a great variety of genetic alterations. Various histological subtypes are recognized, including giant cell, gemistocytic, small cell and granular cell forms 13.
Epithelioid glioblastoma is among the rarest variants of glioblastoma, and is not formally recognized in the World Health Organization (WHO) classification of the central nervous system (CNS) tumors 13, 14, 15. Epithelioid glioblastomas are composed of monotonous, patternless sheets of small, round cells with laterally positioned nuclei and eosinophilic cytoplasm 15. Although epithelioid glioblastomas share similar histological features with rhabdoid glioblastomas, and these terms have been used interchangeably in previously published reports 2, 10, 18, 21, 28, Kleinschmidt‐DeMasters et al proposed that polyphenotypic immunohistochemical expression and focal loss of INI‐1 protein in the rhabdoid areas should differentiate rhabdoid glioblastomas from epithelioid glioblastomas 13. Clinically, epithelioid glioblastomas tend to occur in the first three decades of life, and the prognosis seems to be poorer than in ordinary glioblastomas 4, 14.
BRAF V600E mutation has been found commonly in certain CNS tumors, including pleomorphic xanthoastrocytomas (PXA) (60%), PXA with anaplastic features (60%), gangliogliomas (20%–60%), and extracerebellar pilocytic astrocytomas (20%) 6, 8, 16, 31. Recently, BRAF V600E mutation was also found in epithelioid glioblastomas at the relatively high frequency of 54% (7/13 cases) 14.
In the same series of epithelioid glioblastoma reported by Kleinschmidt‐DeMasters et al, except for a single case of secondary epithelioid glioblastoma with IDH1 mutation, a molecular signature of the common type of secondary glioblastomas, which progressed from oligoastrocytoma WHO grade II, the others were clinically primary/de novo epithelioid glioblastomas and none of them were positive for IDH1 mutation 14. However, original imaging reports of some primary cases with BRAF V600E mutation suggested preexisting low‐grade or long‐standing tumors, despite the fact that possible precursor lesions could not be identified in the resected specimens 14.
In this report, we present a case of primary epithelioid glioblastoma with small areas either of diffusely infiltrating less atypical astrocytoma cells with a lower cell density or spindle cells. We investigated genetic associations among the histologically distinct tumor cells, performing molecular and cytogenetic analyses separately for each component.
Materials and Methods
Case history
A 22‐year‐old man presented with headache. Magnetic resonance imaging (MRI) showed subcortical hemorrhage in the right occipital lobe, and the patient was treated with conservative management. The first follow‐up MRI at 4 months showed enlargement of the hematoma without clear evidence of a tumor; however, the second follow‐up MRI at 6 months disclosed a heterogenously enhanced mass with hemorrhagic components and prominent surrounding edema in the right occipital lobe (Figure 1). A subtotal resection of the mass was then performed. After establishing a diagnosis, extended focal radiotherapy and chemotherapy with temozolomide and interferon‐beta were delivered. From 5 months after surgery, local recurrence, extensive dissemination in the intracranial and spinal subarachnoid space, and metastases to vertebral bodies, the right thoracic wall, right lung and liver were found consecutively. Palliative radiotherapy was appropriately administered, and the patient was still alive 24 months after the diagnosis. This study was approved by the Ethics Committee of Gunma University.
Figure 1.
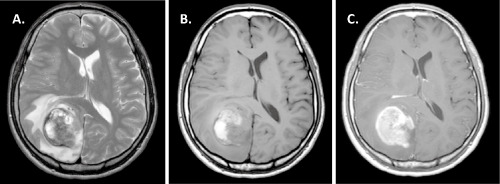
Magnetic resonance imaging. A. Axial T2‐weighted images demonstrated a heterogenous mass with high‐low signal intensity measuring 50 mm in the right occipital lobe. Prominent peritumoral edema and midline shift were seen. B. On axial T1‐weighted images, heterogenous high signal intensity suggesting a hemorrhagic component was seen. C. Axial gadolinium‐enhanced T1‐weighted images showed homogeneous enhancement with strong ring‐like enhancement.
