Abstract
Mutations in isocitrate dehydrogenase 1 (IDH1) are found in a high proportion of glial tumors and have a significant prognostic impact. Although direct sequencing has been considered to be the gold‐standard method to detect this mutation, the sensitivity of this technique has been questioned especially because specimens from glial tumors may contain large numbers of non‐tumor cells. We screened 141 cases of oligodendroglial tumors for IDH1 mutations using peptide nucleic acid (PNA)‐mediated clamping polymerase chain reaction (PCR) and compared the results with the results of direct sequencing, pyrosequencing, and immunohistochemistry (IHC). Nested PCR was only performed in cases having mutant IDH1 only discovered by clamping PCR. Using dilution experiments mixing IDH1 wild‐type and mutant DNA samples, clamping PCR detected mutations in samples with a 1% tumor DNA composition. Using PNA clamping PCR, we detected 138 of 141 (97.9%) cases with mutant IDH1 in our series, which is significantly higher (P = 0.016; PNA clamping vs. direct sequencing) than those of direct sequencing (74.5%), pyrosequencing (75.2%) and IHC (75.9%). From our results, almost all oligodendroglial tumors have IDH1 mutations, and this suggests that IDH1 mutation is an early and common event especially in the development of oligodendroglial tumors.
Keywords: isocitrate dehydrogenase 1, oligodendroglial tumors, PNA‐mediated clamping PCR, pyrosequencing
Introduction
Mutations in isocitrate dehydrogenase 1 (IDH1) are frequently and selectively found in a high proportion of gliomas 4, 21. They are remarkably specific to a single codon in the highly conserved and functionally important Arg132 residue in IDH1 gene. Most of these mutations are G395A (Arg132His), while C394G (Arg132Ser), G395T (Arg132Leu), C394G (Arg132Gly) and C394T (Arg132Cys) constitute a minor proportion of IDH1 mutations 25. Recent studies have demonstrated the prognostic impact of IDH1 mutations in World Health Organization (WHO) grade II, III and IV gliomas 10, 25, and now IDH1 mutation has become one of the most significant and clinically relevant issues in the current neuro‐oncology field.
Direct sequencing has been considered to be the gold standard for the detection of IDH1 mutation. However, the sensitivity of this method is influenced by sample quality, requiring at least 50% of cells in a sample to be tumor cells. In addition, the threshold of detection of mutant DNA in a wild‐type environment is around 25% 6, 20. Gliomas are innately infiltrative tumors that are often admixed with a normal cell population. Thus, detection of IDH1 mutations requires an assay that is more sensitive than conventional polymerase chain reaction (PCR)‐direct sequencing, and such as assay would be a great asset to the clinical laboratory. Several techniques for the detection of IDH1 mutations including pyrosequencing 7, single‐strand conformation polymorphism 30, PCR‐ and restriction endonuclease‐based detection 15, immunohistochemistry (IHC) 24, melting curve analysis performed on real‐time PCR 11 and the PCR‐based SNaPshot® assay 22 have been introduced for clinical applications, but these techniques appear not to be significantly better for IDH1 mutation detection than conventional direct sequencing methods.
Peptide nucleic acid (PNA) oligomers were developed to detect minimal amounts of mutant DNA in clinical samples 19. In PNA‐mediated clamping PCR, PNA oligomers suppress the amplification of the complementary sequence by a pair of DNA oligonucleotide primers, as PNA is not a substrate for DNA polymerase (Figure 1). Thus, a PNA‐clamped probe assay is more sensitive than direct sequencing, and the sensitivity and validity of this technique have been previously demonstrated in the detection of other genetic mutations 17, 26, 27, 29. In the present study, we describe a simple and highly sensitive PNA‐mediated real‐time PCR clamping technique for the detection of IDH1 mutations and discuss the clinical implications of this sensitive technique in the detection of oligodendroglial tumors.
Figure 1.
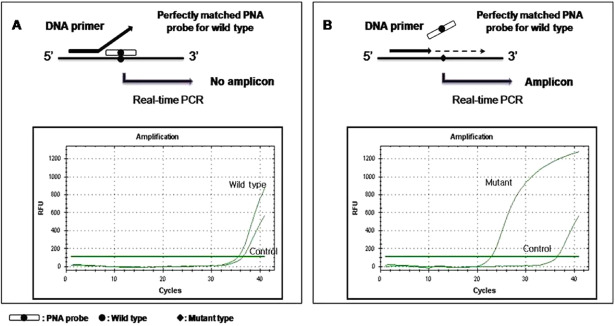
The peptide nucleic acid (PNA) clamping system. The PNA oligomer was designed to bind to the bottom strand of the wild‐type sequence, spanning mutational hot spot of the isocytrate dehydrogenase 1 gene. The forward polymerase chain reaction (PCR) primer partially overlapped the PNA binding site. (A) A PNA/DNA hybrid with a perfect match prevents annealing of the PCR primer and amplification of wild‐type DNA. (B) A PNA/DNA hybrid with a single‐base pair mismatch does not suppress annealing of the PCR primer or amplification of mutant alleles.
