Short abstract
This report examines various vaccine platforms including inactivated vaccines, protein-based vaccines, viral vector vaccines, and nucleic acid (DNA or mRNA) vaccines, and their ways of producing immunogens in cells.
1. Introduction
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),1 the world has taken significant measures to cope with this disease, from increasing personal protection equipment (PPE) production and emphasizing the importance of social distancing/masking to the Emergency Use Authorization (EUA) of remdesivir/therapeutic antibodies and the application of the well-known corticosteroid dexamethasone.2−5 However, the disease is still spreading in an unrelenting fashion and has caused widespread health, social, and economic disruption. Therefore, effective vaccines are urgently needed to end this pandemic and help society return to normalcy. Indeed, many COVID-19 candidate vaccines have been researched, developed, tested, and evaluated at an unprecedented speed. As of the end of February 2021, several vaccines have been conditionally approved, and others are close to such approval. It is likely that many more still in clinical trials will come to market in the next few years.
Vaccination is considered one of the greatest medical achievements of modern civilization. The eradication of smallpox is one of the best examples of how vaccination stopped a deadly disease and saved millions of lives.6 Many childhood diseases, such as polio and measles, have also been drastically reduced in incidence due to worldwide adoption of vaccination.7−9 Annual influenza vaccination is now a common practice to keep people from contracting seasonal flu. However, traditional vaccine development often takes 15 years or more from the initial design stage to the clinical studies.6−10 Vaccines are complex biological products that need to be designed for a wide range of healthy people. Thus, the development and evaluation of vaccines are time-consuming because careful study and monitoring are necessary to ensure safe deployment of any vaccine. Clinical trials of vaccines are often costly, requiring recruitment of large numbers of volunteers with diverse ethnicities, ages, and health conditions. In addition, long-term monitoring is necessary in order to establish vaccine efficacy and to rule out rare safety issues.
The fact that several COVID-19 vaccine candidates entered into clinical trials in less than 6 months and were conditionally approved in 10 months since the beginning of the COVID-19 outbreak demonstrates a record-breaking speed in vaccine development history.11,12 This unprecedented speed was facilitated by the timely release of the viral genomic sequence, the availability of cutting-edge vaccine technologies, active collaboration among the global scientific community, adequate funding from various sources, and the huge/urgent market demand. Since the beginning of the pandemic, governmental agencies around the world, nonprofit organizations, and various vaccine developers have committed vast resources to COVID-19 vaccine development. Despite the high speed, safety standards for the development and approval process of the recently available vaccines were consistent with those for previous vaccines, with rigorous review of their clinical data by government regulatory agencies and external review panels such as the U.S. Vaccines and Related Biological Products Advisory Committee (VRBPAC).13
Many COVID-19 candidate vaccines were designed to use the SARS-CoV-2 spike protein (S protein) or part of it as the immunogen, an agent capable of inducing immune responses. The S protein is the viral surface protein that binds to the angiotensin-converting enzyme 2 (ACE2), a protein receptor on the surface of human cells that mediates entrance of the virus into the human cells. The S protein, with a total of 1273 amino acids, consists of two major regions: the S1 and S2 domains. The S1 domain, where the receptor-binding domain (RBD) resides, is responsible for the binding of S protein with the ACE2 receptor.14,15 The use of S protein as the immunogen was supported by evidence from previous SARS and MERS vaccine development; vaccines that can induce strong antibody responses against the S protein often have a significant effect on blocking viral entry into host cells during natural infection.16 This observation was further validated in studies in which most of the SARS-CoV-2 neutralizing antibodies from COVID-19 convalescent patients were against either the S protein or its RBD domain.15,16 Previous research experience with SARS and MERS coronaviruses identified two proline substitutions on S protein, which stabilize the antigenically optimal prefusion conformation of this protein and were employed in the COVID-19 vaccine design.17,18 The prior vaccine research with SARS and MERS also helped establish suitable animal models for vaccine efficacy testing.19,20 Technologies for mRNA and vector vaccines allowed researchers to precisely design the antigen yet avoid complicated protein purification steps and a high-risk viral culture process.21
In this report, we first provide an overview of the vaccine development landscape with a discussion of the advantages and disadvantages of different vaccine platforms. We then focus on vaccine adjuvants as well as nanotechnology in vaccine delivery systems, as both are relatively underdeveloped areas. Lastly, we analyze the research trends in published documents related to COVID-19 vaccine development and provide a landscape of these documents with highlights on the most notable journal articles. We hope this report can serve as a useful resource for people who want to get a quick understanding of the technologies involved in COVID-19 vaccines, as well as an appreciation of the global effort of COVID-19 vaccine development.
2. Current COVID-19 Vaccine Landscape
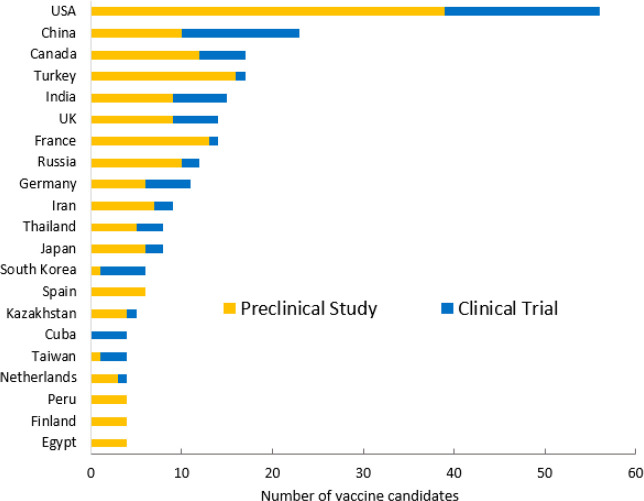
Since the beginning of the COVID-19 pandemic, there has been a worldwide race to develop vaccines against this disease. By the end of February 2021, more than 40 countries and regions were working on developing COVID-19 vaccines, and at least half of these countries have had one or more vaccines in clinical trials, based on the analysis of vaccine development data from the World Health Organization (WHO).22 As shown in Figure 1, the United States leads many other countries and regions in the number of both clinical (17) and preclinical (39) vaccine candidates. China is ranked second with 13 candidates in clinical trials and 10 in preclinical studies. Canada places third with 5 candidates in clinical trials and 12 in preclinical studies. Among those in clinical trials, two vaccines from the United States (Moderna and Johnson & Johnson/Janssen), one codeveloped by the United States (Pfizer) and Germany (BioNTech), one from the UK (AstraZeneca/Oxford), four from China, and one from Russia have been granted conditional authorizations in one or more countries.
Figure 1.
Top 21 countries by number of COVID-19 candidate vaccines in the development pipelines.
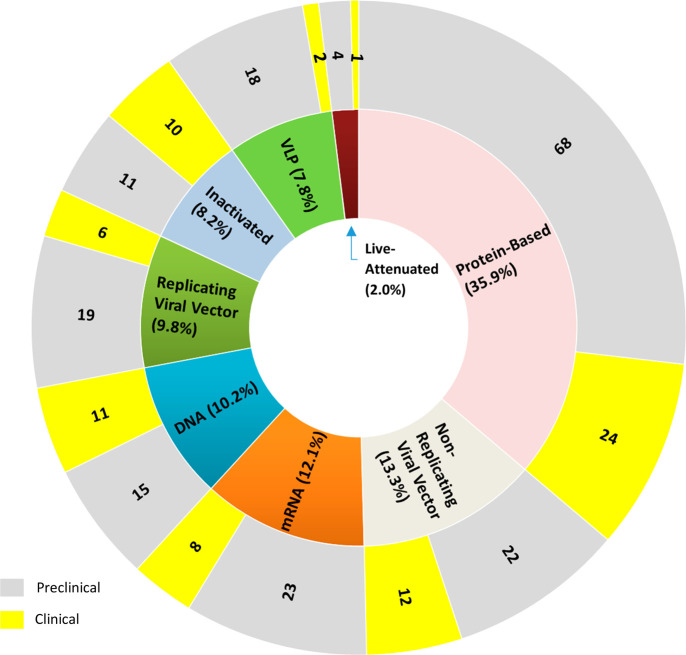
By the end of February 2021, a total of 256 COVID-19 vaccine candidates have been developed, with 74 in clinical trials and 182 in preclinical studies. Of the 74 candidates, 16 are undergoing further validations of safety and efficacy in Phase 3 or Phase 4 clinical trials with large numbers of volunteers (more details in Table 6).22 These vaccine candidates were developed based on a variety of approaches, including conventional whole virus vaccines (live attenuated or inactivated vaccines), recombinant protein-based vaccines (protein subunit vaccines, virus-like particles (VLP)), viral vector vaccines, and nucleic acid vaccines (DNA- and mRNA-based vaccines). Figure 2 illustrates the distribution of various COVID-19 vaccine candidates among different platforms. Protein-based vaccines constitute the largest category, accounting for 35.9% of all the COVID-19 vaccine candidates being developed, with 24 in clinical trials and 68 in preclinical studies. The nonreplicating viral vector, mRNA, DNA, replicating viral vector, and inactivated vaccines account for 13.3%, 12.1%, 10.2%, 9.8%, and 8.2%, respectively.
Table 6. Exemplary COVID-19 Vaccine Candidates with Conditional Approval Granted or in Phase 3 Trials.
| vaccine platform | vaccine | developer | efficacy | delivery route and dosage | storage | Phase 3 and 4 trial size | Clinical Trial Registration Number |
|---|---|---|---|---|---|---|---|
| mRNA | BNT162b2a | Pfizer/BioNTech | 95% | IM (2), 3 weeks apart | –70 °C | 43,998 (age 12+) | NCT04368728; NCT04760132 |
| mRNA-1273a | Moderna | 94.1% | IM (2), 4 weeks apart | –20 °C | 30,420 (age 18+); 3,000 (12–<18) | NCT04470427; NCT04649151; NCT04760132 | |
| Nonreplicating viral vector | AZD1222a | AstraZeneca/Oxford University | 70.4% | IM (2), 12 weeks apart | 2–8 °C | 12,390 (age 18+) | NCT04400838; NCT04760132 |
| JNJ-78436735 | Johnson & Johnson) | 66% | IM (1) | 2–8 °C | 44,325 (age 18+) | NCT04505722 | |
| Convidecia | CanSino Biologics | 65.3% | IM (1) | 2–8 °C | 40,000 (age 18+) | NCT04526990; NCT04756830 | |
| Sputnik V | Gamaleya Research Institute | 91.6% | IM (2), 3 weeks apart | –20 °C or 2–8 °C | 33,758 (age 18+) | NCT04530396 | |
| Inactivated | CoronaVaca | Sinovac | 50.7% | IM (2), 2 weeks apart | 2–8 °C | 12,688 (age 18+) | NCT04456595; NCT04756830; NCT04747821 |
| BBIBP-CorV | Sinopharm | 79.3% | IM (2), 3 weeks apart | 2–8 °C | 3,000 (age 18+) | NCT04560881 | |
| BBV152 | Bharat Biotech | N.A. | IM (2), 4 weeks apart | 2–8 °C | 25,800 (age 18+) | NCT04641481 | |
| Protein subunit vaccine | NVX-CoV2373 | Novavax | 89.7% | IM (2), 3 weeks apart | 2–8 °C | 30,000 (age 18+) | NCT04611802 |
Phase 4 clinical trial in progress.
Figure 2.
Distribution of COVID-19 vaccine candidates among different vaccine platforms and their development stages.
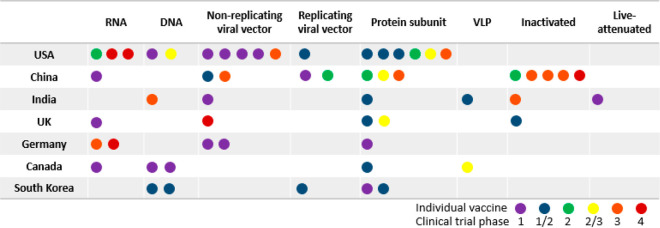
Figure 3 lists the top seven countries according to the number of COVID-19 vaccine candidates in clinical trial. The United States, China, the UK, and Germany have the vaccines in the most advanced clinical trial stage, Phase 4 (post market studies). Individual vaccines as represented by colored dots showing the stages of clinical trials are displayed among different vaccine platforms by country. India is the only country where a live-attenuated COVID-19 vaccine is being developed. China, India, and the UK each has inactivated COVID-19 vaccine candidates. All seven countries are developing protein subunit-based vaccines. With the exception of Germany, these countries are also developing viral vector-based COVID-19 vaccines. In addition, the United States and China have the highest numbers of vaccines in clinical trials, and they both cover a diverse range of platforms, although no live attenuated or inactivated vaccine is being developed in the United States. Both Germany and the UK have COVID-19 vaccine candidates in advanced clinical trial stages using the viral vector or mRNA platform. South Korea and Canada seem to have devoted their efforts to developing protein subunit-, viral vector-, and DNA-based vaccines, although these are still in early clinical trial stages. This interesting distribution pattern of vaccine platforms among top vaccine-developing countries may be related to factors such as (1) availability of vaccine technologies in individual countries, (2) consideration for expedited mass production and the presence of facilities for such production, and (3) variation in the regulatory concerns/stringency in different countries.
Figure 3.
Distribution of COVID-19 vaccine candidates in clinical trials among top seven countries regarding the trial stages and vaccine platforms.
3. Vaccine Platforms
Multiple vaccine platforms have been explored for COVID-19 vaccine development as each vaccine platform has unique advantages and disadvantages (Table 1 and Figure 4).22 It is very likely that the world will need more than one type of approved vaccine to combat this pandemic with assurance of broad target population coverage, production quantities, and storage and transportation requirements on top of vaccine safety and effectiveness.