Conventional histological analysis
Tumor sections were fixed with 10% formalin and embedded in paraffin. Three‐micrometer‐thick tissue sections were cut and stained with hematoxylin and eosin (H&E). Some sections were double‐stained with H&E and periodic acid‐methenamine silver. Immunohistochemical staining was performed on formalin‐fixed, paraffin‐embedded sections. Four primary antibodies directed against the following antigens were applied: glial fibrillary acidic protein (GFAP) (1:5000) 24, Olig2 (1:5000) 37, vimentin (V9; 1:200; Dako, Glostrup, Denmark), cytokeratin (CAM5.2; 1:5; BD Bioscience, San Jose, CA, USA), BAF47/INI1 (BAF47; 1:100; BD Bioscience) and Ki‐67 (MIB‐1; 1:100; Dako). For coloration, a commercially available biotin‐streptavidin immunoperoxidase kit (Histofine, Nichirei, Tokyo, Japan) and diaminobenzidine were employed.
DNA extraction
DNA was extracted from paraffin‐embedded sections separately from an area with epithelioid tumor cells, an area of less atypical astrocytoma cells with a lower cell density and a spindle cell area, as previously described 12, 26.
Array comparative genomic hybridization (CGH)
Array CGH analysis was carried out using a 4 × 180 K CGH oligonucleotide microarray (AgilentTechnologies, Santa Clara, CA, USA), as described previously 12, 26. The log2 ratio of <−1.0 at the region of interest was considered to represent homozygous deletion, and a value of −1.0 to −0.2 was considered to represent heterozygous deletion 32.
Differential polymerase chain reaction (PCR)
A search for homozygous deletion in intron 3 of the LSAMP gene was carried out employing differential PCR, as previously reported 29, using two primer sets (fragments #1 and #2) located in intron 3 of the LSAMP gene with the CF gene sequence as a reference 33. The primer sequences were as follows: 5′‐AAC AGG CCG TGA ATA AAC ACA‐3′ (sense) and 5′‐ACC AAA TGC GGA TGA ATA GAG A‐3′ (antisense) for fragment #1 (PCR product, 110 bp), and 5′‐CAA TGG ATA GGC AAA ATC AGG A‐3′ (sense) and 5′‐TTC AAG CCT TGT TGC TTG CTT‐3′ (antisense) for fragment #2 (PCR product, 105 bp). The mean signal ratio of fragments #1 and #2 to CF of normal DNA (14 non‐tumoral tissues from healthy individuals) was 0.96 and 0.98, with standard variations of 0.16 and 0.10, respectively. Samples with a ratio of ≤0.2 were considered to show homozygous deletion 23.
Fluorescence in situ hybridization (FISH) analysis
Dual‐probe hybridization using an intermittent microwave irradiation method was applied to paraffin‐embedded, 4‐μm‐thick tissue sections, as described previously 36. A 4q34.3–4q35.1 probe was prepared from a bacterial artificial chromosome (BAC) clone, RP11‐713O21, labeled with ENZO Orange‐dUTP (Abbott Molecular Inc., Des Plaines, IL, USA), and a 4p15.2 probe was prepared from a BAC clone RP11–758 M15 labeled with ENZO Green‐dUTP (Abbott Molecular Inc.).
Reverse transcription (RT)‐differential PCR
An RNeasy FFPE kit (Qiagen, Hilden, Germany) was used to isolate total RNA from paraffin‐embedded sections, separately from an area with epithelioid tumor cells and from an area of diffusely infiltrating less atypical astrocytoma cells with a lower cell density. First‐strand cDNA was synthesized from total RNA using the SuperScript VILO cDNA Synthesis Kit (Life Technologies Carlsbad, CA, USA). Then, differential PCR was performed as previously reported 11, using a primer set covering exon boundary 3–4 of the LSAMP mRNA with the GAPDH mRNA sequence as a reference 20. The primer sequences were as follows: 5′‐CCT GAA CCT GTT ATC ACC TGG A‐3′ (sense) and 5′‐GAC CTC GTT GGC AGC TTT G‐3′ (antisense) for LSAMP (PCR product, 135 bp). Human brain‐derived BioBank cDNA (PrimerDesign, Southampton, UK) was used as a normal control.