Materials and Methods
Patients and tumor samples
A total of 141 oligodendroglial tumor cases of WHO grade II and III were retrieved from the tumor registry of the Samsung Medical Center. Tumors consisted of 55 oligodendrogliomas (OII), 26 oligoastrocytomas (OAII), 37 anaplastic oligodendrogliomas (OIII) and 23 anaplastic oligoastrocytomas (OAIII). Histological diagnoses were made from formalin‐fixed, paraffin‐embedded tissue following the current WHO classification guidelines 14 by two neuropathologists (DL and Y‐LS). The percentage of tumor cells corresponds to the ratio of tumor cells to all cells (tumor and non‐tumor cells) on a slide. All samples included in this study were thoroughly selected to contain a tumor cell composition of at least 50%. Twenty nonneoplastic brain tissue samples obtained from epilepsy surgeries were blindly tested by direct sequencing and PNA clamping PCR for negative controls. In addition, to evaluate the IDH1 mutation statuses in other brain tumors by PNA clamping method, we obtained tumor samples of 20 diffuse astrocytomas (AII), 44 primary glioblastomas (GBMs), 10 medulloblastomas, 15 pilocytic astrocytomas (PAs) and 10 pleomorphic xanthoastrocytomas (PXAs). The DNA we used in this study was all extracted from formalin‐fixed paraffin‐embedded (FFPE) tissues, and we used the QIAamp DNA FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. This study was approved by the Institutional Review Board of Samsung Medical Center.
The following clinical data were determined only for the OIII and OAIII patients: age at the time of first operation, gender, type of primary treatment (gross total resection, partial resection, biopsy), type of adjuvant treatment (chemotherapy, radiation therapy), time of the last follow‐up and patient status at the last follow‐up (alive or deceased). Median follow‐ups were 1529 days (range 4–5561) and 1179 days (range 205–4015) for OIII and OAIII patients, respectively. A total of 19 patients (31.7%) died during the follow‐up period. The major clinical data of these 60 patients are summarized in Supporting Information Table S1.
PNA‐mediated clamping PCR for detection of IDH1 mutations
The IDH1 mutation was tested using the PNAClamp IDH1 Mutation Detection Kit (Panagene, Inc, Daejeon, Korea). All reactions had a total reaction volume of 20 μL and contained template DNA, primer and PNA probe sets and SYBR Green PCR master mix (KapaBiosystems, Woburn, MA, USA). All required reagents were included with the kit. Real‐time PCR reactions of PNA‐mediated clamping PCR were performed using a CFX96 (Bio‐Rad, Hercules, CA, USA). PCR cycling conditions were as follows: 5 minutes at 94°C followed by 40 cycles of 94°C for 30 s, 70°C for 20 s, 63°C for 30 s and 72°C for 30 s. In this assay, PNA probes and DNA primers were used together in the clamping reaction. Positive signals were detected by intercalation of SYBR Green fluorescent dye. The PNA probe, which is complementary to the wild‐type sequence, suppresses amplification of the wild‐type target. This suppression results in preferential amplification of the mutant sequences by competitively inhibiting the binding of DNA primers to wild‐type DNA. PCR efficiency was determined by measuring the threshold cycle (Ct) value. Ct values for control and mutant assays were obtained from SYBR Green amplification plots. Calculations of the delta Ct (ΔCt) value were done as follows: ΔCt1 = [Standard Ct] − [Sample Ct], ΔCt2 = [Sample Ct] − [Non PNA mix Ct]. The gene was considered to be mutated when ΔCt1 values were more than 2.0. When ΔCt1 values were between 0 and 2, a ΔCt2 value was then calculated. The gene was considered to be mutated if the calculated ΔCt2 value was ≤6. PNA clamping PCR for IDH1 mutations can be applied to any qualified laboratories using FFPE tumor samples, and the entire procedure including DNA extraction can be performed within approximately 3 h (Figure 2)
Figure 2.
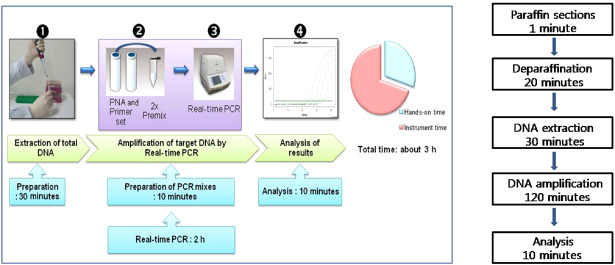
The flowchart and time schedule of the peptide nucleic acid (PNA) clamping method used for the detection of IDH1 mutations in routinely processed glioma tissue specimens. Note that the total time required from cutting paraffin sections to obtaining sequence results amounts to approximately 3 h.
Pyrosequencing analysis
Pyrosequencing for mutational analysis was performed using a PyroMark Q24 (Qiagen, Germantown, MD, USA). Pyrosequencing primer sets were designed to amplify specific target regions of genes using PSQ Assay Design Software (Biotage, Uppsala, Sweden). Each DNA segment was amplified using 0.5 μmol/L of each primer and a 2X PCR premix (Solgent, Daejeon, Korea) in a TP600‐PCR thermal cycler (Takara, Tokyo, Japan). DNA sequences of primers, PNA oligomer and probe sets are summarized in Table 1. Cycling conditions entailed an initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation (95°C for 30 s), annealing (58°C for 40 s) and elongation (72°C for 30 s), with a final elongation step at 72°C for 5 minutes. The cycling protocol contained 40 cycles for the purpose of depleting biotin‐labeled primers in order to prevent unincorporated primer from binding and competing with the amplicon on the streptavidin‐coated beads during pyrosequencing. PCR products were visualized on 2% agarose gel electrophoresis with ethidium bromide staining. Single‐stranded products were prepared from 15–20 μL biotinylated PCR products using streptavidin Sepharose® HP beads (Amersham Biosciences, Eubuckingahamshire, UK) following the PSQ 96 sample preparation guide using multichannel pipettes. Fifteen picomoles of the respective sequencing primers were added for analysis. Pyrosequencing was performed on a PyroMark ID system with the Pyro Gold reagents kit (Biotage, Uppsala, Sweden) and was used according to the manufacturer's instructions without further optimization. All experiments included a negative control without a template.