Table 1. Vaccine Platforms and Their Potential Advantages and Disadvantages.
| vaccine platform | advantages | disadvantages | existing vaccine examples |
|---|---|---|---|
| Live-attenuated | • Strong and long-lasting immune response | • Potential risk of disease | • Smallpox |
| • Broad antigenic profile | • Requirement for biosafety facilities | • Tuberculosis (BCG) | |
| • Measles | |||
| • Polio (OPV) | |||
| Inactivated | • Broad antigenic profile | • Reduced immune response | • Hepatitis A |
| • Requirement for biosafety facilities | • Polio (IPV) | ||
| • Lower purity | • Rabies | ||
| • Influenza | |||
| Protein subunit | • Noninfectious | • Limited capability in inducing cell-mediated immunity | • Hepatitis B (HBV) |
| • Targeting key antigens | • Adjuvant often needed | • DTP (diphtheria, tetanus, and pertussis) | |
| • Challenges in large-scale production | |||
| VLP | • Noninfectious | • Limited immunogenicity | • Hepatitis B (HBV) |
| • Broad antigenic profile | • Lower purity | • Papillomavirus (HPV) | |
| Nonreplicating viral vector | • Fast to produce | • Pre-existing immunity against the vector | N.A. |
| • Reusable platform | • Risk of adverse reactions | ||
| • Strong in both cell- and antibody-mediated immune responses | |||
| Replicating viral vector | • Fast to produce | • Pre-existing immunity against the vector | • Ebola (EUA) |
| • Lower doses/single dose | • Risk of adverse reactions | ||
| • Reusable platform | |||
| • Strong in both cell- and antibody-mediated immune response | |||
| • Less infectious | |||
| DNA | • Fast to produce | • May need special delivery devices | N.A. |
| • Scalable | |||
| • Noninfectious | |||
| • Reusable platform | |||
| • Stable at room temperature | |||
| mRNA | • Fast to produce | • May need extremely low temperatures for storage and transportation | • COVID-19 (EUA) |
| • Noninfectious | • May need special delivery systems | ||
| • No genome integration risk | |||
| • Reusable platform | |||
| • Stimulates strong T cell response | |||
| • Simple formulations |
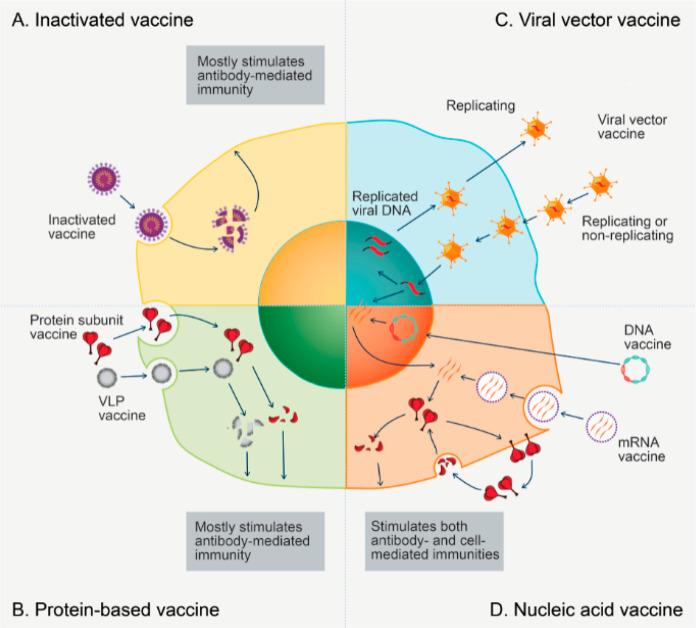
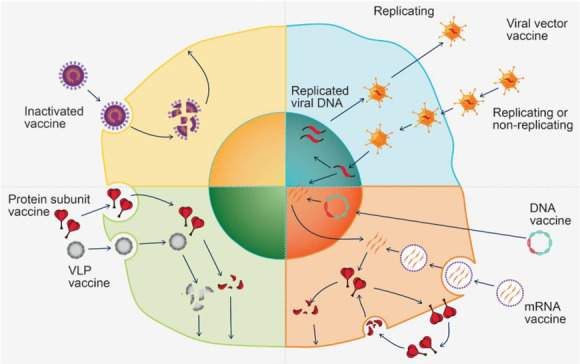
Figure 4.
Vaccine platforms and their ways of producing immunogen in cells. (A) Inactivated vaccine results in a broader spectrum of antigens when it is taken up and broken down by cells. (B) Protein-based vaccine produces a more focused response to a targeted antigen when it is taken up and processed into multiple epitopes by cells. (C) Viral vector vaccine delivers antigen-encoding DNA to cells and enhances the inflammatory response and immunity. (D) Nucleic acid vaccine enters cells and serves as the transcriptional/translational template for protein antigen synthesis.
3.1. Conventional Whole Virus Vaccines
Conventional whole virus vaccines, including live-attenuated and inactivated vaccines, are the oldest and most well-established types of vaccine, used in smallpox, BCG, and measles vaccines. In the case of vaccines developed against COVID-19, the attenuated or inactivated whole SARS-CoV-2 virus is administered to individuals to elicit the immune responses. The immune responses are likely to target not only the S protein of SARS-CoV-2, but also many other viral proteins. Manufacturing of these COVID-19 vaccines is more time-consuming and difficult because it takes time to grow the virus into large quantities and requires dedicated biosafety level 3 production facilities.23
3.1.1. Live-Attenuated Vaccines
Live-attenuated vaccines are traditionally produced by serial passage of disease-producing viruses in cultured cells with selection for a weakened replication capability and thus reduced virulence. The virulence of viruses in live-attenuated vaccines is reduced, but the viruses still retain the ability to replicate. These vaccines usually produce strong and long-lasting antibody-mediated (humoral) and cell-mediated immune responses by mimicking natural infection, but extensive safety evaluation is required. Although live-attenuated viral vaccines do not normally cause disease, they are rarely used in immunocompromised patients because of the possibility of infection by the live viruses in the vaccine. Interestingly, a live-attenuated candidate vaccine against COVID-19 was developed via codon deoptimization by Codagenix/Serum Institute of India. It has entered into Phase 1 clinical evaluation with a single dose administered intranasally (Table 2).24 Four other candidates are undergoing preclinical studies. However, it is worth noting that concerns have been raised that such vaccines may revert to virulence in some cases.25
Table 2. Live-Attenuated and Inactivated Vaccine Candidates against COVID-19 in Clinical Trials.
| vaccine platform (no. of vaccines in clinical trials) | developer/manufacturer | vaccine (CAS Registry Number) | clinical stage | route of administrationa (no. of doses) |
|---|---|---|---|---|
| Live-attenuated virus (1) | Codagenix/Serum Institute of India (India) | COVI-VAC | Phase 1 | IN (1–2) |
| Inactivated (10) | Sinovac (China) | CoronaVac (2480309-93-9) | Phase 4 | IM (2) |
| Wuhan Institute of Biological Products/Sinopharm (China) | N.A. | Phase 3 | IM (2) | |
| Beijing Institute of Biological Products/Sinopharm (China) | BBIBP-CorV (2503126-65-4) | Phase 3 | IM (2) | |
| Bharat Biotech (India) | Covaxin/BBV152 (2501889-19-4) | Phase 3 | IM (2) | |
| Institute of Medical Biology, Chinese Academy of Medical Sciences (China) | N.A. | Phase 3 | IM (2) | |
| Research Institute for Biological Safety Problems (Kazakhstan) | QazCovid-in (2541708-50-1) | Phase 3 | IM (2) | |
| Beijing Minhai Biotechnology Co (China) | N.A. | Phase 2 | IM (1, 2, 3) | |
| Valneva, National Institute for Health Research (UK) | VLA2001 | Phase 1/2 | IM (2) | |
| Erciyes University (Turkey) | ERUCOV-VAC | Phase 1 | IM (2) | |
| Shifa Pharmed Industrial Co (Iran) | N.A. | Phase 1 | IM (2) |
IM: intramuscular; IN: intranasal.
3.1.2. Inactivated Vaccines
Inactivated vaccines, also called killed vaccines, are produced by first growing the virus in a culture medium and then inactivating it by treatment with chemicals, heat, or radiation. Vaccines against hepatitis A, polio, and measles fall into this category. The viruses in these vaccines cannot replicate and so have no risk of causing infection, even in immunocompromised patients. Compared with live-attenuated vaccines, they are less effective, mainly eliciting the antibody-mediated immune response with minimal cell-mediated immune response, and thus usually require multiple doses to boost immunity. Of the 21 inactivated COVID-19 vaccines, 11 are in preclinical studies, and 10 have entered clinical trials with 5 in Phase 3 (Table 2). For example, CoronaVac from Sinovac was developed with β-propiolactone as an inactivating agent and formulated with aluminum hydroxide as an adjuvant (more details in the adjuvant section). With encouraging safety and immunogenicity results from a Phase 1/2 clinical trial,26 this vaccine has achieved an overall efficacy of 50.7% in the Phase 3 trial and has received conditional marketing authorization (CMA) in China.27,28 Another vaccine, BBIBP-CorV produced by Sinopharm in China, exhibited satisfactory early clinical trial results and has transitioned into Phase 3 clinical trials.29 It has been reported to have a 79.3% efficacy and has received CMA approval in China.30 BBV152 from Bharat Biotech is presently in a Phase 3 clinical trial and has received EUA in India.31
3.2. Recombinant Viral Protein-Based Vaccines
Although the whole virus vaccines represent a well-established platform, they require stringent production standards and procedures. Viral protein-based vaccines, however, are produced by recombinant technologies and contain only viral proteins (immunogens), but no genetic materials, and thus are safer in production and cannot cause infection because no intact virus is involved in the process.
3.2.1. Protein Subunit Vaccines
Protein subunit vaccines are protein-based vaccines that contain a viral protein or its segment as the antigen to elicit immune responses. This is a well-developed platform, and many approved vaccines are in this category, including HBV and DTP (Table 1). Because it is inherently safe and uses well-established techniques of protein purification, these vaccines comprise the largest category in COVID-19 vaccine development, as shown in Figure 2. Most of these vaccines utilize either the S protein or its receptor-binding domain (RBD) as the antigen. Because the S protein is membrane-bound with multiple subunits, the expression and production of the full-length S protein can be challenging. The RBD fragment, like the full-length S protein, elicits potent neutralizing antibodies and is much smaller and easier to produce. However, it lacks other potentially important epitopes presented by the full-length S protein. As a result, the RBD-based vaccines may not be as effective as the S protein-based vaccines.23 Similar to inactivated vaccines, protein subunit vaccines elicit mainly antibody-mediated immunity with a weak induction of T-cell response. Adjuvants are usually needed for this type of vaccine to boost the immune response and enhance vaccine efficacy (more information in the adjuvant section).
A total of 24 COVID-19 protein subunit vaccine candidates have entered clinical trials, including those by Novavax, Anhui Zhifei Longcom Biopharmaceutical, Kentucky Bioprocessing, and Sanofi/GlaxoSmithKline (GSK) (Table 3), while 68 more are in preclinical evaluation (Figure 2). NVX-CoV2373 is a recombinant S protein nanoparticle vaccine with the saponin-containing Matrix M1 adjuvant developed by Novavax. It demonstrated 89.3% efficacy in an interim result of Phase 3 trial with satisfactory safety and immunity data observed in prior clinical trials.32,33 In March 2021, Novavax revealed that the UK Phase 3 trial demonstrated efficacies of 96.4% and 86.3% against the original virus strain and the UK (B.1.1.7) variant, respectively, yielding an overall efficacy of 89.7%. Notably, no patients in the treatment of NVX-CoV2373 developed a severe infection.34 UB-612, a multiepitope peptide-based COVID-19 vaccine developed by COVAXX, targets the RBD and several other epitopes from the viral structural membrane and nucleocapsid proteins to promote B-cell and cytotoxic T-cell immune responses.35
Table 3. Recombinant Viral Protein-Based Vaccine Candidates against COVID-19 in Clinical Trials.
| vaccine platform (no. of vaccines in clinical trials) | developer/manufacturer | vaccine (CAS Registry Number) | clinical stage | route of administrationa (no. of doses) |
|---|---|---|---|---|
| Protein subunit (24) | Novavax (USA) | NVX-CoV2373 (2502099-58-1) | Phase 3 | IM (2) |
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences (China) | ZF 2001 (2609662-31-7) | Phase 3 | IM (2 or 3) | |
| Kentucky Bioprocessing, Inc. (USA) | KBP-COVID-19/KBP-201 (2543206-35-3) | Phase 1/2 | IM (2) | |
| Sanofi Pasteur (France)/GSK (UK) | N.A. | Phase 1/2 | IM (2) | |
| Biological E Ltd. (India) | N.A. | Phase 1/2 | IM (2) | |
| West China Hospital, Sichuan University (China) | N.A. | Phase 2 | IM (2) | |
| Clover Biopharmaceuticals Inc.(China)/GSK (UK)/Dynavax (USA) | SCB-2019 (2541906-99-2) | Phase 2/3 | IM (2) | |
| Vaxine Pty Ltd. (Australia)/Medytox (South Korea) | COVAX-19 (2543231-22-5) | Phase 1 | IM (1) | |
| Medigen Vaccine Biologics Corporation (Taiwan)/NIAID/Dynavax (USA) | MVC-COV1901 (2565776-92-1) | Phase 2 | IM (2) | |
| Center for Genetic Engineering and Biotechnology (Cuba) | Soberana 01 (2543410-63-3) | Phase 1/2 | IM (3) | |
| Center for Genetic Engineering and Biotechnology (Cuba) | Soberana 02 (2543416-58-4) | Phase 1/2 | IM (3) | |
| FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo (Russia) | N.A. | Phase 1/2 | IM (2) | |
| University Hospital Tuebingen (Germany) | CoVAC-1 (2543517-71-9) | Phase 1 | SC (1) | |
| COVAXX (USA)/United Biomedical Inc. Asia (Taiwan) | UB-612 (2543531-06-0) | Phase 2/3 | IM (2) | |
| Adimmune Corporation (Taiwan) | N.A. | Phase 1 | ND | |
| Nanogen Pharmaceutical Biotechnology (Vietnam) | N.A. | Phase 1/2 | IM (2) | |
| Shionogi Inc. (Japan) | S-268019 | Phase 1/2 | IM (2) | |
| Instituto Finlay de Vacunas (Cuba) | FINLAY-FR1 | Phase 1/2 | IM (2) | |
| Instituto Finlay de Vacunas (Cuba) | FINLAY-FR2 | Phase 2 | IM (2) | |
| University Medical Center Groningen (Netherlands) + Akston Biosciences Inc. (USA) | SARS-CoV-2-RBD-Fc fusion protein | Phase 1/2 | SC or IM (ND) | |
| University of Saskatchewan (Canada) | COVAC-1 and COVAC-2 subunit vaccine (spike protein) + SWE adjuvant | Phase 1/2 | IM (2) | |
| SK Bioscience Co., Ltd. (South Korea) | GBP510 | Phase 1/2 | IM (2) | |
| Razi Vaccine and Serum Research Institute (Iran) | Razi Cov Pars, recombinant spike protein | Phase 1 | IM and IN (3) | |
| The University of Queensland (Australia) | MF59 adjuvanted SARS-CoV-2 Sclamp vaccine | Phase 1 | IM (2) | |
| VLP (2) | Medicago Inc. (Canada) | N.A. | Phase 2/3 | IM (2) |
| SpyBiotech/Serum Institute of India (India) | N.A. | Phase 1/2 | IM (2) |
IM: intramuscular; SC: subcutaneous; IN: intranasal.