Direct DNA sequencing for BRAF and IDH1/2 mutations
Genomic DNA separately extracted from an area with epithelioid tumor cells and a small area of diffusely infiltrating less atypical astrocytoma cells with a lower cell density was amplified by PCR using primers for exon 15 of the BRAF gene and exon 4 of the IDH1 and IDH2 genes. The primer sequences were previously reported 1, 31, 34. PCR products were sequenced on a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) with the Big Dye Terminator v.1.1 Cycle Sequencing Kit (Applied Biosystems) following standard procedures.
Results
Pathological findings
On histopathologic examination, the tumor was composed mainly of discohesive sheets of medium‐sized, uniform cells with round cytoplasmic contours and eosinophilic cytoplasm devoid of cytoplasmic stellate processes (Fig 2A,B). The nuclei were large, mostly eccentric, and variable in shape: round, ovoid, reniform, crescent or ring‐like (Figure 2B). The cells often had less defined hyalinized cytoplasmic inclusions, some of which contained fine basophilic granules (Figure 2B). Small cytoplasmic vacuoles were also found in some tumor cells. Multiple mitotic figures were found. Interspersed neuropil was absent (Figure 2B). Prominent hemorrhage and extensive coagulative necrosis were seen (Figure 2C left). Microvascular proliferation was absent. Tumor‐cell invasion into the vascular wall was observed (Figure 2C right). These characteristics are consistent with the descriptions of epithelioid glioblastoma by Kleinschmidt‐DeMasters et al 13, 14. These epithelioid tumor cells accounted for more than 90% of the tumor tissue.
Figure 2.
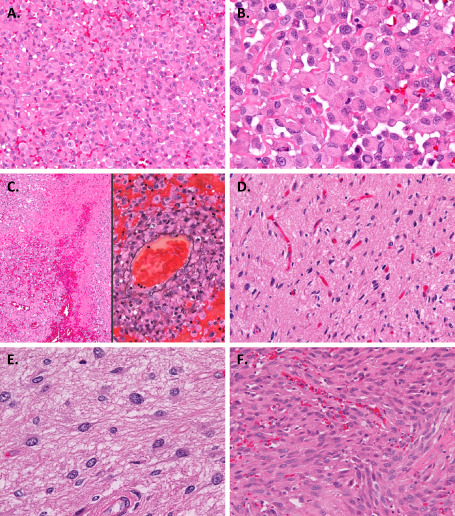
Microscopic appearance. A. The tumor was composed mainly of discohesive sheets of medium‐sized uniform cells. B. The tumor cells had rounded cytoplasmic contours and eosinophilic cytoplasm. The nuclei were large and mostly eccentric, and variable in shape: round, ovoid, reniform, crescent or ring‐like. The cells often had hyalinized cytoplasmic inclusions, some of which contained fine basophilic granules. C. Hemorrhage and coagulative necrosis (left). Tumor‐cell invasion into the vascular wall demonstrated by staining with both hematoxylin and eosin and periodic acid‐methenamine silver (right). D, E. A small area of the tumor displayed the proliferation of well‐differentiated neoplastic fibrillary astrocytes. F. A small part of the tumor extending within the subarachnoid space showed spindle‐shaped cells. Original magnification: ×100 (C left), ×200 (A, C right, D, F), ×400 (B, E).
In addition, a small area of the tumor displayed the proliferation of well‐differentiated neoplastic fibrillary astrocytes in the background of a loosely structured matrix (Figure 2D,E). Histological features of pilocytic astrocytoma or pleomorphic xanthoastrocytoma, such as bipolar piloid processes, a microcystic structure, cellular pleomorphism, Rosenthal fibers or eosinophilic granular bodies, were not obvious. Mitotic figures were absent.
Furthermore, another small part of the tumor extending within the subarachnoid space showed spindle‐shaped cells (Figure 2F). These cells had discernible nucleoli, and three mitoses were detected in 10 high‐power fields.
GFAP immunoreactivity was identified in a limited number of epithelioid cells (Figure 3A), but was diffusely obserbed in less atypical astrocytoma cells (Figure 3B). The cytoplasm of epithelioid cells showed diffuse and strong staining with vimentin (Figure 3C). Nuclear Olig2 staining was noted only in a small number of epithelioid cells (Figure 3D). Cytoplasmic immunostaining of cytokeratin CAM5.2 was not identified in epithelioid cells (Figure 3E). The retention of nuclear staining of INI‐1 was observed throughout the specimen (Figure 3F). MIB‐1 labeling indexes of epithelioid tumor cells, less atypical astrocytoma cells with a lower cell density, and spindle tumor cells were 22.6%, 2.4% and 8.3%, respectively.