Table 1.
DNA sequences of primers, PNA oligomer and probe sets for pyrosequencing and direct/nested PCR sequencing
| IDH1 gene | Primer | Size (bp) | |
|---|---|---|---|
| Pyrosequencing | Forward | 5′‐ CGGTCTTCAGAGAAGCCATT‐3′ | 131 |
| Biotinylated‐reverse | 5′‐ GCAAAATCACATTATTGCCAAC‐3′ | 131 | |
| Pyrosequencing primer | 5′‐GGGTAAAACCTATCATCA‐3′ | — | |
| Sequence to analyze | TAGGTCGT(GGT, CAT, CTT, AGT, TGT)CA | — | |
| Direct/nested PCR sequencing | PNA probe (clamping) | N′‐ AGCATGACGACCTAT‐C′ | 131 |
| Forward | 5′‐ CGGTCTTCAGAGAAGCCATT‐3′ | 131 | |
| Reverse | 5′‐ GCAAAATCACATTATTGCCAAC‐3′ | 131 | |
| Sequencing primer | 5′‐ CGGTCTTCAGAGAAGCCATT‐3′ | — | |
Direct/nested PCR sequencing
We used a specially designed IDH1 clamping PNA probe for direct and nested PCR sequencing. PCR was initially performed with 30 ng of clinical sample DNA per reaction, which was amplified using 0.5 μmol/L of each sequencing primer, 5 μmol/L of the IDH1 clamping probe and 2X PCR premix in a TP600‐PCR thermal Cycler (Takara, Tokyo, Japan). The following conditions were used for DNA amplification: initial denaturation at 94°C for 5 minutes, followed by 40 cycles of denaturation (94°C for 30 s), PNA annealing (70°C for 20 s), primer annealing (63°C for 30 s) and elongation (72°C for 30 s). After the first round of PCR, consecutive PCR amplification was performed using the PCR products from the initial DNA amplification. Cycling conditions entailed an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles of denaturation (95°C for 30 s), annealing (58°C for 40 s) and elongation (72°C for 30 s), with a final elongation at 72°C for 5 minutes. Finally, a direct sequencing reaction was performed using an ABI PRISM 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA) system with Bigdye® Terminator v3.1 Cycle Sequencing Kit (ABI, Carlsbad, CA, USA) according to the manufacturer's instruction.
IHC
IHC was carried out on FFPE, 4‐μm thick tissue sections. Anti‐IDH1 R132H was used as the primary antibody (clone H09, Dianova, Hamburg, Germany). Immunostaining was performed using a Ventana BenchMark XT® autoimmunostainer (Ventana Medical Systems, Tucson, AZ, USA) with a cell conditioner 1 for 60 minutes. Slides were then incubated with 1:150 anti‐IDH1 R132H supernatant at 37°C for 32 minutes, followed by standard Ventana signal amplification, counterstaining with hematoxylin for 4 minutes and staining with a bluing reagent for 4 minutes. Slides were then removed from the immunostainer, mounted and examined by light microscopy. Strong cytoplasmic staining in any number of cells was scored as positive. Slides processed without the primary antibodies were used as negative controls.
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 18.0, SPSS Inc, Chicago, IL, USA). Fisher's exact test was used to compare the quantitative data. Overall survival (OS) time was defined as the interval between first surgery and death or last follow‐up visit. The survival rates were estimated using the Kaplan‐Meier method, and the survival curves were compared by the log‐rank test. A P‐value less than 0.05 was considered statistically significant. All the reported P‐values are two‐sided.
Results
We screened 141 oligodendroglial tumors for the presence of IDH1 mutations by PNA clamping PCR (n = 141), direct sequencing (n = 141), pyrosequencing (n = 141), IHC (n = 141) and nested PCR (n = 27). Table 2 provides an overview of the results of IDH1 mutations from the different types of oligodendroglial tumors included in this study. Complete IDH1 mutation data from all detection methods are presented in the Supporting Information Table S2. The frequencies of detected IDH1 mutations were similar between direct sequencing (74.5%), pyrosequencing (75.2%) and IHC (75.9%). Only the clamping PCR method showed remarkably high sensitivity and detected 138 of 141 (97.9%) cases with an IDH1 mutation in our series (P = 0.016; PNA clamping vs. direct sequencing). The three cases of wild‐type IDH1 assessed by clamping PCR were also found to have wild‐type IDH1 by direct sequencing, pyrosequencing and IHC. All diagnostic methods for the detection of IDH1 mutations presented here were tested at least twice.
Table 2.