3.2.2. Virus-like Particle (VLP) Vaccines
VLP vaccines represent an evolution of protein subunit vaccinology and may also be regarded as a specific class of protein subunit vaccines. These particles are composed of viral capsid/coat proteins that, when recombinantly expressed from a host cell, can self-assemble into a capsid-like structure in the absence of the viral genome and other nonstructural virus proteins. As such, these noninfective particles provide a scaffold onto which multiple copies of an antigen (or epitope) can be chemically coupled or arrayed. This clustering of antigens/epitopes on the VLP surface allows for enhanced cognate activation of B-cells and antibody responses.36 Of the two COVID-19 VLP vaccine candidates in clinical evaluation, one is a vaccine adjuvanted with AS03 from Medicago Inc., and the other is from SpyBiotech/Serum Institute of India (Table 3).
3.3. Viral Vector Vaccines
In contrast to conventional whole virus vaccines and protein-based vaccines that directly introduce antigenic proteins to stimulate host immune responses, vector vaccines use nonpathogenic viral vectors to deliver antigen-coding DNA fragments to host cells for expression of the antigen using cellular protein-making machinery. The decision to use a particular viral vector initially depends on the size (in base pairs) of the antigen gene to be inserted as some viral vectors are more accommodating than others. There are also concerns as to pre-existing immunity and safety in the target population as well as genetic stability of the vector and issues related to manufacturing.37 They can be broadly divided into two types: nonreplicating viral vector vaccines and replicating viral vector vaccines. Because viral vector vaccines result in endogenous antigen production, they are more likely to induce both humoral and cellular immune responses. Viral vector vaccines can be developed and produced quickly on a large scale and do not require very low temperatures for transportation and storage. However, pre-existing immunity to the vector can limit the ability of the vector to deliver genetic material to host cells and thus reduce the effectiveness of the vaccine.
3.3.1. Nonreplicating Viral Vector Vaccines
Nonreplicating viral vector vaccines represent a relatively new approach, and no vaccine of this type was approved before this pandemic. However, 12 vaccine candidates of this type are being evaluated in clinical trials against COVID-19 (Table 4). Many of them use genetically modified adenoviral (Ad) vectors that are unable to replicate in the human body. This is typically achieved by deletion of a gene within the vector that encodes a viral structural protein, thus preventing virion assembly in an infected cell. Assembly of the vaccine vector requires providing the missing structural protein through use of a helper virus or a transgenic host cell. Ad5 nCoV from CanSino Biologics uses human adenovirus 5 and has an overall efficacy of 65.3% after 4 weeks following a single dose vaccination.38 JNJ-78436735, a single-dose vaccine from Johnson & Johnson, uses human adenovirus 26 and shows 66% overall efficacy in preventing moderate to severe COVID-19 at 4 weeks following vaccination.39 AZD1222 developed by AstraZeneca in collaboration with the University of Oxford uses a chimpanzee adenovirus against which humans likely have no pre-existing immunity.40 An interim analysis of a Phase 2/3 trial of this vaccine confirmed its safety and efficacy (70.4%).41 All three of these viral vector vaccines have indeed been granted conditional approval in one or more countries. In addition, VXA-CoV2-1 developed by Vaxart is an adenovirus 5 vector vaccine in a Phase 1 trial that can be administered orally.
Table 4. Viral Vector Candidate Vaccines against COVID-19 in Clinical Trials.
| vaccine platform (no. of vaccines in clinical trials) | developer/manufacturer | vaccine (CAS Registry Number) | clinical stage | route of administrationa (no. of doses) |
|---|---|---|---|---|
| Nonreplicating viral vector (12) | University of Oxford/AstraZeneca (UK) | AZD1222; ChAdOx1-S; ChAdOx1 nCoV-19 (2499737-08-3) | Phase 4 | IM (1–2) |
| CanSino Biological Inc./Beijing Institute of Biotechnology (China) | Ad5 nCoV (2540656-88-8) | Phase 3 | IM (1) | |
| Gamaleya Research Institute (Russia) | rAd26-S+rAd5-S/Gam-COVID-Vac/Sputnik V (2541629-85-8) | Phase 3 | IM (2) | |
| Johnson & Johnson (USA) | Ad26.COV2.S/JNJ-78436735 (2541607-046-7) | Phase 3 | IM (1–2) | |
| ImmunityBio, Inc. & NantKwest Inc. (USA) | hAd5-COVID-19/hAd5-S-Fusion+N-ETSD | Phase 1 | Oral (1) | |
| ReiThera (Italy)/LEUKOCARE (Germany)/Univercells (Belgium) | Gard-CoV2 (2543636-44-6) | Phase 1 | IM (1) | |
| City of Hope (USA) | COH04S1 | Phase 1 | IM (1–2) | |
| Vaxart (USA) | VXA-CoV2-1 (2543668-36-4) | Phase 1 | Oral (2) | |
| Ludwig-Maximilians - University of Munich (Germany) | MVA-SARS-2-S (2543700-32-7) | Phase 1 | IM (2) | |
| Shenzhen Geno-Immune Medical Institute (China) | LV-SMENP-DC vaccine | Phase 1/2 | SC & IV (1) | |
| Altimmune, Inc. (USA) | AdCOVID | Phase 1 | IN (1–2) | |
| Bharat Biotech International Limited (India) | BBV154 | Phase 1 | IN (1) | |
| Replicating viral vector (6) | Jiangsu Provincial Center for Disease Prevention and Control (China) | DelNS1-2019-nCoV-RBD-OPT1 (Intranasal flu-based-RBD) | Phase 2 | IN (1) |
| Shenzhen Geno-Immune Medical Institute (China) | Covid-19/aAPC vaccine | Phase 1 | SC (3) | |
| Israel Institute for Biological Research/Weizmann Inst. of Science (Israel) | VSV-S | Phase 1/2 | IM (1) | |
| Aivita Biomedical, Inc. (USA) | Dendritic cell vaccine AV-COVID-19 | Phase 1/2 | IM (1) | |
| Cellid Co., Ltd. (South Korea) | AdCLD-CoV19 | Phase 1/2 | IM (ND) | |
| Mahidol University; The Government Pharmaceutical Organization (GPO); Icahn School of Medicine at Mount Sinai (Thailand) | NDV-HXP-S, Newcastle disease virus vector | Phase 1/2 | IM (2) |
IM: intramuscular; IN: intranasal; IV: intravenous; SC: subcutaneous.
Although adenoviral vectors may induce immune responses against vector components and attenuate antigen-induced responses, heterologous prime-boost vaccination with two different vectors could minimize this effect.42,43 Sputnik V developed by the Gamaleya Research Institute has two forms, one based on adenovirus 26 vector, and the other based on adenovirus 5 vector. These two forms are administered separately in a three-week interval. The interim analysis of the Phase 3 clinical trial in Russia shows 91.6% efficacy against COVID-19. This vaccine can be produced as a frozen (storage temperature is −18 °C) or freeze-dried (storage temperature is 2–8 °C) formulation.44
3.3.2. Replicating Viral Vector Vaccines
Unlike nonreplicating viral vectors, replicating viral vectors are able to multiply themselves in host cells, such that a smaller dose may be sufficient to induce immunity as compared with nonreplicating viral vector vaccines. However, there can be safety concerns, particularly for immunocompromised individuals, due to the persistence or pathogenicity of the replicating viral vector vaccines.45
Among six replicating viral vector vaccine candidates in clinical trials, an intranasal flu-based-RBD replicating viral vector vaccine is being developed by Jiangsu Provincial Center for Disease Prevention and Control (Table 4). In addition, VSV-S developed by Israel Institute for Biological Research/Weizmann Institute of Science uses the recombinant vesicular stomatitis virus (rVSV), a vector platform similar to the one Merck used in the development of the approved Ebola vaccine ERVEBO.46,47 In humans, wild-type VSV infection is usually asymptomatic or causes mild flu-like symptoms.
3.4. Nucleic Acid Vaccines
Similar to viral vector vaccines, nucleic acid vaccines introduce genetic instructions (DNA or mRNA encoding disease-specific antigens) to host cells and utilize the host cells’ protein-making machinery to generate immunogens. The in situ synthesis of these foreign immunogens within the host cells effectively elicits both antibody production and T-cell induction, which are important parameters of protection as observed in convalescent COVID-19 patients.48−51 In addition, nucleic acid vaccines can more easily be manufactured on a large scale.
3.4.1. DNA Vaccines
DNA vaccines use plasmid DNA containing a mammalian expression promoter and a transgene encoding the protein antigen, such as S protein in the case of COVID-19 vaccines. They are easy to produce in vitro and are very stable at room temperature, which simplifies the storage and distribution of this type of vaccine and may be more practical for use in endemic areas of developing countries.52 The platform can also be easily adapted to produce a new vaccine for another antigen. In early clinical studies, DNA vaccines are associated with low immunogenicity when they were delivered by traditional needle injection without the use of adjuvants or other delivery instruments.53 To compensate, physical delivery devices such as electroporators or gene guns, have been introduced during vaccination to improve the immunogenicity of DNA vaccines.54 No DNA vaccine has yet been approved, but 11 candidates against COVID-19 are in clinical trials (Table 5). The intradermally delivered DNA vaccine INO-4800, developed by Inovio Pharmaceuticals, encodes a full-length S protein and entered the Phase 2 segment of a Phase 2/3 trial in December 2020.55 A preliminary report from a Phase 1 clinical trial showed that INO-4800 is safe and well tolerated in the participants. It is also immunogenic in all subjects in terms of generation of neutralizing antibodies and/or T cell responses.56 AG0301-COVID19 and AG0302-COVID19 developed by AnGes/Osaka University and ZyCoV-D developed by Cadila Healthcare Limited are also in late stage clinical trials (Table 5).
Table 5. Nucleic Acid Candidate Vaccines against COVID-19 in Clinical Trials.
| vaccine platform (no. of vaccines in clinical trials) | developer/manufacturer | vaccine (CAS Registry Number) | clinical stage | route of administrationa (no. of doses) |
|---|---|---|---|---|
| DNA (11) | Inovio Pharmaceuticals/International Vaccine Institute (USA) | INO-4800 (2535490-43-6) | Phase 2/3 | ID (2) |
| Osaka University/AnGes/Takara Bio (Japan) | AG0301-COVID19 (2541593-92-2); AG0302-COVID19 (2541593-93-3) | Phase 2/3 | IM (2) | |
| Cadila Healthcare Limited (India) | ZyCoV-D (2541524-47-2) | Phase 3 | ID (3) | |
| Genexine Consortium (South Korea) | GX-19 (2541485-67-8) | Phase 1/2 | IM (2) | |
| Symvivo (Canada) | bacTRL-Spike | Phase 1 | Oral (1) | |
| Providence Health & Services (USA) | CORVax | Phase 1 | ID (2) | |
| Entos Pharmaceuticals Inc. (Canada) | Covigenix VAX-001 | Phase 1 | IM (2) | |
| GeneOne Life Science, Inc. (South Korea) | GLS-5310 | Phase 1/2 | ID (2) | |
| University of Sydney, Bionet Co., Ltd. Technovalia (Australia) | COVIGEN | Phase 1 | IM (2) | |
| Takis/Rottapharm Biotech (Italy) | COVID-eVax | Phase 1/2 | IM (ND) | |
| Takis/Rottapharm Biotech (Italy) | COVID-eVax | Phase 1/2 | IM (ND) | |
| RNA (8) | Moderna/NIAID (USA) | mRNA-1273 (2457298-05-2) | Phase 4 | IM (2) |
| BioNTech (Germany)//Pfizer (USA) | BNT162b1 (2417899-75-1), BNT162b2 (2417899-77-3) | Phase 4 | IM (2) | |
| Curevac (Germany) | CVNCOV (2541470-90-8) | Phase 3 | IM (2) | |
| Arcturus (USA)/Duke-NUS (Singapore) | ARCT-021 (2541451-24-3) | Phase 2 | ND (ND) | |
| Imperial College London (UK) | LNP-nCoVsaRNA (2545641-90-3) | Phase 1 | IM (2) | |
| Shulan (Hangzhou) Hospital/Center for Disease Control and Prevention of Guangxi Zhuang Autonomous Region (China) | SARS-CoV-2 mRNA vaccine | Phase 1 | IM (2) | |
| Chulalongkorn University (Thailand) | ChulaCov19 | Phase 1 | IM (2) | |
| Providence Therapeutics (Canada) | PTX-COVID19-B | Phase 1 | IM (2) |
ID: Intradermal; IM: intramuscular.
3.4.2. mRNA Vaccines
The mRNA vaccines are comprised of mRNA carrying the genetic instruction of the protein antigens with the mRNA encapsulated in lipid nanoparticles (LNPs). Upon vaccination, LNP-mRNA is delivered to the cytosol of the host cells and the mRNA is subsequently used as a template for protein antigen synthesis. In many COVID-19 mRNA vaccine candidates, the genetic code of the full-length S protein is delivered and translated into S protein using the host cells’ protein-making machinery within the cytosol.57−59 Analogous to the vector vaccines and DNA vaccines, the mRNA vaccines also have the capability to induce both antibody production and T-cell responses, since the protein antigen is produced in the cells of the vaccinated person. In addition, antigen expression after mRNA vaccination is transient, limiting its persistence in the body. These features suggest that mRNA can be a fast, safe, and efficient platform for vaccine development.21
Currently, two COVID-19 mRNA vaccines, from Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273), have been conditionally approved in multiple countries. Six more vaccines are undergoing clinical evaluation (Table 5). Remarkably, it took Moderna only 2 months since the release of the viral genomic sequence to develop mRNA-1273 for clinical trial. In July 2020, the company confirmed its safety and protective immune response after the conclusion of the Phase 1 trial.57 Meanwhile, Pfizer/BioNTech confirmed BNT162b2 safety and immunogenicity data from a Phase 1 trial and moved this vaccine to a Phase 2/3 trial.60 In November, both Moderna and Pfizer/BioNTech announced excellent results for their mRNA vaccines’ efficacy (94.1%61 and 95%,62 respectively) and safety from their Phase 3 trials. In addition, Moderna’s vaccine exhibited 100% efficacy against severe COVID-19.63
Given the unstable nature of RNA molecules, some mRNA candidates require strict cold chain management for distribution and storage. Pfizer/BioNTech and Moderna mRNA vaccines require storage at −70 °C64 and −20 °C, respectively. However, the German company CureVac announced that their mRNA vaccine candidate CVnCOV, currently in Phase 2 study, is stable for three months at 5 °C.65 In addition, Arcturus Therapeutics has developed a single-dose, self-amplifying mRNA vaccine, ARCT-021, that can be stored at a normal refrigerator temperature as a freeze-dried formulation.66
3.5. Summary of the Most Promising COVID-19 Vaccine Candidates
Table 6 summarizes the key features of selected vaccine candidates that, with the exception of NVX-CoV2373, have been conditionally approved by at least one country. Interestingly, the vaccine platforms for these vaccines include only mRNA, nonreplicating viral vector, inactivated, and protein subunit. All these need to be administered via intramuscular injections. Whereas most listed vaccines require two doses, the viral vector-based vaccines developed by Johnson & Johnson and CanSino Biologics need only one dose. Although mRNA vaccines were the first kind to be conditionally approved, their stringent temperature storage condition limits the deployment of these vaccines in rural or undeveloped areas. All other types of vaccines have relatively permissive temperature requirements, which allow them an easier deployment process in less developed areas.