Figure 3.
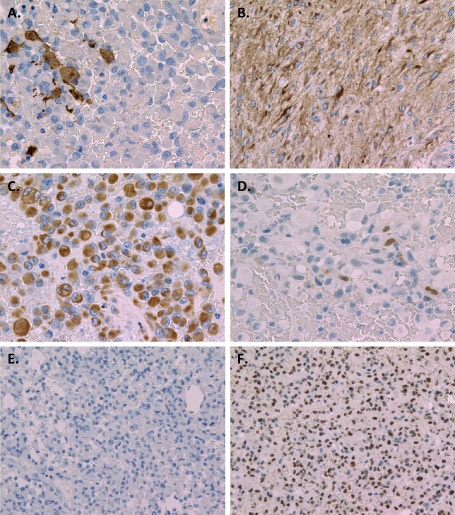
Immunohistochemistry. Glial fibrillary acidic protein immunoreactivity was identified in a limited number of epithelioid cells (A), but was diffusely observed in less atypical astrocytoma cells (B). C. Diffuse and strong vimentin staining was seen in the cytoplasm of epithelioid cells. D. Nuclear Olig2 staining was found only in a small number of epithelioid cells. E. Cytoplasmic immunostaining of CAM5.2 was not identified in epithelioid cells. F. Retention of nuclear staining of INI‐1 was observed in epthelioid tumor cells. Original magnification: ×200 (D, E, F), ×400 (A, B, C).
Array CGH
Array CGH showed 11 copy number alterations (CNAs). Eight CNAs were detected in all three histological areas analyzed (Table 1), while the others (a homozygous deletion in LSAMP, a heterozygous deletion in ODZ3, and a heterozygous deletion in LRP1B) were found only in epithelioid tumor cells (Figure 4A–C). The homozygous deletion in LSAMP was in intron 3, and its size was 43 kb.
Table 1.
Abberations common to all histological areas analyzed by array comparative genomic hybridazation
| Chromosomal location | Loc. Start* | Loc. Stop* | Length (Kb) | Aberration** | Gene names |
|---|---|---|---|---|---|
| 3q26.1 | 162514534 | 162619141 | 104.6 | Deletion (−1.849218) | |
| 4q13.2 | 69392545 | 69462438 | 69.9 | Deletion (−1.831354) | UGT2B17, UGT2B15 |
| 6p22.1 | 29854870 | 29896710 | 41.8 | Deletion (−1.236831) | HLA‐H, HCG2P7, HCG4P6 |
| 8p11.22 | 39237438 | 39345479 | 108.0 | Amplification (0.671442) | ADAM5P, ADAM3A |
| 9p21.3 | 21944037 | 22176231 | 232.2 | Deletion (−1.62978) | C9orf53, CDKN2A, CDKN2BAS, CDKN2B |
| 15q11.1–q11.2 | 20575646 | 21933378 | 1357.7 | Amplification (0.937772) | GOLGA6L6, GOLGA8C, BCL8, POTEB, NF1P1, LOC646214 |
| 20p12.1 | 14903016 | 15015311 | 112.3 | Deletion (−0.880196) | MACROD2 |
| 22q11.23 | 24347959 | 24390254 | 42.3 | Deletion (−1.449985) | LOC391322, GSTT1, GSTTP2 |
Loc. = location on chromosame.
*Genome mapping based on genome build hg19.
**The numbers in parentheses are the average log2 ratio of each aberration.
Figure 4.
