Clinical and Radiological Findings
A 63‐year‐old woman developed deterioration of her general condition over a three‐week period, with apathy and drowsiness. Major history included rheumatoid arthritis (RA) treated by corticoids and anti‐TNFα and right breast tumorectomy. Physical examination revealed tachypnea, dyspnea, Cushingoid facies, bilateral rhonchi, and bilateral edema of the lower limbs. Neurologic examination revealed drowsiness. Further tests brought out atrial fibrillation, cardiac failure, and inflammatory syndrome.
CT scan of the brain showed a large area of hypodensity in the right frontal lobe with mass effect associated with meningeal enhancement (Figure 1A). MRI revealed an area of high signal intensity on FLAIR images in the right frontal lobe as well as in the left frontal sulci (Figure 1B). On T1‐weighted images with contrast a cortical and leptomeningeal enhancement was also found (Figure 1C). These images suggested bilateral frontal meningoencephalitis, predominating over the right side, consistent with an infectious or an inflammatory disease.
Figure 1.
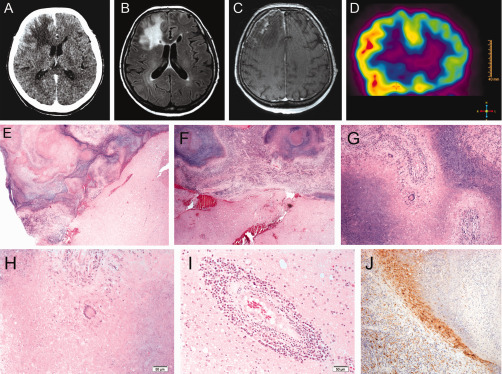
CSF protein level was 59 mg/dl, glucose level at 93 mg/dl and cytology showed a reactive process with presence of numerous lymphocytes and plasma cells. At this stage, differential diagnoses were progressive multifocal leukoencephalopathy (PML), lymphoma and vasculitis. FDG‐PET of the head revealed high glucose uptake in right frontal lobe, consistent with a lymphomatous process or encephalitis (Figure 1D). Biopsies of brain tissue and dura mater from right frontal lobe were obtained.
Pathology
Grossly, the biopsy specimen was a fragment of dura mater and multiple solid and yellowish fragment of brain tissue, altogether measuring 3 x 3 x 1 cm. Smears revealed glial cells without atypia, lymphocytes, plasma cells, and neutrophils within a necrotic background. Histologically, the fragment of dura mater was thickened with necrotizing granulomas surrounded by histiocytes, lymphoplasmocytic infiltrates, and giant cells involving the leptomeninges with extension to the brain parenchyma (Figures 1E–H). Vasculitis and spongiosis were also observed in the parenchyma (Figure 1I). Special stains (PAS, Gomori‐Grocott and Ziehl‐Neelsen) were negative for fungi and mycobacterium. CD68 immunohistochemical staining confirmed the presence of histiocytes surrounding granulomas’ necrosis (Figure 1J). CD3 and CD20 immunohistochemical stainings confirmed the polymorphous population of lymphocytes (T and B). What is your diagnosis?
Diagnosis
Rheumatoid meningitis (RM).
Discussion
RA is a chronic multisystem disease with many extra‐articular manifestations. Typical neurologic sequelae of RA are usually secondary to musculoskeletal involvement. Atypical sequelae directly involving the central nervous system include parenchymal and meningeal vasculitis, rheumatoid nodules, and meningitis 2. From these complications, RM is therefore a rare but one of the most severe, with only 56 cases described in the literature between 1954 and 2014 (Supporting Information: please visit http://path.upmc.edu/divisions/neuropath/bpath/cases/case327/dx.html for the 56 references of previous reports). Various symptoms are seen in RM: altered mental status, cranial neuropathies, hemiparesis, gait imbalance, memory loss, seizures and headaches 2. Diagnosis of RM is therefore difficult. CSF analysis is generally characterized by nonspecific findings with mild pleiocytosis and an elevated protein level 2, 4. Our case matches this description, with protein level at 59 mg/dl and presence of numerous lymphocytes and plasmocytes. Some authors report hypoglycorrhachia as a characteristic of RM 2, but on the basis of a literature overview, the level of glucose is variable. Introduction of MRI examination of the brain has resulted in enhanced early diagnosis of RM, and significantly improved the prognosis of these patients. Because use of immunosuppressive therapy is the gold standard in RA, a biopsy is required to exclude infectious causes of chronic meningitis 4. Pathological findings of RM are characterized by vasculitis, rheumatoid nodules and infiltration of mononuclear cells such as lymphocytes and plasma cells on leptomeninges 3. All these criteria are met in our case. Differential diagnosis of granulomatous meningitis are infectious causes (bacterial as tuberculosis, fungal, parasites or viral as VZV) or noninfectious causes such as sarcoidosis, systemic lupus erythematosus, neoplastic meningitis, granulomatous angiitis, idiopathic chronic pachymeningitis or Wegener's disease. To exclude infectious causes, special stains such as PAS, Ziehl‐Neelsen, Grocott and Giemsa are required on biopsy material or centrifuged CSF sediment.
In conclusion, our case illustrates that RM is a difficult diagnosis, often established by exclusion on biopsy material. The diagnosis of RM should be considered during the differential diagnosis stage in patient with chronic meningitis.
References
- 1. Hildebrand J, Aoun M (2003) Chronic meningitis: still a diagnostic challenge. J Neurol 250:653–660. [DOI] [PubMed] [Google Scholar]
- 2. Jones SE, Belsley NA, McLoud TC, Mullins ME (2006) Rheumatoid meningitis: radiologic and pathologic correlation. AJR Am J Roentgenol 186:1181–1183. [DOI] [PubMed] [Google Scholar]
- 3. Kato T, Hoshi K, Sekijima Y, Matsuda M, Hashimoto T, Otani M et al (2003) Rheumatoid meningitis: an autopsy report and review of the literature. Clin Rheumatol 22:475–480. [DOI] [PubMed] [Google Scholar]
- 4. Shimada K, Matsui T, Kawakami M, Hayakawa H, Futami H, Michishita K et al (2009) Diffuse chronic leptomeningitis with seropositive rheumatoid arthritis: report of a case successfully treated as rheumatoid leptomeningitis. Mod Rheumatol 19:556–562. [DOI] [PubMed] [Google Scholar]