4. Adjuvants, mRNA Sequence Modifications, Formulations, and Delivery Systems
4.1. Commonly Used Adjuvants
Adjuvants contribute to the prophylactic and therapeutic efficacy of vaccines by decreasing the amount of antigen required to elicit a durable immune response, eliciting an inflammatory cytokine and chemokine milieu, and polarizing the helper T-cell response along type 1 vs type 2 differentiation pathways. They are mostly used for protein subunit vaccines. The mechanisms by which these occur are not yet elaborated but are generally recognized to involve a promotion of antigen uptake, an increase in antigen presentation, recruitment of immune cells, and stimulation of the innate immune system via Toll-like receptors that function as a warning system against microbial infection. The adjuvants utilized in most vaccines, including COVID-19 vaccines, are shown in Table 7.
Table 7. Adjuvants Utilized in the Majority of Vaccines.
| adjuvant | trade or trivial name | CAS Registry Number | vaccine (indication) |
|---|---|---|---|
| aluminum hydroxide | Alhydrogel | 21645-51-2 | Infanrix (DTP) |
| Havrix (hepatitis A) | |||
| aluminum phosphate | AdjuPhos | 7784-30-7 | Tenivac (tetanus, diphtheria) |
| UB-612 (COVID-19) | |||
| aluminum hydroxyphosphate sulfate | N.A. | 150828-31-2 | PedvaxHIB (Haemophilus)Gardasil (HPV) |
| oil-in-water emulsion of squalene | MF59 | 172889-84-8 | Fluad (influenza) |
| monophosphoryl lipid A and QS-21 saponin | AS01b | 807365-66-8 | Shingrix (herpes zoster) |
| squalene/α-tocopherol/Tween 80 mixture | AS03 | 880261-17-6 | Pandemrix (influenza) |
| SCB-2019 (COVID-19) | |||
| oil-in-water emulsion of squalene | AF03 | 1244029-44-4 | Humenza (influenza) |
| mixture of saponins | Matrix M | 1235341-17-9 | NVX-CoV2373 (COVID-19) |
| monophosphoryl lipid A + aluminum hydroxide | AS04 | 832690-19-4 | Cervarix (HPV) |
| glucopyranosyl lipid A | GLA-SE | 1246298-63-4 | ID93 (tuberculosis) |
| phosphorothioate oligodeoxyribonucleotide | CpG 1018 | 937402-51-2 | Heplisav-B (hepatitis B) |
| SCB-2019 (COVID-19) | |||
| MVC-COV1901 (COVID-19) | |||
| inulin | Advax | 9005-80-5 | COVAX-19 (COVID-19) |
| potassium aluminum sulfateb | Aluma | 10043-67-1 | N.A. |
| imidazoquinoline derivativesb | 3M-052 | 1359993-59-1 | N.A. (HIV, tumor) |
| squalane-in-water emulsion of sucrose fatty acid sulfate ester | CoVaccine HT | 872176-43-7 | CiVax (COVID-19) |
| water-in-oil emulsion of mannide monooleate surfactant | Montanide ISA-51 | 190396-06-6 | Galinpepimut-S (mesothelioma) |
| SurVaxM (neuroendocrine tumor) |
Collective term for aluminum salts.
Various studies have explored the use of these compounds as vaccine adjuvants, and yet no vaccine with an identifiable lab code or trade name has been reported.
The adjuvants of Table 7 represent traditional and well-established agents. However, many natural products (e.g., polysaccharides, α-galactosylceramide),67 endogenous cytokines (e.g., interleukins, interferons, GM-CSF),68 and synthetic compounds (e.g., saponin derivatives)69 have been examined in preclinical and experimental settings. As the molecular pathways underlying the effects of these adjuvants have been investigated, it has become clearer that the pattern recognition receptor (PRR) system of the innate immune system is targeted by many of these agents.
Pattern recognition comprises systems evolved to detect pathogen-associated molecular patterns (PAMPs) and host danger-associated molecular patterns (DAMPs) by way of receptors of the inflammasome, stimulator of interferon genes protein (STING), and Toll-like receptor (TLR) pathways. Many of the foregoing are known to elicit immune response via Toll-like receptors in addition to the NLR family pyrin domain-containing protein 3 (NLRP3)-inflammasome.70,71 Compounds such as imidazoquinoline derivatives (Table 7) that elicit/enhance immune responses via such a pathway have also been explored as potential vaccine adjuvants.
The global market for vaccine adjuvants was $300–400 M in 2016, and by some estimates will approach $1B by 2027.72,73 Therefore, it is not surprising that many pharmaceutical firms and research institutions are invested in this area. In particular, agonists of Toll-like receptors 3, 4, 7, and 8 have received considerable attention,74,75 and a recent publication from Tsinghua University demonstrated that a cyclic nucleotide agonist of STING could enhance the IgG and T-cell responses to SARS-CoV-2 spike protein over that achieved using aluminum hydroxide as an adjuvant.76
4.2. Modifications of IVT mRNA Sequences to Enhance the Functionality of mRNA Vaccines
Many strategies have been introduced to optimize mRNA sequences to increase their stability and translational efficiency and to minimize intrinsic immunogenicity. Like mammalian mRNA, mRNA vaccines produced by in vitro transcription (IVT) contain an open reading frame (ORF) flanked by 5′ and 3′ untranslated regions (UTRs), a 5′-cap, and a 3′ polyadenylation (poly(A)) tail, as shown in Figure 5. The cap, poly (A) tail, and UTRs are crucial for ribosome-mediated translation and stability of mRNA.
Figure 5.
Schematic illustration of an mRNA molecule with the structural elements.
Efficient translation of mammalian mRNA requires a functional 5′-cap structure in the form of a 7-methylguanosine joined to the first nucleotide of mRNA via a 5′–5′ triphosphate linkage. The process of 5′ capping is crucial for initiation of mRNA translation.77,78 Indeed, mRNA cap analogues have been explored and developed to increase the stability and translational efficiency of mRNA vaccines. In addition, without a cap, the IVT mRNA would be recognized as foreign RNA by the host immune system through a mechanism mediated by Toll-like receptors 7/8.79 Capping of IVT mRNA would prevent its recognition by the host immune system and reduce immunogenicity caused by mRNA molecule itself.77,80
The elongated poly (A) tail at the 3′ end is important for the stability and subsequent translation of mRNA.81 Incorporation of UTR sequences enhances mRNA half-life and translation efficiency.82 For example, incorporating a human β-globin UTR to both 5′- and 3′-UTR of the mRNA has been shown to enhance the stability and translational efficiency,83 whereas insertion of an α-globin UTR to the 3′-UTR region increased the mRNA stability.84 Both 3′- and 5′-UTR regions can also inhibit decapping and degradation of mRNA.83,84 As such, sequence modifications in both 5′- and 3′-UTRs have been explored to enhance the mRNA vaccine functionality.85
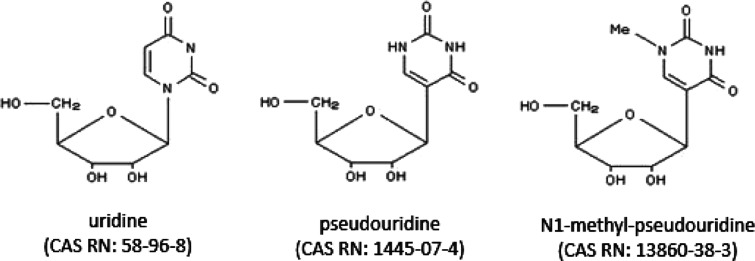
In addition, unmodified IVT mRNA sequences can stimulate Toll-like receptors (TLRs) and activate the innate immune system.86 To reduce immunogenicity of the IVT mRNA, a variety of modified nucleotides have been introduced into the ORF region.86,87 For instance, designed incorporation of pseudouridine (ψ) (Figure 6) has been shown to reduce immunogenicity and improve translation.88 Additionally, mRNA containing N1-methyl-pseudouridine (m1ψ) (Figure 6) exhibited an increase in translational efficiency.89 Recent studies have shown that this increase is related to N1-methyl-pseudouridine induced enhancement of ribosome recycling to the same mRNA.90
Figure 6.
Chemical structures of uridine, pseudouridine, and N1-methyl-pseudouridine.
Aside from the nucleotide modifications, optimizing the codons with GC-rich sequences may also reduce mRNA immunogenicity.91 In an erythropoietin production model without chemical modification, the ORF of mRNA was engineered by adapting GC-rich codons used for each amino acid. The result showed GC-enriched mRNA may achieve meaningful biological effects without causing inappropriate immunostimulation.91
4.3. Nanoparticle-Based Delivery Vehicles for COVID-19 Vaccines
Recent progress in nanotechnology has significantly advanced the development of novel vaccine delivery systems and adjuvants. A major challenge in successfully achieving the full potential of mRNA vaccines and therapeutics is ensuring their efficient delivery. The physicochemical features of nucleic acids, such as negative charge and hydrophilicity, impede passive diffusion across the plasma membrane. Additionally, a series of barriers, such as association with serum proteins, uptake by phagocytes, and degradation by endogenous nucleases, obstruct efficient delivery of nucleic acids. Thus, they require a delivery vehicle for efficient cellular uptake and degradation protection.
Both the Pfizer/BioNTech and Moderna mRNA vaccines are formulated with lipid nanoparticles as the delivery vehicle for the mRNA, with formulations including ionizable cationic lipid. Lipid nanoparticles comprising synthetic cationic lipids, which form nanoscale complexes with polyanionic nucleic acids, are presently the most widely used nonviral nucleic acid carriers. Draped by positively charged lipids, the mRNA is more stable and resilient to RNase degradation and forms self-assembled nanoparticles (Figure 7). Once endocytosed, the lipid nanoparticles escape the endosomes and deliver their cargo in the cytosol, where the mRNA is translated into antigenic proteins, prompting the immune system to produce antibodies. Thus, the basic steps of the nucleic acid delivery include (i) adsorption and endocytosis of lipid nanoparticles inside the cell, followed by (ii) release of the nucleic acid. The lipid nanoparticles’ adsorption and fusion with the cell membrane is electrostatically promoted since the biomembranes commonly bear a negative charge. The unbinding of the nucleic acid from a cationic lipid carrier when the nanoparticles get inside the cell has been identified as one of the key steps in the nucleic acid delivery. It is supposedly a result of charge neutralization by the cellular anionic lipids, which triggers unbinding of nucleic acid from nanoparticle carriers in two distinct ways: by neutralizing cationic lipid charge, thus eliminating the nucleic acid–lipid electrostatic attraction and by disruption of the nanoparticle architecture and formation of nonlamellar structures. It has been suggested that the delivery efficacy of the cationic lipids correlates with their ability to modulate the phase behavior of the biomembrane lipids, specifically to induce formation of nonlamellar lipid phases.92,93
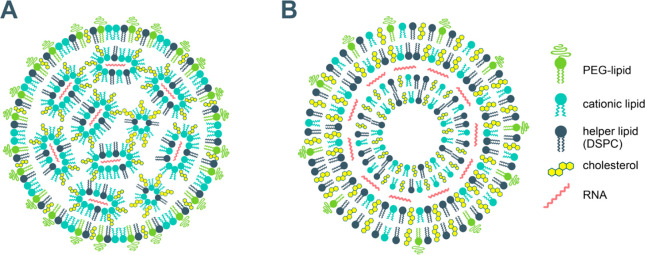
Figure 7.
Suggested structures of lipid nanoparticle vaccine carriers: mRNA organized in inverse lipid micelles inside the nanoparticle97−99 (A); mRNA intercalated between the lipid bilayers100 (B).
The compositions of the lipid nanoparticles of the Pfizer/BioNTech and Moderna mRNA vaccines are similar: ionizable cationic lipid, PEGylated lipid, cholesterol, and the phospholipid distearoylphosphatidylcholine (DSPC) as a helper lipid,11,12,94−96 as shown in Figure 7. Cationic lipids, as mentioned above, are key components in the nucleic acid nonviral drug delivery vehicles as they form complexes with the anionic nucleic acids. PEGylated lipids confer longer systemic circulation of the vehicles by reducing uptake by the macrophages (“stealth” liposomes). Cholesterol and phosphatidylcholines are common biomembrane components and are the most widely used constituents of the lipid drug delivery systems.