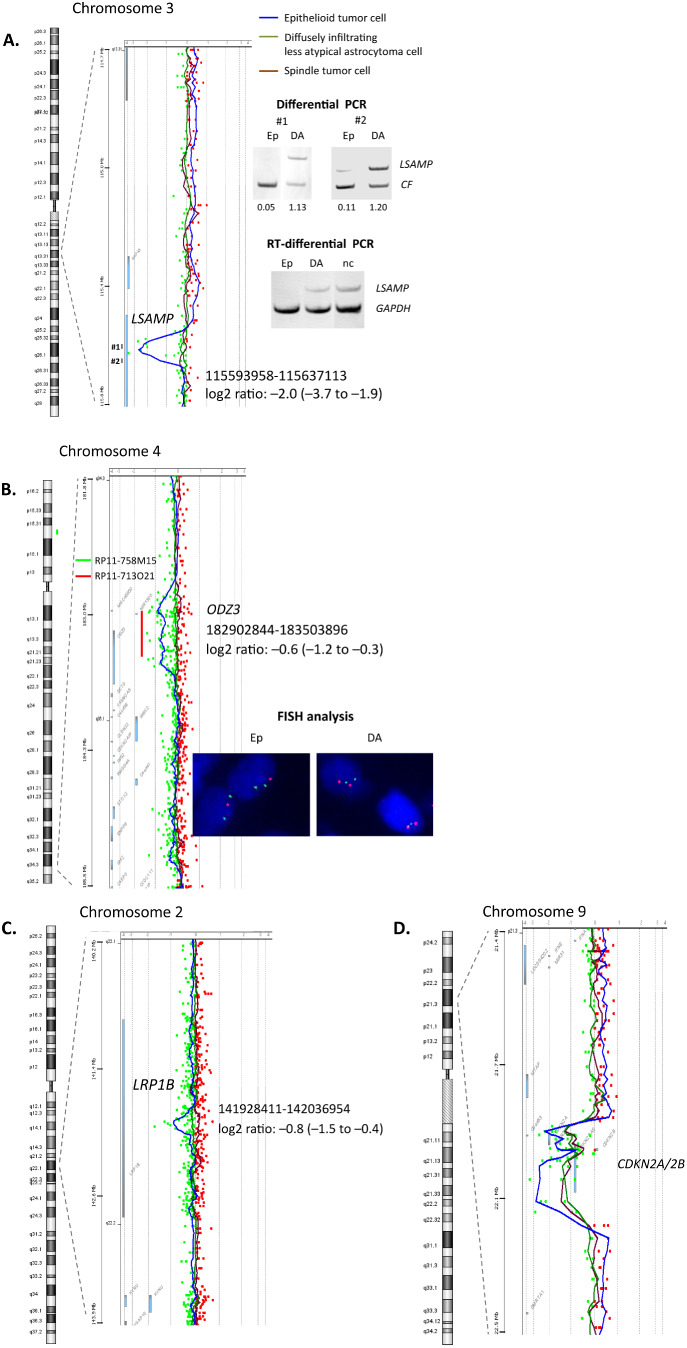
Three aberrations found only in epithelioid tumor cells (A–C) and one example of aberrations detected in all histological areas (D) by array CGH. A. A homozygous deletion in intron 3 of the LSAMP. The deletion was validated by differential PCR using two primer sets (fragments #1 and #2) located in the deleted part shown by array CGH. Numbers below each band of differential PCR indicate the ratio of amplification of the target compared with the CF reference gene. RT‐differential PCR showed LSAMP mRNA expression in diffusely infiltrating less atypical astrocytoma cells but not in epithelioid tumor cells. #1, 2 = fragments #1, 2. B. A heterozygous deletion in ODZ 3. The deletion was validated by FISH analysis. BAC clones used for the target (RP11‐713O21; 4q34.3–4q35.1) and reference (RP11–758 M15; 4p15.2) probes are indicated with red and green bars, respectively. C. A heterozygous deletion in LRP1B. D. A homozygous deletion in CDKN 2A/2B. Abbreviations: BAC = bacterial artificial chromosome; CGH = comparative genomic hybridization; DA = diffusely infiltrating less atypical astrocytoma cells; Ep = epithelioid tumor cells; FISH = fluorescence in situ hybridization; nc = normal control; PCR = polymerase chain reaction; RT = reverse transcription.
Validation of array CGH data
Differential PCR was carried out to assess the homozygous deletion in intron 3 of the LSAMP gene, which was detected by array CGH, in epithelioid tumor cells and diffusely infiltrating less atypical astrocytoma cells using two primer sets (fragments #1 and #2), and the deletion was confirmed in epithelioid tumor cells (Figure 4A).
FISH analysis was carried out to assess the heterozygous deletion in ODZ3, which was detected by array CGH, in epithelioid tumor cells and less atypical astrocytoma cells, and the deletion was revealed only in epithelioid tumor cells (Figure 4B).