Summary of IDH1 mutation prevalence according to the various techniques used in grade II and III oligodendroglial tumors. Abbreviations: OII = oligodendroglioma; OAII = oligoastrocytoma; OIII = anaplastic oligodendroglioma; OAIII = anaplastic oligoastrocytoma; IHC = immunohistochemistry
| Detection tool | Number of IDH1 mutations | |||||
|---|---|---|---|---|---|---|
| OII (%) | OAII (%) | OIII (%) | OAIII (%) | Mutant, total (%) | ||
| Direct sequencing | Mutant | 42 (76.4) | 19 (73.1) | 28 (75.7) | 16 (69.6) | 105 (74.5) |
| R132H | 40 | 16 | 28 | 15 | ||
| R132G | 2 | 2 | — | 1 | ||
| R132S | — | 1 | — | — | ||
| Wild | 13 | 7 | 9 | 7 | ||
| Pyrosequencing | Mutant | 43 (78.2) | 19 (73.1) | 28 (75.7) | 16 (69.6) | 106 (75.2) |
| R132H | 41 | 16 | 28 | 15 | ||
| R132G | 2 | 2 | — | 1 | ||
| R132S | — | 1 | — | — | ||
| Wild | 12 | 7 | 9 | 7 | ||
| IHC | Positive | 47 (85.5) | 18 (69.2) | 27 (73) | 15 (65.2) | 107 (75.9) |
| Negative | 8 | 8 | 10 | 8 | ||
| Nested PCR | R132H | 4 | 3 | 3 | 5 | — |
| R132G | — | — | 2 | — | ||
| Wild | — | 2 | 1 | 1 | ||
| Fail | 3 | — | 3 | — | ||
| Clamping PCR | Mutant | 54 (98.2) | 25 (96.2) | 37 (100) | 22 (95.7) | 138 (97.9) |
| Wild | 1 | 1 | 0 | 1 | ||
| Total | 43 | 26 | 37 | 23 | 141 | |
To evaluate the validity and sensitivity of PNA clamping PCR, two additional experiments were performed. First, direct sequencing and clamping PCR were tested in 20 cases of nonneoplastic brain tissue from epilepsy surgery as a negative control. All cases were found to possess wild‐type IDH1 by both methods (Supporting Information Table S3). Second, we performed dilution experiments by mixing wild‐type (from U87‐MG human glioblastoma‐astrocytoma cell line) 5 and mutant IDH1 (from a mutant clone) DNA samples in different proportions (Figure 3). These results showed that clamping PCR was able to detect IDH1 mutations with a mutant allele frequency of 1% or more.
Figure 3.
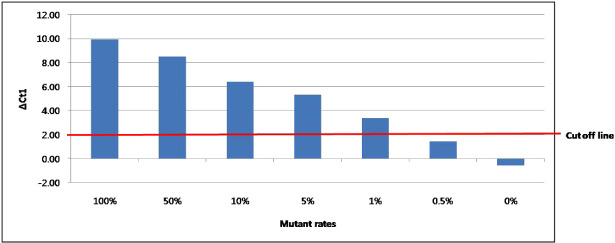
Results of dilution experiments mixing IDH1 wild‐type (from U87‐MG human glioblastoma‐astrocytoma cell line) and mutant (from a mutant clone) DNA samples in different proportions. These results indicate that the PNA clamping method is able to detect IDH1 mutations with a mutant allele frequency of 1% or more by our standards (ΔCt1 > 2.0).
Next, we performed quantitative mutational analysis using the results from pyrosequencing and clamping PCR. Pyrosequencing detected a median of 53.3% (range 0–81.4%) of residual wild‐type alleles in tumors carrying an IDH1 mutation. This tumor population had a median percentage of mutant alleles of 46.7% (range 18.6–100%). In tumors carrying a wild‐type IDH1 as assessed by pyrosequencing, the median percentage of tumors with the wild‐type allele was 87.5% (range 84.1–98%), while the median percentage of residual mutant alleles was 12.5% (range 2–15.9%). These results indicate that cases which have been presumed to have wild‐type IDH1 still possess a low percentage of mutant alleles, an important finding that may be missed by other conventional detection methods. Alternatively, in tumors carrying mutated IDH1, clamping PCR detected mutant alleles of an estimated median of 25% (range 1–80%). The three tumors having wild‐type IDH1 as detected by clamping PCR appeared to possess almost all wild‐type IDH1 alleles only.
We had hypothesized that the three cases without an IDH1 mutation by clamping PCR in our cohort might have an IDH2 mutation. Because we did not develop IDH2 clamping kit yet, we only performed direct sequencing and pyrosequencing for the detection of IDH2 mutations on these three cases and found only one case with an IDH2 mutation (R172K).
Using clamping PCR, 27 additional cases of IDH1 mutation were detected that had been missed by direct sequencing and pyrosequencing (Table 3). All of these cases had a ΔCt1 value of less than 4. It was interesting that the percentages of these cases increased in an ascending order according to the histological diagnoses: OII (7/55, 12.7%), OAII (5/26, 19.2%), OIII (9/37, 24.3%), OAIII (6/23, 26.1%). The estimated median percentage of mutant alleles found by clamping PCR was 1% (range 1–25%) in these cases. To confirm the presence of mutations in these cases, nested PCR was performed. Aside from the six cases in which we failed to amplify DNA, 17 of the remaining 21 cases (81%) demonstrated IDH1 mutations (R132H 15 cases, R132G 2 cases). In addition, 5 of these 27 cases (18.5%) were tested positive for anti‐IDH1‐R132H, and all of these five cases showed only a few positive cells.
Table 3.