The constituents of the lipid nanoparticle delivery vehicles of the two approved mRNA vaccines are summarized in Table 8. The molar ratios of the cationic lipid/PEG-lipid/cholesterol/DSPC are (46.3:1.6:42.7:9.4) for Pfizer/BioNTech and (50:1.5:38.5:10) for Moderna vaccines,101 respectively. Particle sizes between 80 and 100 nm have been reported.102 The number of encapsulated mRNA molecules per lipid nanoparticle is usually on the order of 100.103
Table 8. Constituents of the Lipid Nanoparticle Vehicles of Approved mRNA Vaccines.
| lipid name | abbreviation or lab code | CAS Registry Number |
|---|---|---|
| Pfizer/BioNTech vaccine12,94,95 | ||
| ((4-hydroxybutyl) azanediyl) bis(hexane-6,1-diyl) bis(2-hexyldecanoate) | ALC-0315 | 2036272-55-4 |
| 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide | ALC-0159 | 1849616-42-7 |
| 1,2-distearoyl-sn-glycero-3-phosphocholine | DSPC | 816-94-4 |
| cholesterol | 57-88-5 | |
| Moderna vaccine11,95,96 | ||
| heptadecan-9-yl 8-((2-hydroxyethyl)(6-oxo-6-(undecyloxy)hexyl)amino)octanoate | SM-102 | 2089251-47-6 |
| 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol 2000 | PEG2000-DMG | 1397695-86-1 |
| 1,2-distearoyl-sn-glycero-3-phosphocholine | DSPC | 816-94-4 |
| cholesterol | 57-88-5 | |
The two cationic lipids used—ALC-0315 (Pfizer/BioNTech) and SM-102 (Moderna)—are proprietary for the two companies, respectively. The structures of Moderna proprietary cationic lipids optimized for mRNA delivery104,105 and the vaccine formulations were discussed previously.106 It is noteworthy that the molecular structure of the cationic lipids including branched hydrocarbon chains is appropriate for promoting nonlamellar phase formation, which is closely related to membrane fusion and subsequent cargo release, thus allegedly enhancing the delivery efficacy of the lipid carrier.93
Various other nanotechnology strategies have been explored for vaccine design.107,108 In one such approach, nanoparticles were constructed to display the receptor-binding domain (RBD) of SARS-CoV-2 along with several (4–8) different RBDs from animal betacoronaviruses.109 These constructs, termed mosaic nanoparticles, were produced using SpyTag/SpyCatcher technology for conjugation of recombinant proteins.110 This technology employs a 13-amino-acid peptide SpyTag that spontaneously reacts with an engineered protein domain called SpyCatcher (12.3 kDa) to form an intermolecular peptide bond. This system was used to prepare multimeric SARS-CoV-2 RBD nanoparticles that elicited high titers of neutralizing antibodies.109,111,112
Another kind of nanoparticle vaccine was produced using self-assembling ferritin nanoparticles to display SARS-CoV-2 S protein or its subunits.113,114 This type of vaccine may prompt robust neutralizing antibodies and cellular immune responses in animal models. Therefore, these nanoparticles are considered a promising vaccination approach against SARS-CoV-2 and other coronaviruses.
5. COVID-19 Vaccine-Related Publication Trend Analysis
A significant amount of research has been conducted with regard to the development of COVID-19 vaccines. To gain a better insight into the ongoing research in this area, we performed a series of analyses based on journal articles and patents in the CAS content collection.
5.1. Overall Journal Publications by Country and Organization
An analysis of the CAS content collection, as of the end of February 2021, was performed in order to assess COVID-19 vaccine-related research with regard to countries and organizations. Over 4000 published journal articles related to COVID-19 vaccine development were identified. The United States, China, the UK, India, and Italy are the top five countries, accounting for over 50% of the total publications. Figure 8 depicts the relative number of COVID-19 vaccine-related papers (articles per thousand) classified by country (A) and organization (B). The University of California, University of Oxford, and the National Institutes of Health (USA) have published the highest number of documents on COVID-19 vaccine-related research (Figure 8B).
Figure 8.
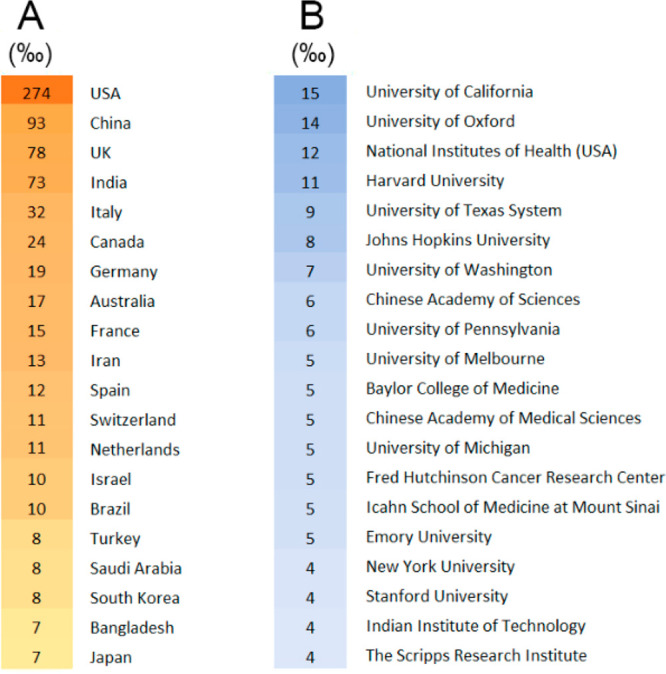
COVID-19 vaccine-related journal article publications classified according to country (A) and organization (B); values are presented as number per thousand (‰). The increase in color intensity reflects the increase in the number of publications.
5.2. Distribution of COVID-19-Related Journal Articles in Different Vaccine Research Areas, Including Vaccine Platforms
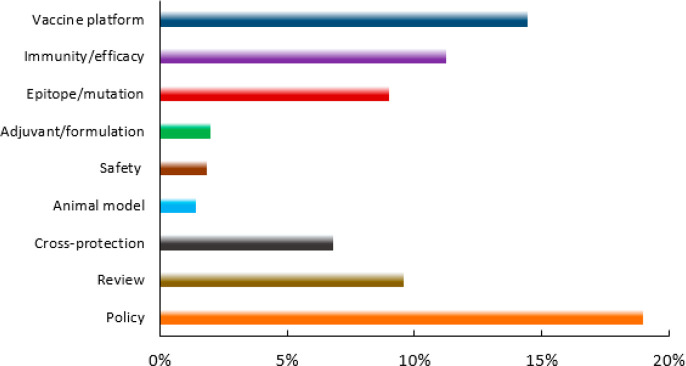
These identified documents were further analyzed with respect to their scientific focus to assess how research efforts were distributed. As shown in Figure 9, about 15% of the published journal articles have been devoted to exploration of various vaccine platforms. There have also been significant efforts exploring the parameters of immunity/efficacy and epitope/mutations. Adjuvants/formulations, safety, and animal models are also important areas of investigation. In addition, researchers have noticed correlations between COVID-19 severity/morbidity and the status of previous vaccinations. Cross-protection by other vaccines has been explored in a sizable number of published documents. After the first vaccines were approved and the vaccination process began, a considerable portion of the articles (∼18%) addressed vaccination policies, including the vaccine administration program and strategy, and its social and psychological perspectives. Because an individual document may have more than one covered scientific focus, the sum of percentage values is greater than 100%.
Figure 9.
Distribution of COVID-19 vaccine-related journal publications in different research areas.
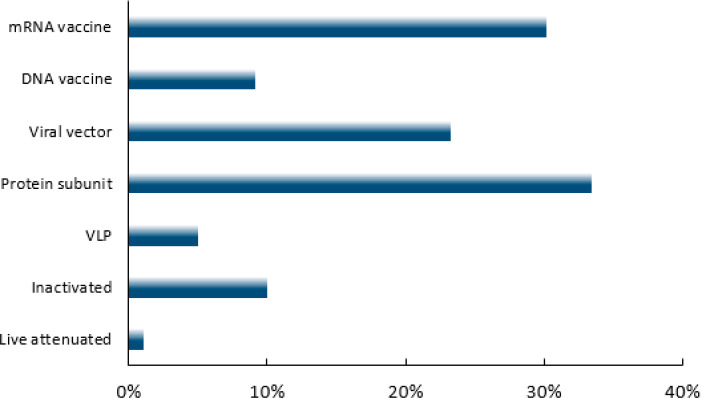
Those documents related to vaccine platforms were further analyzed to reveal the percentage of publications associated with each platform (Figure 10). Publications about the protein subunit vaccine platform account for the largest number of articles. Documents related to mRNA vaccines represent the second largest group, followed in decreasing order by those about viral vector vaccines, inactivated vaccines, DNA vaccines, and VLP vaccines.
Figure 10.
Distribution of COVID-19-related journal publications among vaccine platforms.
At the beginning of the pandemic, researchers noticed correlations between COVID-19 severity and morbidity and the status of previous vaccinations. As Figure 9 shows, there is a significant number of publications regarding vaccine cross-protection. According to a Mayo Clinic research study, various vaccines, from influenza to measles, etc., appear to provide partial protection against COVID-19.115 The exact mechanisms underlying the beneficial off-target effects of the vaccines are not well understood, but it is believed that vaccines induce metabolic and epigenetic changes that enhance the innate immune response to infections, a process termed trained immunity.116,117
Those documents related to COVID-19 cross-protection by other vaccines, as shown in Figure 9, were further analyzed to illustrate the distribution of publications per existing vaccines (Figure 11). Bacillus Calmette–Guérin (BCG) is a live-attenuated vaccine against tuberculosis. Epidemiological analyses have suggested a negative association between national BCG vaccination programs and the prevalence and mortality of COVID-19 disease.118−120 Analysis of the CAS content collection identified 212 documents indicating a possible epidemiological correlation. More than half of these documents suggest that routine BCG immunization programs correlate with lower case fatality rates for COVID-19, while 36 documents assert there is no such correlation. It is noteworthy that virtually all documents, even those advocating the ability of BCG to confer protection against COVID-19, emphasize that convincing proof from extensive preclinical and clinical studies is required. Influenza and pneumococcal vaccines may have a beneficial effect in minimizing the severity of COVID-19.121−124 It has been suggested that influenza vaccination could act as a nonspecific immunity stimulator in patients with COVID-19 leading to early activation of the immune system to attack SARS-CoV-2 before it invades cells. Here again, more than half of the documents discussing the relation of influenza vaccine and COVID-19 disease severity ascertain a positive effect. Further studies are needed to advance our understanding of the role of influenza vaccines in controlling the course of SARS-CoV-2 infection. Evidence indicates that countries with recent vaccination campaigns against MMR (measles, mumps, rubella) have fewer COVID-19 deaths.125 Sequence homology has been identified between the SARS-CoV-2 S glycoprotein and both the measles virus fusion (F1) glycoprotein and the rubella virus envelope (E1) glycoprotein, suggesting that the MMR vaccine may mitigate COVID-19 spread and severity.125,126
Figure 11.
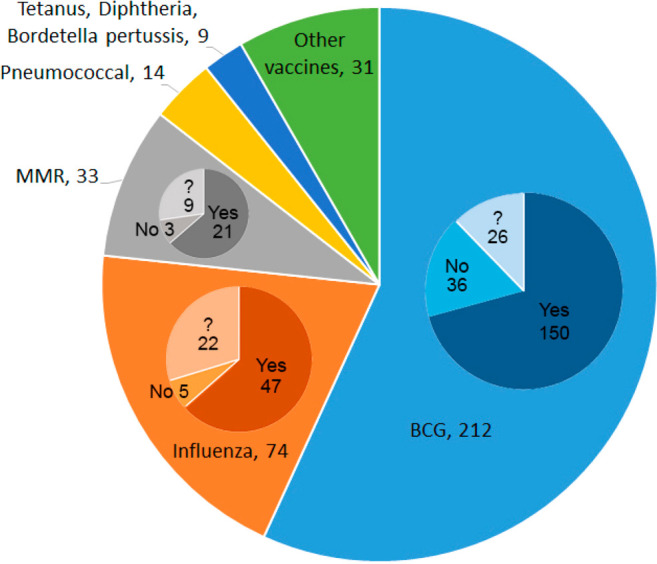
COVID-19 cross-protection by other vaccines. Numbers of documents are next to the vaccine names. In the inset pie-charts for BCG, influenza, and MMR, “Yes” indicates documents suggesting cross-protection, “No” indicates documents suggesting there is no cross-protection; “?” indicates documents which are inconclusive. “Other vaccines” include encephalitis, hepatitis, Newcastle disease, rotavirus, polio, and other routine childhood vaccines.
Although several COVID-19 vaccines have already been approved, the accumulated evidence suggests that vaccines can have cross-protective effects on unrelated infections and diseases as additional routes to prevent and reduce the spread of diseases in the future. This could also buy time for targeted vaccines or effective therapies to be developed or identified. In addition, exploring the mechanism of cross-protection may provide valuable information on the molecular bases of diseases.
5.3. SARS-CoV-2 Mutations and Vaccine Effectiveness
Previous studies have shown that RNA viruses, especially those responsible for respiratory diseases, pose a significant threat to global health.127 The spread of these RNA viruses among the population may select for mutations that change these viruses’ pathogenesis, virulence, and transmissibility, or a combination of these.128 As Figure 9 shows, there are a significant number of publications regarding SARS-CoV-2 mutations in the context of vaccine development. It is not surprising to see that this virus, after over a year circulating in the global population, has accumulated mutations that have produced several variants with stronger transmissibility and possibly altered S protein antigen. Most of the leading COVID-19 vaccines, including those from Moderna, Pfizer/BioNTech, Johnson & Johnson, and Novavax, were developed based on the genetic sequence of the prototype S protein published in January 2020, with minimal amino acid substitutions for conformation stabilization and establishing protease resistance.33,57,58,60,106,129−136
Studies have shown that the variant that emerged in early 2020 with the D614G mutation was recognizable by the antibodies elicited by mRNA-1273 vaccine developed by Moderna.106 In addition, three recently emerged variants (B.1.1.7, B.1.351, and P.1) associated with D614G mutation have raised concerns about the effectiveness of certain approved COVID-19 vaccines. The B.1.1.7 variant, also known as 501Y.V1 with key mutations of N501Y, A570D, D614G, and P681H, emerged in September 2020 in southeast England. Because of its enhanced transmissibility and possibly higher virulence, this variant quickly dominated the UK and has spread to more than 50 countries.137−140 Although this new variant has accumulated several mutations on the S protein, including the most significant N501Y mutation, recent studies demonstrated that sera obtained from individuals vaccinated with mRNA-1273 or BNT162b2 showed similar neutralizing activities against B.1.1.7 variant when compared to the wild type form.141
The B.1.351 variant, also known as 501Y.V2, with key mutations of K417N, E484K, N501Y, and D614G, emerged in late 2020 in South Africa, and since then has become the dominant variant locally.140 A recent study showed that the two leading mRNA vaccines (mRNA-1273 and BNT162b2) may not have comparable effectiveness to this variant as compared to the wild type form.141 Further studies showed that the E484K mutation acquired by this variant on the receptor binding domain (RBD) of the S protein may be the major contributor of the antigenic change leading to the loss of neutralizing activity of the current leading vaccines.141
Another variant, known as P.1 or 501Y.V3, with key mutations of K417N/T, E484K, N501Y, and D614G, is rapidly emerging in Brazil and contains the similar key mutations to the B.1.351 variant.142,143 It is anticipated that this variant may escape the protective effects of the leading mRNA vaccines like the B.1.351 variant.141 Interestingly, recent studies done in South Africa suggested that the antibody response in patients infected with B.1.135 variant has a broad specificity and that vaccines designed with this variant sequence may elicit more cross-reactive responses.144
To cope with the mutations accumulated on the S protein, several leading vaccine developers are already in the process of adjusting their vaccine sequences accordingly. In this regard, vaccines such as the mRNA vaccines that are based on viral antigen sequences and produced in a cell-free environment can be readily adjusted to generate new vaccines against new variants within a short period of time.145 Regardless, viral mutations need to be vigilantly monitored and vaccine antigens adjusted accordingly in order to sustain high efficacy of any newly developed COVID-19 vaccine.