LSAMP mRNA expression
RT‐differential PCR detected LSAMP mRNA expression in a small area of diffusely infiltrating less atypical astrocytoma cells (28% reduction compared with normal control), but the expression was below the detection limit in an area with epithelioid tumor cells (Figure 4A).
BRAF and IDH1/2 mutations
The heterozygous BRAF V600E mutation was found both in an area with epithelioid tumor cells and in a small area of diffusely infiltrating less atypical astrocytoma cells (Figure 5). No mutation in exon 4 of IDH1 and IDH2 was observed in either area analyzed (data not shown).
Figure 5.
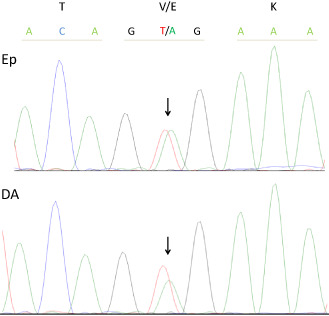
Illustration of the heterozygous BRAF V600E mutation (arrow) found in both an area with epithelioid tumor cells (upper) and an area of diffusely infiltrating less atypical astrocytoma cells (lower).
Discussion
In the current study, array CGH was performed separately for epithelioid tumor cells, which accounted for the majority of the tumor, a small area of diffusely infiltrating less atypical astrocytoma cells with a lower cell density, and a spindle cell area in a single case of epithelioid glioblastoma. The results demonstrated that 8 of 11 CNAs were identical in all tumor areas analyzed (Table 1, Figure 4D). Among 3 CNAs specific to epithelioid tumor cells, we focused on the only homozygously deleted gene, that is, LSAMP at 3q13.31. Deletion of LSAMP has been reported to be involved in tumorgenesis 5, 17, 27, 35. The LSAMP gene has been established as a tumor suppressor gene, which was frequently deleted, down‐regulated or epigenetically silenced in osteosarcoma and renal cell carcinoma 5, 17, 27, 35. Recent data from The Cancer Genome Atlas, analyzing 578 samples of glioblastoma, showed infrequent single gene deletion involving LSAMP 3. In the present report, a homozygous deletion of LSAMP found in epithelioid cells was detected in an intron, but was shown to lead to a loss of LSAMP mRNA expression (Figure 4A). Taken together, the possibility cannot be ruled out that the regional loss of LSAMP led to the aggressive nature of epithelioid cells in the present case. Moreover, whether or not the inactivation of LSAMP is a frequent event in this rare type of glioblastoma needs to be further investigated. The other hemizygously deleted genes, ODZ3 and LRP1B were also reported to be potent tumor supressor genes 7, 19, 22, 25, and their biological significance in tumorigenesis requires further investigation as well.
Along with PXA, gangliogliomas and extracerebellar pilocytic astrocytomas, frequent BRAF V600E mutation was also reported in epithelioid glioblastomas 14. In the present case, BRAF V600E mutation was observed not only in an area with epithelioid tumor cells, but also in an area of less atypical astrocytoma cells with a lower cell density (Figure 5), which may mean that the acquisition of the BRAF mutation occurred at tumor initiation. Concurrent BRAF V600E mutation and CDKN2A homozygous deletion have been found in a subset of diffuse astrocytomas without IDH1/2 mutations in children and young adults 9, 30. This combination of alterations, which was observed in the present case (Table 1, Figure 4D), has also been detected in astrocytic tumors of higher malignancy grades in the same age category 30.
The present case showed extensive systemic spread including dissemination in the intracranial and spinal subarachnoid space, and metastases to vertebral bodies, the right thoracic wall, right lung and liver. Some epithelioid glioblastomas have reported to show cerebrospinal fluid dissemination and/or disease progression at sites outside the CNS: peritoneal tumor deposites and multiple liver metastases 4, 14, 15. These behaviors are not typical of conventional glioblastomas 13, but seem not to be infrequent in epithelioid glioblastomas.
In conclusion, our results suggest that the regional loss of LSAMP led to the aggressive nature of epithelioid cells in the present case of epithelioid glioblastoma. Large cohorts will have to be tested to explore additional pathobiologic and prognostic differences associated with epithelioid glioblastomas.
Acknowledgments
We thank Ms. Machiko Yokota and Mr. Koji Isoda (Gunma University) for their excellent technical assistance.