Results of cases demonstrating a wild‐type IDH1 from the analyses with direct sequencing and pyrosequencing, but showing a mutant IDH1 when assessed by clamping PCR. Abbreviations: OII = oligodendroglioma; OAII = oligoastrocytoma; OIII = anaplastic oligodendroglioma; OAIII = anaplastic oligoastrocytoma
| Case No. | Diagnosis | Direct sequencing | Pyrosequencing | IHC | Nested PCR | Clamping PCR | ΔCt1 | ΔCt2 |
|---|---|---|---|---|---|---|---|---|
| 1 | OII | Wild | Wild | − | R132H | Mutant | 2.55 | 6.03 |
| 2 | OII | Wild | Wild | + | R132H | Mutant | 2.44 | 4.32 |
| 3 | OII | Wild | Wild | − | R132H | Mutant | 2.7 | 5.27 |
| 4 | OII | Wild | Wild | + | Fail | Mutant | 2.75 | 2.05 |
| 5 | OII | Wild | Wild | + | R132H | Mutant | 0.87 | 1.91 |
| 6 | OII | Wild | Wild | + | Fail | Mutant | 0.77 | 2.61 |
| 7 | OII | Wild | Wild | − | Fail | Mutant | 2.6 | 7.12 |
| 8 | OAII | Wild | Wild | − | Wild | Mutant | 1.7 | 5.51 |
| 9 | OAII | Wild | Wild | − | Wild | Mutant | 3.06 | 6.77 |
| 10 | OAII | Wild | Wild | + | R132H | Mutant | 2.79 | 5.04 |
| 11 | OAII | Wild | Wild | − | R132H | Mutant | 2.34 | 7.49 |
| 12 | OAII | Wild | Wild | − | R132H | Mutant | 2.58 | 5.95 |
| 13 | OIII | Wild | Wild | − | R132G | Mutant | 2.65 | 5.45 |
| 14 | OIII | Wild | Wild | − | R132H | Mutant | 2.55 | 6.16 |
| 15 | OIII | Wild | Wild | − | R132G | Mutant | 1.1 | 5.49 |
| 16 | OIII | Wild | Wild | − | Fail | Mutant | 1.97 | 5.81 |
| 17 | OIII | Wild | Wild | − | Wild | Mutant | 2.45 | 7.45 |
| 18 | OIII | Wild | Wild | − | Fail | Mutant | 3.54 | 6.14 |
| 19 | OIII | Wild | Wild | − | R132H | Mutant | 2.25 | 5.21 |
| 20 | OIII | Wild | Wild | − | R132H | Mutant | 2.36 | 5.37 |
| 21 | OIII | Wild | Wild | − | Fail | Mutant | 2.86 | 6.5 |
| 22 | OAIII | Wild | Wild | − | R132H | Mutant | 3.93 | 5.1 |
| 23 | OAIII | Wild | Wild | − | R132H | Mutant | 3.42 | 6.76 |
| 24 | OAIII | Wild | Wild | − | R132H | Mutant | 3 | 7.78 |
| 25 | OAIII | Wild | Wild | − | R132H | Mutant | 3.23 | 7.65 |
| 26 | OAIII | Wild | Wild | − | R132H | Mutant | 3.71 | 6 |
| 27 | OAIII | Wild | Wild | − | Wild | Mutant | 3.73 | 6.65 |
To evaluate the effect of PNA clamping method for the prevalence of IDH1 mutations in other brain tumors than oligodendroglial tumors, we performed clamping PCR and direct sequencing in a total of 99 cases of brain tumors. IDH1 mutation was detected in 4 of 20 (25%) cases of AII by both methods. Direct sequencing found only one case of PA with an IDH1 mutation (1/15, 6.7%), while PNA clamping detected two additional cases having an IDH1 mutation (3/15, 20%). Of 44 cases of primary GBMs, 2 cases (4.5%) having an IDH1 mutation was screened by direct sequencing, and the number was doubled by clamping method (4 cases, 9.1%). IDH1 mutation was not detected even by clamping PCR in all the cases of PXAs and medulloblastomas tested (10 cases each), as previously documented 1.
Then we asked for the clinical usefulness of IDH1 mutations detected by this sensitive technique. We divided the patients with OIII and OAIII into two groups by ΔCt1 values. The cutoff values for each group were set to best discriminate the patients' survival statistically. The median OS of OIII patients with ΔCt1 > 2.7 was significantly longer (P = 0.016) as compared with OIII patients with ΔCt1 ≤ 2.7 (Figure 4A). In line with this, the median survival of OAIII patients with ΔCt1 > 4 was significantly longer (P = 0.002) as compared with OAIII patients with ΔCt1 ≤ 4 (Figure 4B). Even though PNA clamping method is not a quantitative test, these results suggest that the relative amount of IDH1 mutant alleles influence the clinical outcomes and can stratify the patients.
Figure 4.
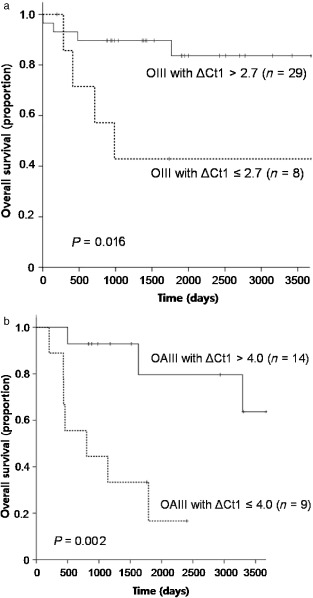
Kaplan‐Meier survival curves for overall survival in patients with (i) anaplastic oligodendroglioma (OIII); and (ii) anaplastic oligoastrocytoma (OAIII) according to the ΔCt1 values from clamping PCR.
Direct sequencing and pyrosequencing revealed similar IDH1 mutation detection results in our cohort. Indeed, results were identical in OAII, OIII and OAIII, while differences were found in only a few cases (three cases) of OII. The overall IHC sensitivity (75.9%) was similar to the sensitivity of other techniques, but there existed some minor differences. For example, five cases found to be positive by IHC tested negative by direct sequencing and pyrosequencing, as described earlier. These five cases carried mutant IDH1 as determined by clamping PCR: three cases had R132H mutation as confirmed by nested PCR, and in the other two cases, nested PCR failed to amplify the DNA. Mutant IDH1 was detected by direct sequencing or pyrosequencing in six cases; however, IHC for these cases was negative even after repetitive staining. Furthermore, although the IMab‐1 antibody was designed to selectively detect R132H, three cases were tested positive but were found to have R132G mutation. Cases with other types of IDH1 mutations such as R132G (two cases) and R132S (one case) had negative results by IHC. Samples that had different results by the various detection methods described previously were analyzed in triplicate independent experiments.