5.4. Most Notable Journal Publications Related to COVID-19 Vaccines
Among the over 4000 COVID-19 vaccine-related journal publications in the CAS content collection, 20 articles were selected to highlight notable information regarding the vaccine platform, design, and formulation, as well as vaccine safety and efficacy (Table 9). The journal impact factor, number of citations, and/or online access/downloads were also important considerations.
Table 9. Most Notable Journal Publications Related to COVID-19 Vaccines.
| paper title | journal | key feature |
|---|---|---|
| Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals146 | Cell (May 20, 2020) | identified and quantified circulating SARS-CoV-2-specific CD8+ and CD4+ T cells in ∼70% and 100% of COVID-19 convalescent patients, respectively. CD4+ T cell responses to the viral S protein were robust and correlated with the magnitude of the anti-SARS-CoV-2 IgG and IgA titers. |
| Development of an inactivated vaccine candidate for SARS-CoV-2147 | Science (July 3, 2020) | inactivated SARS-CoV-2 virus vaccine, PiCoVacc, against SARS-CoV-2 strain in mice, rats, and nonhuman primates |
| Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice148 | Nature Communications (July 9, 2020) | mRNA vaccine encoding the SARS-CoV-2 S protein encapsulated in a lipid nanoparticle |
| An mRNA vaccine against SARS-CoV-2 - preliminary report57 | The New England Journal of Medicine (July 14, 2020) | results from a Phase 1 clinical trial for Moderna vaccine, mRNA-1273, in three different doses |
| Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, Phase 2 trial149 | Lancet (July 20, 2020) | single-dose, nonreplicating adenovirus vector vaccine expressing the full-length SAR-CoV-2 S protein gene |
| Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques129 | Nature (July 30, 2020) | preclinical study on immunogenicity and protective efficacy of a single dose of adenovirus serotype 26 (Ad26) vector-based vaccine expressing the SARS-CoV-2 S protein in nonhuman primates |
| A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity150 | Nature (July 29, 2020) | recombinant protein subunit vaccine tested in nonhuman primates |
| A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice151 | Immunity (July 30, 2020) | two mRNA-LNP vaccines encoding: (1) the full-length SARS-CoV-2 S protein with deleted furin cleavage site and (2) receptor-binding site of the S protein, respectively |
| A universal design of beta-coronavirus vaccines against COVID-19, MERS, and SARS152 | Cell (August 6, 2020) | structure-guided design of protein subunit vaccine composed of tandem repeat single-chain dimer of the RBD of coronavirus |
| A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge153 | Nature Communications (August 14, 2020) | nonreplicating human adenovirus vector-based vaccine encoding SARS-CoV-2 S protein (Ad5-nCoV) tested in mice |
| Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters132 | Nature Medicine (September 3, 2020) | adenovirus vector-based vaccine expressing a stabilized SARS-CoV-2 S protein |
| Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates58 | The New England Journal of Medicine (October 14, 2020) | nucleotide-modified (1-methylpseudouridine) mRNA vaccine by Pfizer/BioNTech encoding the SARS-CoV-2 S protein |
| A platform incorporating trimeric antigens into self-assembling nanoparticles reveals SARS-CoV-2-spike nanoparticles to elicit substantially higher neutralizing responses than spike alone112 | Nature (October 23, 2020) | two nanoparticle platforms, lumazine synthase and ferritin, for the display of trimeric viral protein immunogens using the SpyTag:SpyCatcher system with added N-linked glycosylation sites to nanoparticle monomers to allow the production of the specified nanoparticles in mammalian cell culture |
| Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity154 | Cell (November 12, 2020) | a combined examination of SARS-CoV-2-specific CD4+ and CD8+ T cell and neutralizing antibody responses in acute and convalescent subjects |
| Eliciting B cell immunity against infectious diseases using nanovaccines155 | Nature Nanotechnology (November 16, 2020) | review of nanovaccine transport, localization, and antibody responses as well as its promises and challenges |
| Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK156 | Lancet (December 8, 2020) | evaluated the safety and efficacy of the adenovirus-vectored vaccine (AZD1222) expressing the SARS-CoV-2 S protein in a pooled interim analysis of four trials |
| Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine62 | The New England Journal of Medicine (December 10, 2020) | result from a multinational, randomized, placebo-controlled, observer-blinded clinical trial for a two-dose regimen of BNT162b2 |
| Safety and efficacy of the mRNA-1273 SARS-CoV-2 vaccine61 | The New England Journal of Medicine (December 30, 2020) | result from the Phase 3 randomized, observed-blinded, placebo-controlled clinical trial of mRNA-1273 conducted in the U.S. |
| Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection157 | Science (February 5, 2021) | assessed multiple compartments of circulating immune memory to SARS-CoV-2 in hundreds of COVID-19 cases |
| Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomized, double-blind, placebo-controlled trial158 | Lancet (February 2021) | reported vaccine SCB-2019 developed by Clover Biopharmaceuticals which contains a stabilized trimeric form of the S protein combined with two different adjuvants |
| Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7141 | Nature (March 8, 2021) | tested the effectiveness of neutralizing antibodies and mRNA vaccines mRNA-1273 and BNT162b2 against two SARS-CoV-2 variants |
5.5. Analysis of Patents Related to COVID-19 Vaccine Development
This study also examined 114 COVID-19 vaccine-related patents in the CAS content collection as of the end of February 2021. Most of these patents (92) were filed by organizations in China and only filed to the Chinese Patent Office. There were 10 and 6 patents from organizations in Russia and the United States, respectively. The rest include India (3), Singapore (1), South Korea (1), and Estonia (1). CAS curates patent applications after they are published by patent offices. We anticipate that more patents in this area will be available in the future. Highlighted below are four intriguing patents.
Patent application WO2020198337159 by Dong et al. at The Ohio State University discloses several COVID-19 mRNA candidate vaccines engineered to enhance the expression of antigenic proteins derived from these mRNA molecules. These mRNA vaccine candidates are comprised of the following four components: (1) an RPS27A 5′ untranslated region (5′-UTR) sequence; (2) a heterologous nucleic acid sequence encoding either the full length of SARS-CoV-2 spike protein, or the receptor-binding domain (RBD), or the envelope protein, or the membrane protein, or the nucleocapsid protein; (3) RPS27 3′ untranslated region (3′-UTR) sequence; and (4) a poly(A) tail. Pseudouridine was used to replace uridine triphosphate (UTP) in the in vitro transcription.
Patent application CN111218459A160 by Chen et al. at the Chinese Military Medical Research Institute discloses a simple and fast method for preparing a nonreplicating viral vaccine using human adenovirus (Ad5) as a vector carrying the gene encoding the optimized SARS-CoV-2 S protein. Testing in mouse and guinea pig models shows quick induction of both antibody and cellular immune response and good protection against lung invasion by the SARS-CoV-2 virus. It is claimed that this method can be easily adapted to large-scale production.
Patent application CN111603556A161 by Liu et al. at Sun Yat-Sen University of China discloses the preparation, formulation, and application of a protein subunit vaccine comprising the S1 subunit of the SARS-CoV-2 spike protein, monophosphoryl lipid A, CpG oligonucleotide, a cationic lipid, and an auxiliary lipid to generate vaccines as nanoparticles. As compared with antigen mixed with monophosphoryl lipid A and/or CpG oligonucleotide as adjuvants, or antigen mixed with aluminum adjuvant, this nanoformulation of vaccine exhibited a stronger ability to elicit both humoral and cellular immune responses in animal studies.
Patent application CN111956797A162 by Li et al. at Tsinghua University developed a new vaccine adjuvant that is a chemically modified cyclic dinucleotide. The structure of a representative compound is shown in Figure 12, in which the guanine nucleobase is in the shaded areas. According to the patent, this adjuvant is able to enhance the immunogenicity of various types of SARS-CoV-2 vaccines in terms of both antibody production and T-cell generation and may be superior to aluminum adjuvants.
Figure 12.
Structure of the chemically modified cyclic dinucleotide adjuvant from patent application CN111956797A. Guanine nucleobase is shown in the boxes.
6. Perspectives
Using WHO’s data and CAS-curated data, this report provides a comprehensive review of research and development of COVID-19 vaccines. In particular, it describes the landscape of this ongoing effort and offers comparisons among different vaccine platforms and leading vaccines, as defined by their status in clinical trials.
The devastating impact of COVID-19 has catalyzed unprecedented development of vaccines and vaccine technologies in the fight against this pandemic. Within one year of the outbreak of this disease, many COVID-19 vaccine initiatives are underway worldwide, and more than 70 vaccines have proceeded into clinical trials. A few of these have obtained conditional approval, and more hold the promise to gain such approval in 2021. Whereas many vaccines under development use traditional approaches, several innovative technologies, such as mRNA vaccines and nonreplicating adenovirus vaccines, have quickly risen to a prominent position by leading the race for mass production and distribution and securing conditional approvals. Such achievements can be attributed collectively to decades of pioneering research, timely sharing of the critical information about the virus genome, heightened collaboration among various research entities including universities and pharmaceutical/biotech companies, increased governmental support, and most importantly, the tireless efforts of vaccine scientists who have worked around the clock for the past year.
Despite such remarkable progress in COVID-19 vaccine development, many issues remain to be addressed.163 Although clinical trial data have shown that the COVID-19 vaccines approved so far are able to elicit immunity with a high degree of efficacy, it is not yet known how durable the immunity will be. A recently published study examining multiple components of adaptive immunity in COVID-19 infection cases indicated that SARS-CoV-2 immunity may last at least 8 months following symptom onset.157 On the other hand, a modeling study predicts decay of neutralization titer over the first 250 days after immunization with seven conditionally approved vaccines, although protection from severe disease may be retained.164 More longitudinal studies of immune responses following vaccination will be needed to provide a more definitive answer.165 Since deployment, COVID-19 vaccines have demonstrated protection in vaccinated individuals against the development of COVID-19. Recently released data strongly suggest that certain vaccines can also prevent asymptomatic infection in most cases.166
Finally, mutations of SARS-CoV-2 S protein have been reported, and some variants that bear these mutations may be more infectious or resistant to neutralizing antibodies.141,167,168 Thus, how mutations and the resultant variants would affect the effectiveness of vaccines is a critical issue. Studies addressing this issue have been reported. For example, clinical studies of Johnson & Johnson’s JNJ-78436735 vaccine showed an efficacy of 57% in South Africa where most of the COVID-19 cases were caused by the B.1.351 variant.39 The Novavax vaccine NVX-CoV2373 appeared to have an overall efficacy of 48.6% against key variants, of which the B.1.351 variant was the predominant form.34 In addition, a recently published paper reported that the AstraZeneca/Oxford University ADZ1222 vaccine exhibited only 10.4% efficacy against the B.1.351 variant.169 It is encouraging to know that both Moderna and Pfizer/BioNTech have developed variant B.1.351-specific vaccines which will be tested in clinical trials as booster shots of their variant-specific vaccines.170,171
It is also encouraging that the COVID-19 vaccine pipeline currently features many platforms. Despite high efficacy and relative ease in mass production of mRNA vaccines, the two leading mRNA vaccines still need to be stored and transported at stringent temperatures. This requirement may prevent effective distribution of such vaccines to less developed areas where specialized freezers may not be readily available. Thus, vaccines based on other types of platforms that do not need such stringent storage and transportation conditions are still very much needed. Also, most of the leading vaccines require a booster shot, and therefore effective administration procedures need to be in place to ensure that correct booster shot schedules are followed for widespread vaccination to be accomplished. Vaccines that can elicit durable immunity with a single dose may offer logistical advantages and be more easily adopted in some parts of the world.
In conclusion, although there are still many challenges and unanswered questions, the remarkable breakthroughs in COVID-19 vaccine development have offered the world hope that this pandemic can be defeated in the foreseeable future.
Acknowledgments
We sincerely appreciate the CAS Data, Analytics & Insights team for their assistance in data extraction for this paper. We are thankful to Erica Brown and Xiaohong Wang for enhancing the graphic illustrations and chemical structure representations. We thank Susan Jervey, Robert Bird, Zach Baum, Allison Curtze, Xiang Yu, and Masayo Bechtel for proofreading. We are also very grateful to Manuel Guzman, Gilles Georges, Dawn George, and Dana Albaiu for their encouragement and support.
Author Contributions
# Y.L. and R.T. contributed equally to this paper
The authors declare no competing financial interest.
References
- Valencia D. N. Brief Review on COVID-19: The 2020 Pandemic Caused by SARS-CoV-2. Cureus 2020, 12 (3), e7386. 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Update: FDA Broadens Emergency Use Authorization for Veklury (remdesivir) to Include All Hospitalized Patients for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalized (accessed March 1, 2021).
- The RECOVERY Collaborative Group . Dexamethasone in Hospitalized Patients with Covid-19. New Engl. J. Med. 2020, 384, 693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (accessed March 1, 2021).
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed March 1, 2021).
- Center for Disease Control, History of Smallpox. https://www.cdc.gov/smallpox/history/history.html (accessed December 5, 2020).
- Diseases You Almost Forgot About (Thanks to Vaccines). https://www.cdc.gov/vaccines/parents/diseases/forgot-14-diseases.html (accessed March 1, 2021).
- Greenwood B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. B-Biol. Sci. 2014, 369, 1645. 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre F. E.; Booy R.; Bock H. L.; Clemens J.; Datta S. K.; et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. 10.2471/blt.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccines & Immunizations. https://www.cdc.gov/vaccines/ (accessed March 12, 2021).