The authors declare no conflict of interest
References
- 1. Arai M, Nobusawa S, Ikota H, Takemura S, Nakazato Y (2012) Frequent IDH1/2 mutations in intracranial chondrosarcoma: a possible diagnostic clue for its differentiation from chordoma. Brain Tumor Pathol 29:201–206. [DOI] [PubMed] [Google Scholar]
- 2. Babu R, Hatef J, McLendon RE, Cummings TJ, Sampson JH, Friedman AH, Adamson C (2013) Clinicopathological characteristics and treatment of rhabdoid glioblastoma. J Neurosurg 119:412–419. [DOI] [PubMed] [Google Scholar]
- 3. Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broniscer A, Tatevossian RG, Sabin ND, Klimo P Jr, Dalton J, Lee R et al (2013) Clinical, radiological, histological, and molecular characteristics of paediatric epithelioid. Neuropathol Appl Neurobiol doi: 10.1111/nan.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Lui WO, Vos MD, Clark GJ, Takahashi M, Schoumans J et al (2003) The t(1;3) breakpoint‐spanning genes LSAMP and NORE1 are involved in clear cell renal cell carcinoma. Cancer Cell 4:405–413. [DOI] [PubMed] [Google Scholar]
- 6. Dias‐Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, Borger DR et al (2011) BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic. PLoS ONE 6:e17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K et al (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ et al (2010) Activating mutations in BRAF characterize a spectrum of pediatric low‐grade gliomas. Neuro‐Oncol 12:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW et al (2009) Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol 218:172–181. [DOI] [PubMed] [Google Scholar]
- 10. He MX, Wang JJ (2011) Rhabdoid glioblastoma: case report and literature review. Neuropathology 31:421–426. [DOI] [PubMed] [Google Scholar]
- 11. Huang H, Colella S, Kurrer M, Yonekawa Y, Kleihues P, Ohgaki H (2000) Gene expression profiling of low‐grade diffuse astrocytomas by cDNA arrays. Cancer Res 60:6868–6874. [PubMed] [Google Scholar]
- 12. Kim YH, Lachuer J, Mittelbronn M, Paulus W, Brokinkel B, Keyvani K et al (2011) Alterations in the RB1 pathway in low‐grade diffuse gliomas lacking common genetic alterations. Brain Pathol 21:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kleihues P, Burger PC, Aldape KD, Brat DJ, Biernat W, Bigner DD (2007) Glioblastoma. Chapter 1. In: WHO Classification of Tumours of the Central Nervous System. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), pp. 33–59. IARC Press: Lyon. [Google Scholar]
- 14. Kleinschmidt‐DeMasters BK, Aisner DL, Birks DK, Foreman NK (2013) Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol 37:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kleinschmidt‐DeMasters BK, Alassiri AH, Birks DK, Newell KL, Moore W, Lillehei KO (2010) Epithelioid versus rhabdoid glioblastomas are distinguished by monosomy 22 and immunohistochemical expression of INI‐1 but not claudin 6. Am J Surg Pathol 34:341–354. [DOI] [PubMed] [Google Scholar]
- 16. Koelsche C, Wöhrer A, Jeibmann A, Schittenhelm J, Schindler G, Preusser M et al (2013) Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol 125:891–900. [DOI] [PubMed] [Google Scholar]
- 17. Kresse SH, Ohnstad HO, Paulsen EB, Bjerkehagen B, Szuhai K, Serra M et al (2009) LSAMP, a novel candidate tumor suppressor gene in human osteosarcomas, identified by array comparative genomic hybridization. Genes Chromosomes Cancer 48:679–693. [DOI] [PubMed] [Google Scholar]
- 18. Lath R, Unosson D, Blumbergs P, Stahl J, Brophy BP (2003) Rhabdoid glioblastoma: a case report. J Clin Neurosci 10:325–328. [DOI] [PubMed] [Google Scholar]
- 19. Letouzé E, Rosati R, Komechen H, Doghman M, Marisa L, Flück C et al (2012) SNP array profiling of childhood adrenocortical tumors reveals distinct pathways of tumorigenesis and highlights candidate driver genes. J Clin Endocrinol Metab 97:E1284–E1293. [DOI] [PubMed] [Google Scholar]