Discussion
Technological advances in the life sciences have enabled the development of more sensitive tools than direct sequencing for the detection of DNA mutations. In field of neuro‐oncology, various approaches with comparable sensitivity have been developed to detect IDH1 mutations and have been practically applied 7, 11, 22, 24. PNA clamping PCR is a promising new method for the detection of IDH1 mutations and has been successfully applied for the detection of other DNA mutations such as epidermal growth factor receptor (EGFR) and K‐ras 2, 9. Sensitivity is a critical issue for molecular diagnostic approaches as mutations may only be present in a subset of tumor cells, and wild‐type alleles may still be present in tumor cells with mutations in IDH1. In addition, biopsy specimens from glial tumors often contain considerable numbers of normal cells. In this study, we showed that clamping PCR allows detection of as little as 1% of IDH1 mutant alleles in the background of wild‐type DNA. The high sensitivity of clamping PCR suggests a potential role for this method in assessing diagnostically ambiguous tissue samples with low tumor cellularity, especially in the infiltrating tumor periphery. In addition, PNA clamping PCR is a fast, more simple and economical method for the IDH1 mutation detection than direct sequencing, and this test would be a valuable asset in clinical laboratories.
The prevalence of IDH1 mutations in grades II/III oligodendroglial tumors differs across studies (Table 4). In 2008, Balss et al 1 reported that the frequencies of IDH1 mutations ranged from 67% to 78% in these tumors by direct sequencing. Since then, many investigators have examined IDH1 mutations in glial tumors using different methods. In addition to direct sequencing 10, 25, 30, other techniques such as pyrosequencing and IHC have been used 7, 24. The overall frequencies of IDH1 mutations appear to be somewhat increasing as detection tools become more sensitive. Recently, Capper et al 4 reported a frequency of IDH1 mutation of approximately 90% in grades II/III oligodendroglial tumors when results of IHC and direct sequencing were combined. In the current study, we used PNA clamping PCR and detected higher IDH1 mutation frequencies ranging from 95.7% to 100% in grades II/III oligodendroglial tumors. Only 3 of 141 cases (2.1%) assessed by clamping PCR contained wild‐type IDH1. Initially, this unexpected high mutational frequency suggested a false‐positive result. However, these results were confirmed by two or more repetitive tests. Furthermore, mutant IDH1 was not demonstrated in any of 20 cases of nonneoplastic brain tissues using direct sequencing or clamping PCR, indicating the validity of our results.
Table 4.
Previously reported IDH1 mutation frequencies in oligodendroglial tumors of various histologic subtypes. Abbreviations: OII = oligodendroglioma; OAII = oligoastrocytoma; OIII = anaplastic oligodendroglioma; OAIII = anaplastic oligoastrocytoma; IHC = immunohistochemistry; SSCP = single‐strand conformation polymorphism
| OII (%) | OAII (%) | OIII (%) | OAIII (%) | |
|---|---|---|---|---|
| Balss et al 1 (direct sequencing) | 74 | 78 | 67 | 78 |
| Sanson et al 25 (direct sequencing) | 76 | 76 | 49 | 63 |
| Watanabe et al 30 (SSCP and direct sequencing) | 79 | 94 | 75 | 91 |
| Horbinski et al 11 (melting curve analysis) | 79.2 | 50 | 88 | — |
| Hartmann et al 10 (direct sequencing) | 82 | 81.6 | 69.5 | 66.1 |
| Felsberg et al 7 (pyrosequencing) | 62.5 | — | — | 80 |
| Capper et al 4 (IHC and direct sequencing) | 91 | 79 | 95.7 | 91 |
| Current study (PNA clamping PCR) | 98.2 | 96.2 | 100 | 95.7 |
Recently described next‐generation sequencing technology has influenced cancer genomics enormously, and it affords rapid, cost‐effective “deep sequencing” of cancer genomes, which enables the genome‐wide searches of cancer‐associated somatic mutations 16, 23. For the detection of IDH1 mutations, this new sequencing method appears to be an emerging golden standard. Bettegowda et al 3 performed exome sequencing of seven OII, and found the IDH1 gene was mutated in all the seven tumors. More recently, Yip et al 31 also performed exome sequencing of 16 cases of OII, and revealed that all cases had mutations in either IDH1 (14/16) or IDH2 (2/16), which was validated by deep sequencing. These previous data strongly support our results and the hypothesis that all oligodendroglial tumors might have mutations in IDH genes. To prove this hypothesis, we performed direct sequencing and pyrosequencing for the detection of IDH2 mutations on three cases having wild‐type IDH1 by clamping PCR, but only found one IDH2 mutation. However, if we could have used a more sensitive technique, the results might be changed. Regretfully, we did not develop IDH2 clamp kit yet, we cannot prove this at present and this is our drawback.