- Emergency Use Authorization (EUA) of the Moderna COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) in Individuals 18 Years of Age and Older. https://www.fda.gov/media/144638/download (accessed December 22, 2020).
- Emergency Use Authorization (EUA) of the Pfizer-Biontech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) in Individuals 16 Years of Age and Older. https://www.fda.gov/media/144414/download (accessed December 22, 2020).
- Cohen J.Here’s how the U.S. could release a COVID-19 vaccine before the election—and why that scares some. https://www.sciencemag.org/news/2020/08/here-s-how-us-could-release-covid-19-vaccine-election-and-why-scares-some.
- Huang Y.; Yang C.; Xu X.-f.; Xu W.; Liu S.-w. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S.; Zhu Y.; Liu M.; Lan Q.; Xu W.; et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020, 17, 765–767. 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron-Regalado E. Vaccines for SARS-CoV-2: Lessons from Other Coronavirus Strains. Infect. Dis. Ther. 2020, 9 (2), 255–274. 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen J.; Wang N. S.; Corbett K. S.; Wrapp D.; Kirchdoerfer R. N.; et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E7348–E7357. 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L.; Gao G. F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See R. H.; Zakhartchouk A. N.; Petric M.; Lawrence D. J.; Mok C. P. Y.; et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J. Gen. Virol. 2006, 87, 641–650. 10.1099/vir.0.81579-0. [DOI] [PubMed] [Google Scholar]
- Zhao J. C.; Li K.; Wohlford-Lenane C.; Agnihothram S. S.; Fett C.; et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 4970–4975. 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N.; Hogan M. J.; Porter F. W.; Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discovery 2018, 17, 261–279. 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed December 11, 2020).
- Krammer F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Serum Institute of India Initiates Manufacturing of Codagenix’s Intranasal Live-Attenuated COVID-19 Vaccine Candidate. https://www.prnewswire.com/news-releases/serum-institute-of-india-initiates-manufacturing-of-codagenixs-intranasal-live-attenuated-covid-19-vaccine-candidate-301135221.html (accessed December 11, 2020).
- Dong Y.; Dai T.; Wei Y.; Zhang L.; Zheng M.; et al. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduction and Targeted Therapy 2020, 5, 237. 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zeng G.; Pan H.; Li C.; Hu Y.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinovac Announces Phase III Results of Its COVID-19 Vaccine. http://www.sinovac.com/?optionid=754&auto_id=922 (accessed March 12, 2021).
- Sinovac Receives Conditional Marketing Authorization in China for its COVID-19 Vaccine. http://www.sinovac.com/?optionid=754&auto_id=923 (accessed March 12, 2021).
- Xia S.; Zhang Y.; Wang Y.; Wang H.; Yang Y.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China grants conditional market approval for Sinopharm CNBG’s COVID-19 Vaccine. http://www.sinopharm.com/en/s/1395-4689-38862.html (accessed March 12, 2021).
- Yadav P. D.; Ella R.; Kumar S.; Patil D. R.; Mohandas S.; et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat. Commun. 2021, 12, 1386. 10.1038/s41467-021-21639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3 (accessed March 10, 2021).
- Keech C.; Albert G.; Cho I.; Robertson A.; Reed P.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials. https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0 (accessed March 16, 2021).
- Our Vaccine. https://www.covaxx.com/vaccine (accessed December 11, 2020).
- Mohsen M. O.; Augusto G.; Bachmann M. F. The 3Ds in virus-like particle based-vaccines: ″Design, Delivery and Dynamics. Immunol. Rev. 2020, 296, 155–168. 10.1111/imr.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–556. 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMPA Accepts the Application for Conditional Marketing Authorization of CanSinoBIO’s COVID-19 Vaccine ConvideciaTM. http://www.cansinotech.com/html/1///179/180/651.html (accessed March 12, 2021).
- Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. https://www.jnj.com/johnson-and-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial (accessed March 16, 2021).
- van Doremalen N.; Lambe T.; Spencer A.; Belij-Rammerstorfer S.; Purushotham J. N.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M.; Clemens S. A. C.; Madhi S. A.; Weckx L. Y.; Folegatti P. M.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. Y.; Kang K. W.; Lee E. Y.; Seo D. W.; Kim H. L.; et al. Heterologous prime-boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine 2018, 36, 3468–3476. 10.1016/j.vaccine.2018.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–51. 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D. Y.; Dolzhikova I. V.; Shcheblyakov D. V.; Tukhvatulin A. I.; Zubkova O. V.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura T.; Okuda K.; Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache B. E.; Grobusch M. P.; Agnandji S. T. Safety, immunogenicity and risk-benefit analysis of rVSV-Delta G-ZEBOV-GP (V920) Ebola vaccine in Phase I-III clinical trials across regions. Future Microbiol. 2020, 15, 85–106. 10.2217/fmb-2019-0237. [DOI] [PubMed] [Google Scholar]
- Ebola Vaccines. https://www.niaid.nih.gov/diseases-conditions/ebola-vaccines (accessed December 11, 2020).
- Zhu L. N.; Yang P. H.; Zhao Y. Z.; Zhuang Z. K.; Wang Z. F.; et al. Single-Cell Sequencing of Peripheral Mononuclear Cells Reveals Distinct Immune Response Landscapes of COVID-19 and Influenza Patients. Immunity 2020, 53, 685. 10.1016/j.immuni.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D.; Giles J. R.; Baxter A. E.; Oldridge D. A.; Greenplate A. R.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T.; Perez-Potti A.; Rivera-Ballesteros O.; Stralin K.; Gorin J. B.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158. 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames A. H.; Wolniak K. L.; Stupp S. I.; Jewett M. C. Principles Learned from the International Race to Develop a Safe and Effective COVID-19 Vaccine. ACS Cent. Sci. 2020, 6, 1341–1347. 10.1021/acscentsci.0c00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolotti M.; Kawalekar O.; Shedlock D. J.; Muthumani K.; Weiner D. B. DNA vaccines for targeting bacterial infections. Expert Rev. Vaccines 2010, 9, 747–763. 10.1586/erv.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Wang S.; Lu S. DNA immunization as a technology platform for monoclonal antibody induction. Emerging Microbes Infect. 2016, 5, e33. 10.1038/emi.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.; Evans K.; McElwaine-Johnn H.; Sharpe M.; Oxford J.; et al. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine 2009, 27, 2506–12. 10.1016/j.vaccine.2009.02.061. [DOI] [PubMed] [Google Scholar]
- INOVIO Doses First Subject in Phase 2 Segment of its INNOVATE Phase 2/3 Clinical Trial for INO-4800, its DNA Medicine to Prevent COVID-19. http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Doses-First-Subject-in-Phase-2-Segment-of-its-INNOVATE-Phase-23-Clinical-Trial-for-INO-4800-its-DNA-Medicine-to-Prevent-COVID-19/default.aspx (accessed March 12, 2021).
- Tebas P.; Yang S.; Boyer J. D.; Reuschel E. L.; Patel A.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine 2021, 31, 100689. 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L. A.; Anderson E. J.; Rouphael N. G.; Roberts P. C.; Makhene M.; et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E.; Frenck R. W.; Falsey A. R.; Kitchin N.; Absalon J.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay P. F.; Hu K.; Blakney A. K.; Samnuan K.; Brown J. C.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523. 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Frenck R., Falsey A. R., Kitchin N., Absalon J., et al. RNA-Based COVID-19 Vaccine BNT162b2 Selected for a Pivotal Efficacy Study. medRxiv, 2020, 10.1101/2020.08.17.20176651. [DOI] [Google Scholar]
- Baden L. R.; El Sahly H. M.; Essink B.; Kotloff K.; Frey S.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F. P.; Thomas S. J.; Kitchin N.; Absalon J.; Gurtman A.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.‘Absolutely remarkable’: No one who got Moderna’s vaccine in trial developed severe COVID-19. Science, 2020https://www.sciencemag.org/news/2020/11/absolutely-remarkable-no-one-who-got-modernas-vaccine-trial-developed-severe-covid-19. 10.1126/science.abf9360. [DOI]
- Coronavirus (COVID-19) Update: FDA Allows More Flexible Storage, Transportation Conditions for Pfizer-BioNTech COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-allows-more-flexible-storage-transportation-conditions-pfizer (accessed March 16, 2021).
- CureVac’s COVID-19 Vaccine Candidate, CVnCoV, Suitable for Standard Fridge Temperature Logistics. https://www.curevac.com/en/2020/11/12/curevacs-covid-19-vaccine-candidate-cvncov-suitable-for-standard-fridge-temperature-logistics/ (accessed December 11, 2020).
- Flanagan C.One-Shot Wonders. https://www.bloomberg.com/news/newsletters/2020-10-10/one-shot-wonders (accessed December 28, 2020).
- Woods N., Niwasabutra K., Acevedo R., Igoli J., Altwaijry N. A., et al. Chapter 11 - Natural Vaccine Adjuvants and Immunopotentiators Derived From Plants, Fungi, Marine Organisms, and Insects. In Immunopotentiators in Modern Vaccines, 2nd ed.; Schijns V. E. J. C.; O’Hagan D. T., Eds.; Academic Press, 2017; pp 211–229. [Google Scholar]
- Degen W. G. J.; Schijns V. E. J. C.. Chapter 4 - Host-Derived Cytokines and Chemokines as Vaccine Adjuvants. In Immunopotentiators in Modern Vaccines (2nd ed.); Schijns V. E. J. C.; O’Hagan D. T., Eds.; Academic Press, 2017; pp 65–84. [Google Scholar]
- Fernandez-Tejada A.; Tan D. S.; Gin D. Y. Development of Improved Vaccine Adjuvants Based on the Saponin Natural Product QS-21 through Chemical Synthesis. Acc. Chem. Res. 2016, 49, 1741–1756. 10.1021/acs.accounts.6b00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke S.; Thakur A.; Gartlan C.; Bezbradica J. S.; Milicic A. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 2020, 8, 554. 10.3390/vaccines8030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G.; Orr M. T.; Fox C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- Vaccine Adjuvants Market Size And Forecast By Type (Pathogen, Adjuvant Emulsion, Particulate), By Route of Administration (Oral, Intradermal, Intranasal, Intramuscular), By Application, And Trend Analysis, 2018–2025. https://www.grandviewresearch.com/industry-analysis/vaccine-adjuvants-market (accessed January 6, 2021).
- Global vaccine adjuvants market size to surpass USD 1,305 million mark by 2027. https://www.globenewswire.com/news-release/2020/10/27/2114924/0/en/Global-vaccine-adjuvants-market-size-to-surpass-USD-1-305-million-mark-by-2027.html (accessed January 6, 2021).
- Kieffer M. E.; Patel A. M.; Hollingsworth S. A.; Seganish W. M. Small molecule agonists of toll-like receptors 7 and 8: a patent review 2014–2020. Expert Opin. Ther. Pat. 2020, 30, 825–845. 10.1080/13543776.2020.1825687. [DOI] [PubMed] [Google Scholar]
- Liang Z.; Zhu H.; Wang X.; Jing B.; Li Z. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.-J.; Chen Y.-X.; Li Y.-M.. Adopting STING agonist cyclic dinucleotides as a potential adjuvant for SARS-CoV-2 vaccine. bioRxiv, 2020. 10.1101/2020.07.24.217570. [DOI] [Google Scholar]
- Galloway A.; Cowling V. H. mRNA cap regulation in mammalian cell function and fate. Biochim. Biophys. Acta, Gene Regul. Mech. 2019, 1862, 270–279. 10.1016/j.bbagrm.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan A.; Robb G. B.; Chan S.-H. mRNA capping: biological functions and applications. Nucleic Acids Res. 2016, 44, 7511–7526. 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.; Wang L. T.; Chen S. W. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010, 14, 2592–2603. 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K. 5′-Terminal cap structure in eukaryotic messenger ribonucleic-acids. Microbiol. Rev. 1980, 44, 175–205. 10.1128/MR.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann C. R.; Rammelt C.; Wahle E. Control of poly(A) tail length. Wiley Interdisciplinary Reviews-Rna 2011, 2, 348–361. 10.1002/wrna.56. [DOI] [PubMed] [Google Scholar]
- Asrani K. H.; Farelli J. D.; Stahley M. R.; Miller R. L.; Cheng C. J.; et al. Optimization of mRNA untranslated regions for improved expression of therapeutic mRNA. RNA Biol. 2018, 15, 756–762. 10.1080/15476286.2018.1450054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibzadeh S.; Fardaei M.; Takhshid M. A.; Miri M. R.; Rafiei Dehbidi G.; et al. Enhancing Stability of Destabilized Green Fluorescent Protein Using Chimeric mRNA Containing Human Beta-Globin 5′ and 3′ Untranslated Regions. Avicenna J. Med. Biotechnol 2019, 11, 112–117. [PMC free article] [PubMed] [Google Scholar]
- Rodgers N. D.; Wang Z. R.; Kiledjian M. Regulated alpha-globin mRNA decay is a cytoplasmic event proceeding through 3 ’-to-5 ’ exosome-dependent decapping. RNA 2002, 8, 1526–1537. 10.1017/s1355838202029035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson N. A. C.; Kester K. E.; Casimiro D.; Gurunathan S.; DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. npj Vaccines 2020, 5, 11. 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariko K.; Buckstein M.; Ni H. P.; Weissman D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Sahin U.; Karikó K.; Türeci Ö. mRNA-based therapeutics — developing a new class of drugs. Nat. Rev. Drug Discovery 2014, 13, 759–780. 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- Kariko K.; Muramatsu H.; Welsh F. A.; Ludwig J.; Kato H.; et al. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. 10.1586/14760584.2015.973859. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V.; Cheng Y. M.; Chakraborty T.; Presnyak V.; John M.; et al. N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res. 2017, 45, 6023–6036. 10.1093/nar/gkx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thess A.; Grund S.; Mui B. L.; Hope M. J.; Baumhof P.; et al. Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol. Ther. 2015, 23, 1456–1464. 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koynova R.; Tenchov B.. Cationic Lipids: Molecular Structure/Transfection Activity Relationships and Interactions with Biomembranes. In Nucleic Acid Transfection; Bielke W.; Erbacher C., Eds.; Springer-Verlag: Berlin, Heidelberg, 2010; Vol. 296, pp 51–93. [DOI] [PubMed] [Google Scholar]
- Koynova R.; Wang L.; MacDonald R. C. An intracellular lamellar - nonlamellar phase transition rationalizes the superior performance of some cationic lipid transfection agents. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 14373–14378. 10.1073/pnas.0603085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer-Biontech COVID-19 Vaccine - bnt162b2 injection, suspension. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=908ecbe7-2f1b-42dd-94bf-f917ec3c5af8 (accessed December 22, 2020).