- 20. Lv SQ, Kim YH, Giulio F, Shalaby T, Nobusawa S, Yang H et al (2012) Genetic alterations in microRNAs in medulloblastomas. Brain Pathol 22:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Momota H, Iwami K, Fujii M, Motomura K, Natsume A, Ogino J et al (2011) Rhabdoid glioblastoma in a child: case report and literature review. Brain Tumor Pathol 28:65–70. [DOI] [PubMed] [Google Scholar]
- 22. Nagayama K, Kohno T, Sato M, Arai Y, Minna JD, Yokota J (2007) Homozygous deletion scanning of the lung cancer genome at a 100‐kb resolution. Genes Chromosomes Cancer 46:1000–1010. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura M, Watanabe T, Klangby U, Asker C, Wiman K, Yonekawa Y et al (2001) P14Arf deletion and methylation in genetic pathways to glioblastomas. Brain Pathol 11:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakazato Y, Ishizeki J, Takahashi K, Yamaguchi H, Kamei T, Mori T (1982) Localization of S‐100 protein and glial fibrillary acidic protein‐related antigen in pleomorphic adenoma of the salivary glands. Lab Invest 46:621–626. [PubMed] [Google Scholar]
- 25. Nikolaev SI, Rimoldi D, Iseli C, Valsesia A, Robyr D, Gehrig C et al (2011) Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat Genet 44:133–139. [DOI] [PubMed] [Google Scholar]
- 26. Nobusawa S, Lachuer J, Wierinckx A, Kim YH, Huang J, Legras C et al (2010) Intratumoral patterns of genomic imbalance in glioblastomas. Brain Pathol 20:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pasic I, Shlien A, Durbin AD, Stavropoulos DJ, Baskin B, Ray PN et al (2010) Recurrent focal copy‐number changes and loss of heterozygosity implicate two noncoding RNAs and one tumor suppressor gene at chromosome 3q13.31 in osteosarcoma. Cancer Res 70:160–171. [DOI] [PubMed] [Google Scholar]
- 28. Pimentel J, Silva R, Pimentel T (2003) Primary malignant rhabdoid tumors of the central nervous system: considerations about two cases of adulthood presentation. J Neurooncol 61:121–126. [DOI] [PubMed] [Google Scholar]
- 29. Rollbrocker B, Waha A, Louis DN, Wiestler OD, von Deimling A (1996) Amplification of the cyclin‐dependent kinase 4 (CDK4) gene is associated with high cdk4 protein levels in glioblastoma multiforme. Acta Neuropathol 92:70–74. [DOI] [PubMed] [Google Scholar]
- 30. Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley MY, Yu M et al (2010) Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric astrocytomas. Cancer Res 70:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold‐Mende C et al (2011) Analysis of BRAF V600E mutation in 1320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra‐cerebellar pilocytic astrocytoma. Acta Neuropathol 121:397–405. [DOI] [PubMed] [Google Scholar]
- 32. Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A et al (2005) Genome‐wide array‐based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 24:1348–1358. [DOI] [PubMed] [Google Scholar]
- 33. Tohma Y, Gratas C, Biernat W, Peraud A, Fukuda M, Yonekawa Y et al (1998) PTEN (MMAC1) mutations are frequent in primary glioblastomas (de novo) but not in secondary glioblastomas. J Neuropathol Exp Neurol 57:684–689. [DOI] [PubMed] [Google Scholar]
- 34. Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yen CC, Chen WM, Chen TH, Chen WY, Chen PC, Chiou HJ et al (2009) Identification of chromosomal aberrations associated with disease progression and a novel 3q13.31 deletion involving LSAMP gene in osteosarcoma. Int J Oncol 35:775–788. [DOI] [PubMed] [Google Scholar]
- 36. Yokoo H, Kinjo S, Hirato J, Nakazato Y (2006) Fluorescence in situ hybridization targeted for chromosome 1p of oligodendrogliomas (in Japanese). Rinsho Kensa 50:761–766. [Google Scholar]
- 37. Yokoo H, Nobusawa S, Takebayashi H, Ikenaka K, Isoda K, Kamiya M et al (2004) Anti‐human Olig2 antibody as a useful immunohistochemical marker of normal oligodendrocytes and gliomas. Am J Pathol 164:1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]