Watanabe et al 30 analyzed multiple biopsy specimens from the same patients (51 patients) and revealed that there were no instances in which an IDH1 mutation occurred after the acquisition of either TP53 mutation or loss of 1p/19q. This suggests that IDH1 mutations occur early in gliomagenesis and may affect a common glial precursor cell population. Our results support this idea as almost all oligodendroglial tumors had IDH1 mutations by clamping PCR. Moreover, this implies that mutations in IDH1 are basic, underlying genetic alterations in the development of oligodendroglial tumors. As previously documented 10, 25, IDH1 mutational status is one of the most powerful prognostic indicators in patients with low and high grade gliomas. However, with this sensitive technique, IDH1 mutation will no longer have a prognostic value as almost all tumors contained these mutations. We analyzed the newly found IDH1‐mutated tumors (27 cases, 19.1%) by clamping PCR, and all had a ΔCt1 value of less than 4, suggesting a relatively low percentage of mutant alleles. From the results of pyrosequencing, the median percentage of mutant alleles in these tumors was found to be 3.2% (range 2–15.9%). We confirmed that these tumors had a lower percentage of mutant alleles when compared to those of the other cases carrying IDH1 mutations (median 44.3%, range 18.6–100%). In addition, the fact that more tumors with higher grade and/or astrocytic component were included in this group of tumors may suggest that not just the presence or absence of IDH mutation but low percentage of IDH1 mutant alleles is associated with disease progression and clinical outcome. Indeed, we proved this that relative quantitation of IDH1 mutant alleles according to the ΔCt1 value from clamping PCR could stratify the patients' survival. Therefore, quantitative analysis for the IDH1 mutation appears to be necessary sooner or later from this point of view. In the current study, we applied different cutoff values to OIII and OAIII patients. That is because we set the best cutoff values to stratify patients' survival statistically in two different tumor groups, and they were simply different. This difference may be ascribed to (i) different proportions of IDH1 mutant alleles in astrocytic and oligodendroglial tumor cells by nature; (ii) different proportions of astrocytic elements even in the OAIII; and (iii) possible interaction between astrocytic and oligodendroglial tumor elements for the IDH1 mutations.
The most prominent consequence universally accepted in IDH mutation is that altered enzyme acquires neomorphic activity to reduce α‐ketoglutarate (αKG) into a novel oncometabolite, D‐2‐hydroxyglutarate (D‐2HG) in an nicotinamide adenine dinucleotide phosphate (NADP)‐dependent manner 5. Indeed, elevated D‐2HG levels were found in acute myeloid leukemia and brain tumor patients with IDH1 and IDH2 mutations 5, 8. However, exact mechanisms through which IDH1/2 mutations contribute to the pathogenesis of gliomas are still not fully understood. There are only several hypotheses regarding the molecular pathogenesis of IDH mutations have been proposed. For example, D‐2HG may compete with αKG and inhibit prolyl hydroxylase (PHD)‐mediated degradation of hypoxia‐inducible factor (HIF)‐1α. Accumulated HIF‐1α may induce expression of vascular endothelial growth factor (VEGF), promoting angiogenesis, which might enhance tumor growth 32. In the second hypothesis, IDH1/2 mutations promote tumorigenesis by deregulating gene expression from DNA hypermethylation at a number of targeted genes 12, 18. The possibility is also raised that an oxidative DNA damage induced by decreased NADP‐dependent IDH activity may be involved in tumorigenesis 12, 13. However, there are still limitations to explain the gliomagenesis in IDH‐mutated tumors only by these hypotheses yet 12. The biologic function of IDH and its role in the development and progression of glial tumors still needs to be investigated further.
Unlike oligodendroglial tumors, PNA clamping method failed to find such a high frequency of IDH1 mutations in other glial tumors including PAs, AIIs and primary GBMs. Of course, clamping PCR increased the mutation frequency almost the double in PAs and primary GBMs, but the general prevalence was still low, measuring only 20% and 9.1% in these tumors, respectively. This result confirms that IDH1 mutation is highly specific for oligodendroglial tumors, although this mutation also occurs in a small fraction of other glial tumors.
In the current study, different results were derived from direct sequencing, pyrosequencing and IHC in several cases. These differences are likely due to the differing sensitivities of each method or to undiscovered technical problems. Although there is general agreement that PCR is more sensitive than IHC, we found that IHC may be more sensitive in the detection of mutations in IDH1, especially in tumors which have just a fraction of tumor cells having mutant IDH1. In general, investigators apply different cutoff values when they interpret IHC results. Some consider that 10% of tumor cells should be reactive in order to be rated as positive 28, while others consider a strong cytoplasmic staining in just a few cells to be a positive result 4. We used the latter criteria in this study. As discussed earlier, any number of positive cells identified by IHC should be interpreted meaningfully so as not to miss the presence of IDH1 mutations in a small number of tumor cells.
Conclusions
We established a robust method of PNA‐mediated clamping PCR to detect IDH1 mutations with the sensitivity of 1% or more frequency of mutant allele in diagnostic tissue samples. With this technique, we found that 97.5% of oligodendroglial tumors in our cohort had mutant IDH1 genes, a significantly higher figure than results assessed by other methods. As previously suggested, IDH1 mutation seems to be an early and common event especially in the development of oligodendroglial tumors. The biologic function of mutated IDH1 and its role in the development and progression of oligodendroglial tumors needs to be further elucidated in this context.
Conflicts of interest
The authors have no conflicts of interest to declare.
Supporting information
Table S1. The clinical characteristics of the 60 patients with OIII and OAIII.
Table S2. The whole data of results of IDH1 mutations according to different methods.
Table S3. Results of direct sequencing and PNA clamping PCR for IDH1 mutations on non‐tumor brain tissues.
Acknowledgments
This work was supported by a Biomedical Research Institute grant, the Kyungpook National University Hospital (2012) and Kyungpook National University Research Fund (2012).