- Miller K.What’s in the Pfizer and Moderna COVID-19 Vaccines? https://www.prevention.com/health/a35002158/pfizer-vs-moderna-covid-19-vaccine-ingredients/ (accessed December 22, 2020).
- Vaccines and Related Biological Products Advisory Committee Meeting. Moderna COVID-19 Vaccine. FDA Briefing Document. https://www.fda.gov/media/144434/download (accessed December 22, 2020).
- Scheideler M.; Vidakovic I.; Prassl R. Lipid nanocarriers for microRNA delivery. Chem. Phys. Lipids 2020, 226, 104837. 10.1016/j.chemphyslip.2019.104837. [DOI] [PubMed] [Google Scholar]
- Evers M. J. W.; Kulkarni J. A.; van der Meel R.; Cullis P. R.; Vader P.; et al. State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery. Small Methods 2018, 2, 1700375. 10.1002/smtd.201700375. [DOI] [Google Scholar]
- Lam K.; Heyes J.; Judge A.; Palmer L.; Yuen H., et al. Advances in Clinically Viable LNP Compositions for mRNA Delivery. https://investor.arbutusbio.com/static-files/5e2f8172-07ee-4019-b4b8-22c0ab38e4df (accessed January 15, 2021). [Google Scholar]
- Kulkarni J. A.; Thomson S. B.; Zaifman J.; Leung J.; Wagner P. K.; et al. Spontaneous, solvent-free entrapment of siRNA within lipid nanoparticles. Nanoscale 2020, 12, 23959–23966. 10.1039/D0NR06816K. [DOI] [PubMed] [Google Scholar]
- DeFrancesco L. Whither COVID-19 vaccines?. Nat. Biotechnol. 2020, 38, 1132–1145. 10.1038/s41587-020-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis S.; Kumarasinghe E. S.; Salerno T.; Mihai C.; Ketova T.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018, 26, 1509–1519. 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez Arteta M.; Kjellman T.; Bartesaghi S.; Wallin S.; Wu X.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E3351–E3360. 10.1073/pnas.1720542115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett K. J.; Benenato K. E.; Jacquinet E.; Lee A.; Woods A.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther.--Nucleic Acids 2019, 15, 1–11. 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenato K. E.; Cornebise M.. Compounds and compositions for intracellular delivery of therapeutic agents. US 10442756, Oct 15, 2019.
- Corbett K. S.; Edwards D. K.; Leist S. R.; Abiona O. M.; Boyoglu-Barnum S.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune K. D.; Howarth M. New Routes and Opportunities for Modular Construction of Particulate Vaccines: Stick, Click, and Glue. Front. Immunol. 2018, 9, 1432. 10.3389/fimmu.2018.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea R.How Nanotechnology Helped Create mRNA COVID-19 Vaccines. [Online], 2020. https://www.azonano.com/news.aspx?newsID=37659 (accessed January 16, 2021).
- Cohen A. A.; Gnanapragasam P. N. P.; Lee Y. E.; Hoffman P. R.; Ou S. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 2021, 371, 735. 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri B.; Fierer J. O.; Celik E.; Chittock E. C.; Schwarz-Linek U.; et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, E690–E697. 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. K.; Rijal P.; Rahikainen R.; Keeble A. H.; Schimanski L., et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. bioRxiv 2020. 10.1101/2020.08.31.275701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.; Chao C. W.; Tsybovsky Y.; Abiona O. M.; Hutchinson G. B.; et al. A platform incorporating trimeric antigens into self-assembling nanoparticles reveals SARS-CoV-2-spike nanoparticles to elicit substantially higher neutralizing responses than spike alone. Sci. Rep. 2020, 10, 18149. 10.1038/s41598-020-74949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. C.; Zou F.; Yu F.; Li R.; Yuan Y. C.; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315. 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell A. E.; Zhang K.; Sanyal M.; Tang S.; Weidenbacher P. A.; et al. A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice. ACS Cent. Sci. 2021, 7, 183–199. 10.1021/acscentsci.0c01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J.Non-COVID vaccines offer some COVID protection, Mayo Clinic reports [Online], 2020. https://www.startribune.com/non-covid-vaccines-offer-some-covid-protection-mayo-reports/571949932/.
- Netea M. G.; Giamarellos-Bourboulis E. J.; Domínguez-Andrés J.; Curtis N.; van Crevel R.; et al. Trained Immunity: a Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell 2020, 181, 969–977. 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landhuis E.‘Trained Immunity’ Offers Hope in Fight Against Coronavirus. Quanta Magazine, September 14, 2020.
- Escobar L. E.; Molina-Cruz A.; Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 17720–17726. 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.; Reandelar M. J.; Fasciglione K.; Roumenova V.; Li Y., et al. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv 2020, 10.1101/2020.03.24.20042937. [DOI] [Google Scholar]
- Pathak S.; Jolly M. K.; Nandi D.. Protective roles of flu infections and BCG vaccination in lowering Covid-19 mortality 2020. https://www.researchsquare.com/article/rs-33242/v1.
- Paguio J. A.; Yao J. S.; Dee E. C. Silver lining of COVID-19: Heightened global interest in pneumococcal and influenza vaccines, an infodemiology study. Vaccine 2020, 38, 5430–5435. 10.1016/j.vaccine.2020.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M. L.; El-Hennawy D. The possible beneficial adjuvant effect of influenza vaccine to minimize the severity of COVID-19. Med. Hypotheses 2020, 140, 109752. 10.1016/j.mehy.2020.109752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thindwa D.; Quesada M. G.; Liu Y.; Bennett J.; Cohen C.; et al. Use of seasonal influenza and pneumococcal polysaccharide vaccines in older adults to reduce COVID-19 mortality. Vaccine 2020, 38, 5398–5401. 10.1016/j.vaccine.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Hernandez D.; Schwartz R. E.; Nixon D. F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2021, 93, 64–65. 10.1002/jmv.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiq K. R.; Sabir D. K.; Ali S. M.; Kodzius R. Does Early Childhood Vaccination Protect Against COVID-19?. Front. Mol. Biosci. 2020, 7, 120. 10.3389/fmolb.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.; Neumann B.; Mendez R. F.; Reyahi A.; Joannides A., et al. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID-19. medRxiv 2020, 10.1101/2020.04.10.20053207. [DOI] [Google Scholar]
- Lam T. T.; Zhu H.; Guan Y.; Holmes E. C. Genomic Analysis of the Emergence, Evolution, and Spread of Human Respiratory RNA Viruses. Annu. Rev. Genomics Hum. Genet. 2016, 17, 193–218. 10.1146/annurev-genom-083115-022628. [DOI] [PubMed] [Google Scholar]
- Baric R. S. Emergence of a Highly Fit SARS-CoV-2 Variant. N. Engl. J. Med. 2020, 383, 2684–2686. 10.1056/NEJMcibr2032888. [DOI] [PubMed] [Google Scholar]
- Mercado N. B.; Zahn R.; Wegmann F.; Loos C.; Chandrashekar A.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583. 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K. S.; Flynn B.; Foulds K. E.; Francica J. R.; Boyoglu-Barnum S.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.-H., Patel N., Haupt R., Zhou H., Weston S., et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 elicits immunogenicity in baboons and protection in mice. bioRxiv 2020, 10.1101/2020.06.29.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostanoski L. H.; Wegmann F.; Martinot A. J.; Loos C.; McMahan K.; et al. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat. Med. 2020, 26, 1694–1700. 10.1038/s41591-020-1070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. J.; Lyke K. E.; Kitchin N.; Absalon J.; Gurtman A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589. 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Sahin U.; Muik A.; Derhovanessian E.; Vogler I.; Kranz L. M.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Bangaru S.; Ozorowski G.; Turner H. L.; Antanasijevic A.; Huang D.; et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. 10.1126/science.abe1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R.; Rutten L.; van der Lubbe J. E. M.; Bakkers M. J. G.; Hardenberg G. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. Npj Vaccines 2020, 5, 91. 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A.; Loman N.; Pybus O.; Barclay W.; Barrett J., et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. https://virological.org/t/preliminarygenomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-ukdefined-by-a-novel-set-ofspike-mutations/563. (accessed March 11, 2021).
- Washington N. L.; Gangavarapu K.; Zeller M.; Bolze A.; Cirulli E. T., et al. Genomic epidemiology identifies emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States. medRxiv 2021. 10.1101/2021.02.06.21251159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ. 2020, 371, m4857. 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- Tegally H.; Wilkinson E.; Giovanetti M.; Iranzadeh A.; Fonseca V., et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv 2020, 10.1101/2020.12.21.20248640. [DOI] [Google Scholar]
- Wang P.; Nair M. S.; Liu L.; Iketani S.; Luo Y., et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature 2021, 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Nuno R.; Faria N. R.; Claro I. M.; Candido D.; Moyses Franco L. A. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586 (accessed March 11, 2021).
- Naveca F.; Nascimento V.; Souza V.; Corado A.; Nascimento F. Phylogenetic relationship of SARS-CoV-2 sequences from Amazonas with emerging Brazilian variants harboring mutations E484K and N501Y in the Spike protein. https://virological.org/t/phylogenetic-relationship-of-sars-cov-2-sequences-from-amazonas-with-emerging-brazilian-variants-harboring-mutations-e484k-and-n501y-in-the-spike-protein/585 (accessed March 11, 2021).
- Moyo-Gwete T.; Madzivhandila M.; Makhado Z.; Ayres F.; Mhlanga D., et al. SARS-CoV-2 501Y.V2 (B.1.351) elicits cross-reactive neutralizing antibodies. bioRxiv 2021, 10.1101/2021.03.06.434193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scally D.Pfizer-BioNTech Covid-19 vaccine likely to be effective against UK mutation. https://www.irishtimes.com/business/health-pharma/pfizer-biontech-covid-19-vaccine-likely-to-be-effective-against-uk-mutation-1.4444212 (accessed December 29, 2020).
- Grifoni A.; Weiskopf D.; Ramirez S. I.; Mateus J.; Dan J. M.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489. 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q.; Bao L. L.; Mao H. Y.; Wang L.; Xu K. W.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77. 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay P. F.; Hu K.; Blakney A. K.; Samnuan K.; Brown J. C. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523. 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F. C.; Guan X. H.; Li Y. H.; Huang J. Y.; Jiang T.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Y.; Wang W.; Chen Z. M.; Lu S. Y.; Yang F. L.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572. 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- Laczko D.; Hogan M. J.; Toulmin S. A.; Hicks P.; Lederer K.; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724. 10.1016/j.immuni.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L. P.; Zheng T. Y.; Xu K.; Han Y. X.; Xu L. L.; et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell 2020, 182, 722. 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. P.; Zhong G. X.; Zhang J.; Shuai L.; Zhang Z. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderbacher C. R.; Ramirez S. I.; Dan J. M.; Grifoni A.; Hastie K. M.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996. 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat. Nanotechnol. 2021, 16, 16–24. 10.1038/s41565-020-00790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M.; Clemens A. N. K.; Madhi S. A.; Weckx L. Y.; Folegatti P. M.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J. M.; Mateus J.; Kato Y.; Hastie K. M.; Yu E. D. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond P.; Hatchuel L.; Dong M.; Ma B.; Hu B.; et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 682–694. 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Zeng C.; Zhao W.. New engineered mRNA comprises 1st nucleic acid sequence comprising RPS27A 5′ untranslated region (5′UTR) sequence and 2nd nucleic acid sequence comprising heterologous nucleic acid sequence, for increasing protein expression. WO2020198337-A1, 2020.
- Chen W.; Wu S.; Hou L.; Zhang Z.; Wang B., et al. New polynucleotide encoding 2019 new coronavirus S protein, useful in human replication-defective recombinant adenovirus for preparing 2019 new coronavirus vaccine comprising specified nucleotide sequence. CN111218459-A; CN111218459-B, 2020.
- Liu L.; Chen Y.; Chen H.; Liu Z.; Liu H.. New nanoparticle useful in preparation of immunogenic composition for diseases related to severe acute respiratory syndrome (SARS)-coronavirus (CoV)-2 infection, contains SARS-CoV-2 virus spike protein and auxiliary lipid. CN111603556-A.
- Li Y.; Wu J.; Chen Y.. Use of (1R,5R,9R,14R)-2,4,6,10,12,15-hexaoxa-3(5),11(5)-diphosphatricyclo(12.3.0.0(5,9))heptadecane-3,11-dione compounds in preparing vaccine adjuvant and vaccine composition. CN111956797-A, 2020.
- Hodgson S.; Mansatta K.; Mallet t. G.; Harris V.; Emary K. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D. S.; Cromer D.; Reynaldi A.; Schlub T. E.; Wheatley A. K., et al. What level of neutralising antibody protects from COVID-19? medRxiv 2021, 10.1101/2021.03.09.21252641. [DOI] [Google Scholar]
- Widge A. T.; Rouphael N. G.; Jackson L. A.; Anderson E. J.; Roberts P. C.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real-World Evidence Confirms High Effectiveness of Pfizer-BioNTech COVID-19 Vaccine and Profound Public Health Impact of Vaccination One Year After Pandemic Declared. https://www.pfizer.com/news/press-release/press-release-detail/real-world-evidence-confirms-high-effectiveness-pfizer (accessed March 16, 2021).
- Li Q.; Wu J.; Nie J.; Zhang L.; Hao H.; et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell 2020, 182, 1284. 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A. J.; Starr T. N.; Gilchuk P.; Zost S. J.; Binshtein E.; et al. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain that Escape Antibody Recognition. Cell Host Microbe 2021, 29, 44–57. 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S. A.; Baillie V.; Cutland C. L.; Voysey M.; Koen A. L.. et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021. 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer and BioNTech Initiate a Study as Part of Broad Development Plan to Evaluate COVID-19 Booster and New Vaccine Variants. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-part-broad-development (accessed March 10, 2021).
- Moderna Announces it has Shipped Variant-Specific Vaccine Candidate, mRNA-1273.351, to NIH for Clinical Study. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-it-has-shipped-variant-specific-vaccine/ (accessed March 11, 2021).