References
- 1. Balss J, Meyer J, Mueller W, Korshunov A, von Hartmann C, Deimling A (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol (Berl) 116:597–602. [DOI] [PubMed] [Google Scholar]
- 2. Beau‐Faller M, Legrain M, Voegeli AC, Guerin E, Lavaux T, Ruppert AM et al (2009) Detection of K‐Ras mutations in tumour samples of patients with non‐small cell lung cancer using PNA‐mediated PCR clamping. Br J Cancer 100:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bettegowda C, Agrawal N, Jiao Y, Sausen M, Wood LD, Hruban RH et al (2011) Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333:1453–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capper D, Reuss D, Schittenhelm J, Hartmann C, Bremer J, Sahm F et al (2011) Mutation‐specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma‐like morphology. Acta Neuropathol (Berl) 121:241–252. [DOI] [PubMed] [Google Scholar]
- 5. Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM et al (2009) Cancer‐associated IDH1 mutations produce 2‐hydroxyglutarate. Nature 462. pp. 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eberhard DA, Giaccone G, Johnson BE (2008) Biomarkers of response to epidermal growth factor receptor inhibitors in Non‐Small‐Cell Lung Cancer Working Group: standardization for use in the clinical trial setting. J Clin Oncol 26:983–994. [DOI] [PubMed] [Google Scholar]
- 7. Felsberg J, Wolter M, Seul H, Friedensdorf B, Goppert M, Sabel MC, Reifenberger G (2010) Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol (Berl) 119:501–507. [DOI] [PubMed] [Google Scholar]
- 8. Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG et al (2010) Cancer‐associated metabolite 2‐hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 207:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han HS, Lim SN, An JY, Lee KM, Choe KH, Lee KH et al (2012) Detection of EGFR mutation status in lung adenocarcinoma specimens with different proportions of tumor cells using two methods of differential sensitivity. J Thorac Oncol 7:355–364. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1‐mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol (Berl) 120:707–718. [DOI] [PubMed] [Google Scholar]
- 11. Horbinski C, Kelly L, Nikiforov YE, Durso MB, Nikiforova MN (2010) Detection of IDH1 and IDH2 mutations by fluorescence melting curve analysis as a diagnostic tool for brain biopsies. J Mol Diagn 12:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ichimura K (2012) Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol 29:131–139. [DOI] [PubMed] [Google Scholar]
- 13. Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW (2002) Cytosolic NADP(+)‐dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med 32:1185–1196. [DOI] [PubMed] [Google Scholar]
- 14. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) (2007) WHO Classification of Tumors of the Central Nervous System. IARC: Lyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer J, Pusch S, Balss J, Capper D, Mueller W, Christians A et al (2010) PCR‐ and restriction endonuclease‐based detection of IDH1 mutations. Brain Pathol 20:298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morin RD, Mendez‐Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD et al (2011) Frequent mutation of histone‐modifying genes in non‐Hodgkin lymphoma. Nature 476:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagai Y, Miyazawa H, Huqun TT, Udagawa K, Kato M, Fukuyama S et al (2005) Genetic heterogeneity of the epidermal growth factor receptor in non‐small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid‐locked nucleic acid PCR clamp. Cancer Res 65:7276–7282. [DOI] [PubMed] [Google Scholar]
- 18. Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orum H, Nielsen PE, Egholm M, Berg RH, Buchardt O, Stanley C (1993) Single base pair mutation analysis by PNA directed PCR clamping. Nucleic Acids Res 21:5332–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pao W, Ladanyi M (2007) Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res 13:4954–4955. [DOI] [PubMed] [Google Scholar]
- 21. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perizzolo M, Winkfein B, Hui S, Krulicki W, Chan JA, Demetrick DJ (2012) IDH Mutation Detection in Formalin‐Fixed Paraffin‐Embedded Gliomas Using Multiplex PCR and Single‐base Extension. Brain Pathol 22:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD et al (2010) A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Preusser M, Wohrer A, Stary S, Hoftberger R, Streubel B, Hainfellner JA (2011) Value and limitations of immunohistochemistry and gene sequencing for detection of the IDH1‐R132H mutation in diffuse glioma biopsy specimens. J Neuropathol Exp Neurol 70:715–723. [DOI] [PubMed] [Google Scholar]
- 25. Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F et al (2009) Isocitrate dehydrogenase 1 con 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27:4150–4154. [DOI] [PubMed] [Google Scholar]
- 26. Sutani A, Nagai Y, Udagawa K, Uchida Y, Koyama N, Murayama Y et al (2006) Gefitinib for non‐small‐cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid‐locked nucleic acid PCR clamp. Br J Cancer 95:1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taback B, Bilchik AJ, Saha S, Nakayama T, Wiese DA, Turner RR et al (2004) Peptide nucleic acid clamp PCR: a novel K‐ras mutation detection assay for colorectal cancer micrometastases in lymph nodes. Int J Cancer 111:409–414. [DOI] [PubMed] [Google Scholar]
- 28. Takano S, Tian W, Matsuda M, Yamamoto T, Ishikawa E, Kaneko MK et al (2011) Detection of IDH1 mutation in human gliomas: comparison of immunohistochemistry and sequencing. Brain Tumor Pathol 28:115–123. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka T, Nagai Y, Miyazawa H, Koyama N, Matsuoka S, Sutani A et al (2007) Reliability of the peptide nucleic acid‐locked nucleic acid polymerase chain reaction clamp‐based test for epidermal growth factor receptor mutations integrated into the clinical practice for non‐small cell lung cancers. Cancer Sci 98:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yip S, Butterfield YS, Morozova O, Chittaranjan S, Blough MD, An J et al (2012) Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol 226:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P et al (2009) Glioma‐derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF‐1alpha. Science 324:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The clinical characteristics of the 60 patients with OIII and OAIII.
Table S2. The whole data of results of IDH1 mutations according to different methods.
Table S3. Results of direct sequencing and PNA clamping PCR for IDH1 mutations on non‐tumor brain tissues.


